w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Review
article
Thrombin
generation
assays
for
global
evaluation
of
the
hemostatic
system:
perspectives
and
limitations
Rita
Carolina
Figueiredo
Duarte
a,
Cláudia
Natália
Ferreira
a,
Danyelle
Romana
Alves
Rios
b,
Helton
José
dos
Reis
a,
Maria
das
Grac¸as
Carvalho
a,∗ aUniversidadeFederaldeMinasGerais(UFMG),BeloHorizonte,MG,BrazilbUniversidadeFederaldeSãoJoãodel-Rei(UFSJ),Divinópolis,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received15April2016 Accepted30March2017 Availableonline9May2017
Keywords:
Thrombingeneration CATmethod Hemostasis
a
b
s
t
r
a
c
t
Theexistingtechniquestoevaluatehemostasisinclinicallaboratoriesarenotsensitive enoughtodetecthypercoagulableandmildhypocoagulablestates.Underdifferent exper-imental conditions, the thrombin generation test maymeet these requirements. This techniqueevaluatestheoverallbalancebetweenprocoagulantandanticoagulantforcesand hasprovidednewinsightsinourunderstandingofthecoagulationcascade,aswellasof thediagnosisofhypocoagulabilityandhypercoagulabilityconditions.Thrombingenerated inthethrombingenerationtestcanbequantifiedasplatelet-richorplatelet-poorplasma usingthecalibratedautomatedthrombogrammethod,whichmonitorsthecleavageofa fluorogenicsubstratethatissimultaneouslycomparedtotheknownthrombinactivityin anon-clottingplasmasample.Thecalibratedautomatedthrombogrammethodisanopen system,inwhichdifferentantibodies,proteins,enzymesandpeptidescanbeintroducedto answerspecificquestionsregardinghemostaticprocesses.Thethrombingenerationtesthas greatclinicalpotential,suchasinmonitoringpatientstakinganticoagulantsandantiplatelet drugs,screeningforgeneticoracquiredthromboticdisorders,andevaluatingbleedingrisk controlinpatientswithhemophiliausingbypassagentsorreplacementtherapy.Different toconventionalcoagulationtests,thethrombingenerationtestcanbeusedforanoverall evaluationofhemostasis,theresultsofwhichcanthenbeusedtoevaluatespecific char-acteristicsofhemostasis,suchasprothrombintime,activatedpartialthromboplastintime, andlevelsoffibrinogenandothercoagulationfactors.Theintroductionofthismethod willcontributetoabetterunderstandingandevaluationofoverallhemostaticprocesses; however,thismethodstillrequiresstandardizationandclinicalvalidation.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:ClinicalandToxicologicalDepartment,UniversidadeFederaldeMinasGerais(UFMG),Av.AntônioCarlos,6627,
31270-910Pampulha,BeloHorizonte,Brazil.
E-mailaddress:mgcarvalho@farmacia.ufmg.br(M.G.Carvalho). http://dx.doi.org/10.1016/j.bjhh.2017.03.009
Introduction
Different aspects of hemostasis can be studied using the methods that are currently available to researchers and clinicallaboratories.These includecoagulometryand chro-mogenic methods, which assess separate aspects of the hemostaticprocess.However,thesemethodsdonotprovide anoverall evaluationof hemostasis.1 The method of
eval-uation is chosen depending on the patient’s clinical data, andmayseparatelyprovideimportantinformationabout pri-mary,secondaryandtertiaryphasesofhemostasis,inaddition tonatural inhibitors such asprotein C (PC)protein S (PS), antithrombin(AT)andtissuefactorpathwayinhibitor(TFPI). Molecular methods also contribute to the investigation of hemostaticdisorders,inparticular,incasesofgenetic resis-tancetoactivatedPC(aPC),whichisprimarilycausedbyfactor VLeidenand other uncommonmutations,and incasesof hyperprothrombinemiacausedbytheG20210Aprothrombin mutation.2Despitethediversityoflaboratorymethodsused
toevaluatehemostaticprocesses,noneofthecurrently avail-ablemethodsisabletoassessallthephasesofhemostasis. Thus,theresultsobtainedusingtheseconventionalmethods arenotalwaysassociatedwithclinicalmanifestations.2
Traditionallyusedmethodshavesatisfactorysensitivityfor moderateandseverehypocoagulability,butnotfor hyperco-agulableormildhypocoagulablestates.Suchmethodsonly provideinformationaboutthebeginning ofthecoagulation process, and therefore, the resultof the test is not repre-sentativeoftheentireclotformationprocess,asmeasured using the total thrombin generation capacity.2–4 The
typi-calcoagulometricmeasurements,suchasprothrombintime (PT)andactivatedpartialthromboplastintime(aPTT), mea-sure onlythe clottingtime corresponding to the initiation phaseofthecoagulationprocess.Furthermore,theend-point ofthesetestsoccursaftertheformationofonly5%oftotal thrombin.2,4 Therefore, PTand aPTTreflectonlythe initial
coagulation process while the formation of thrombin and fibrin isstill occurring.2,5 A greater amount ofthrombin is
generatedduringtheamplificationandpropagationphases, resulting in an exponential increase in thrombin, which becomesinactivatedbyphysiologicalanticoagulantssuchas alpha-2-macroglobulin,AT,PCandPS.1,2 Therefore,
conven-tionaltestsdonotprovideinformationabouttheamplification andpropagationphasesofthehemostaticsystem.1,2
Theintroductionofamethodabletoevaluatetheentire coagulation process is highly desirable, as this could bet-ter reflect bleeding and thrombotic risks. Many attempts havebeen madeto achieve this goal,which would ideally accuratelyreflectallcomponentsandconditionsofthe hemo-staticprocess,includingplatelets,coagulationfactors,natural inhibitors, theendotheliumand its interactions,aswell as fibrinolysisandbloodflow.Severalauthorshavesuggestedthe needtointroduceinvitromethodsrepresentativeofthemain physiologicalaspectsofhemostasisasapossiblesolution.3,6–8
Attemptstodevelopamethodtocomprehensivelyevaluate hemostasisbeganseveraldecadesago.In1953,MacFarlane and Biggs9 were the first toreport thrombin generation in
thebloodusingalaboriousandtime-consumingtechnique, whichrendereditinapplicableforuseintheclinicalpractice.
Inthesameyear,PitneyandDacie10reportedthe
measure-ment of thrombin generation inplasma. Many years later, convincedoftheneedforacomprehensivetestforthebroader assessmentofhemostasis,theillustriousProfessorCoenraad Hemkeretal.attheUniversityofMaastricht(Netherlands)1,11
improvedandsemi-automatedthethrombingeneration tech-nique,initiallyemployingachromogenicmethod,andlatera fluorogenicmethod.12Thisimprovementcontributedgreatly
tothesuccessfuluseofthistechniqueinnumerousstudies. Duetothelackofinformationaboutglobaltestsof throm-bingeneration,wepresentashortdiscussionofthistechnique withemphasisonthecalibratedautomatedthrombogram® (CAT) method. Inthis concise review,we present method-ological aspectsofthe thrombin generation test(TGT), the evaluationofhemostaticcomponentsundersomeanalytical conditions,theuseofthetestinexperimentalstudies, poten-tialclinicalapplicationsasaglobalcoagulationtest,aswellas itslimitationsandfutureperspectives.
Thrombin
generation
assays
and
the
calibrated
automated
thrombogram
method
Thrombinisakeyproteininvolvedintheregulationof hemo-staticprocesses;ithasbothprocoagulantandanticoagulant properties.13Whileinvivothrombingenerationcanbe
evalu-atedbymeasuringthethrombin-antithrombincomplex(TAT) andprothrombinfragments1+2(F1+2),exvivoTGTaimsto evaluatetheendogenouscapacityoftheoverallhemostatic potential.Therefore,whilehighlevelsofTATandF1+2 rep-resent thepathological activation ofin vivocoagulation,ex vivo thrombin generation reflects the endogenous capacity ofthe hemostaticsystem, and canbeindicative of throm-botic or hemorrhagic risk. TGTcontinuouslymeasures the proteolyticactivityofthrombinformedinplasmausing chro-mogenic or fluorogenic substrates following the activation ofclottingusingatriggeringagent,aswascomprehensively shownbyLecutetal.1Thesyntheticsubstrate,whichis
cou-pledtoachromogenorfluorophore,isselectivelycleavedby thrombin,releasingthechromogenorfluorophore.The out-putsignaliscontinuouslymeasured,andisproportionalto theamountofthrombinpresentinthereaction,thekinetics ofwhichcomprisetwostages.Thefirst,theinitiationstage, correspondingtothecoagulationtimemeasuredusingtests suchasPTandaPTT,canbeinhibitedbyTFPI.Thesecond,the amplification/propagation stage,isfollowedbyaresolution phaseresultingfromtheactionofvariousinhibitorspresent inplasma,suchasaPC,ATandalpha-2-macroglobulin.
TheCAT method,developed byHemkeret al.12 enables
thequantificationofthrombinconcentrationsinplatelet-rich (PRP) or platelet-poor plasma (PPP) bymonitoring the sep-aration ofafluorogenic substrate,whichis simultaneously compared to known thrombin activity in a non-clotting plasmasample.12Thisthrombincalibratorcontainsaknown
Plasma (PPP/PRP)
Thrombin
Cleavage of the fluorescent substrate (Z-Gly-Gly-Arg-7-amino-4methylcoumarin)
Reading of the fluorescence intensity Releasing of
fluorophore
TF + phospholipids + calcium
Figure1–Schematicofthethrombinformationreaction andcleavageofthefluorescentsubstrate.
(TF),phospholipids(amplifytheeffectofTF)andcalciumin theplasma,resultsincoagulationactivationandsubsequent generation of thrombin. Thrombin cleaves the fluorescent substrate(Z-Gly-Gly-Arg7-amino-4-methylcoumarin)thatis addedtothereactioninalaterstep,releasingafluorophore whosefluorescenceintensityovertimeisproportionaltothe concentrationofthrombinformed(Figure1).12,14
Aftermeasurementsaretakenina96-wellplateusinga fluorimeter(Fluoroscan,ThermoScientific),Thrombinoscope BVsoftwareisusedtoconvertfluorescenceunits(RFU)into thrombin concentrations(nM).Thesoftwarealsocalculates thrombogramparameterssuchaspeakandendogen throm-bin potential(ETP) from the area under the curve and the peakthrombinconcentration,respectively.Thefluorescence intensityisconvertedtothrombinconcentration(nM)usinga referencecurvepreparedbymeasuringtheconversionrateof thesubstratewithaknownconcentrationofthrombin.12,14,15
Comparisonofthetwosignals(testandcalibrationsamples) allows a calculation of the thrombin concentration in the plasma(Figure2).
Well (sample)
Fluca
Fluca
PPP HNBSA
Low/High TF
10 min
37ºC 10 min
37ºC Plasma
Plasma
PPP HNBSA
Well (calibrator)
Calibrator
400 700
600 500 400 300 200
Fluorescence (RFU) Fluorescence (RFU) 100
0 300
200
100
0
0 10
Time (min)
Thrombin (nM)
Thrombin generation curve
Time (min)
0 5 10 15 20 25
20
250
200
Peak time
Peak
Leg time
ETP
150
100
50
0
30 40 0 10
Time (min)
20 30 40
Peak
ETP Lag time
0 100
Thrombin (nM)
200 300
10 20
Time (min)
30
Figure3–Parametersofthethrombingenerationcurve usingthecalibratedautomatedthrombogram(CAT) method.
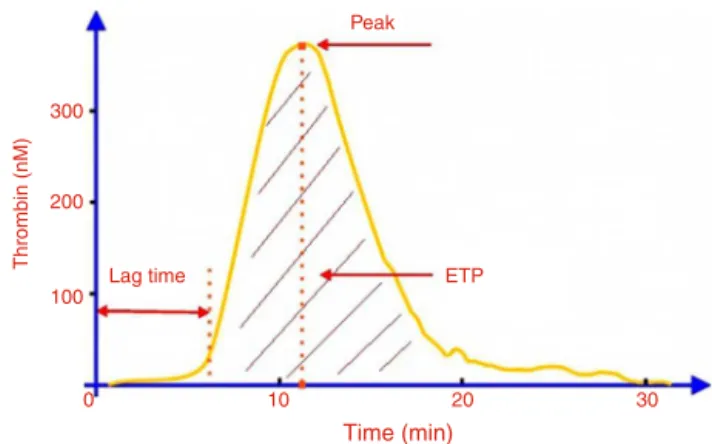
Thethrombingenerationcurve(Figure3)ischaracterized byaninitiationphase (lag-time) followedbythe formation oflargeamountsofthrombin(propagation),culminatingina peakthrombinconcentration,andfinallyinhibitionof throm-bin generation by natural anticoagulants.15 The ETP (area
underthecurve),representstheamountofthrombinformed over60min.11Clotformationisknowntooccurattheendof
thelag-time,therefore,thedurationofthisparameter corre-spondstotheclottingtime.14 Thecoagulationstatecanbe
inferredfromanalysisoftheseparametersfromthe throm-bingeneration curve.A prolongedlag-time and reductions inbothETP andpeak valuesindicate astate of hypocoag-ulability,characterizedbylessthrombin generation.Onthe otherhand,higherthrombingenerationischaracterizedby reducedlag-time,andincreasedETPandpeakvalues, indicat-ingahypercoagulablestate.1
Asmentionedpreviously,theCATmethodisbasedonthe useofasyntheticfluorogenicsubstrate that issensitive to theactionofthrombin.16 However,this syntheticsubstrate
canbecleavednotonlybyfreethrombin,butalsoby throm-binboundtoalpha-2-macroglobulin,potentiallyresultingin highervaluesforthrombingeneration.Toaccountforthis, theCATmethodusesanalgorithmthatnullifiestheactivity ofthrombinlinkedtoalpha-2-macroglobulin.12Similartothe
CATmethod,thereagentsforthisareavailablefrom Diagnos-ticaStago(France).Severalversionsofthistestarecurrently commerciallyavailableforchromogenic(Innovance Endoge-nousThrombinPotential assay,Siemens; Pefakit Thrombin Dynamics Test,Pentapharm, Switzerland) and fluorimetric (TechnothrombinTGA,Germany)techniques.Theadvantages anddisadvantagesofthesemethods,aswellascomparisons, includingthereagentconcentrationsanduseoffluorogenic orchromogenicsubstrates,havebeenreportedelsewhere.1,17
AnadvantageofTGTisthattheoperationmodesarevery flexible, and the experimentalconditions are designed for specificpurposes.Theflexibilityofthismethodallows numer-ous investigations using various types and concentrations ofreagents thatactivatecoagulation,withandwithoutthe additionofdifferentsubstances(e.g.,aPCand thrombomodu-lin),cells(e.g.,platelets)ordifferentbuffers.Anadvantageof thefluorogenicmethodoverthechromogenicmethodisthat
fibrinogendoesnotinfluencetheresults.Asinvivoactivation ofcoagulationoccursinthepresenceofbloodcells,therehas beenmuchresearchintothedevelopmentofthistechnique forusewithwholeblood.18
As mentioned by Lipets and Ataullakhanov,5 although
otherstimulicanbeused,thethrombingenerationreaction isgenerallytriggeredbytheadditionofloworhigh picomo-lar (pM)concentrations ofTF, inaddition to phospholipids andCa2+ions,inordertomeasurethebleedingorthrombotic
potential.Dependingontheintendedpurposeofthetest,the amountofTFaddedmaybetterreflectparticularaspectsofthe hemostaticmechanism.Forexample,whenhighamountsof TF(≥10pM)areadded,thereactionisveryfastwithreduced
sensitivityforfactorsoftheintrinsicpathway.However,atTF concentrationsbetween2and5pM,thereactionismore sen-sitivetodeficienciesoffactor(F)VIII,FIXandFXI.19Therefore,
inPPPsamples,the CATmethodissufficientlysensitiveto detectalldeficienciesofcoagulationfactors(withthe excep-tion ofFXIII)andthe effectofallanticoagulants, including vitaminKantagonists,heparinoids,and directinhibitorsof FXa and thrombin, as previously reported by Brinkman.20
WithPRPsamples,thismethodissensitiveforcasesofvon Willebranddisease,andisabletoshowtheeffectofplatelet inhibitorssuchasaspirinandabciximab.Furthermore, throm-bin generation may beinhibited bythe addition ofaPC or thrombomodulin,reflectingcongenitaloracquireddisorders oftheaPC/PSpathway.12,17,21
Application
of
the
thrombin
generation
test
as
a
potential
tool
for
experimental
and
clinical
studies
Manythrombingenerationassayshavebeencreatedto eval-uatetheclinicalpotentialofthistechnique,particularlythe correlationbetweenTGTparametersandriskofbleeding,and venous andarterialthrombosis.1,18 Ofall thethrombogram
parameters, ETPappearstobe themostwidely used,as it is bettercorrelated tothe clinical phenotype, as described byLipetsandAtaullakhanov.5Anumberofpotentialclinical
applicationsofTGThavebeen identified,suchastoassess treatmentfailureofpatientswhohavethromboticdisorders and are receiving oralanticoagulants, heparin, antiplatelet drugsoracombinationofsuchdrugsandtocomprehensively evaluatethethromboticorhemorrhagicstatusofat-risk indi-viduals.Someofthemostimportantreportsarepresented inTable1.Inthe researchfield,TGThasbeenwidelyused in various parts of the world. Briefly, TGT has been used to help elucidate new hemostatic mechanisms by explor-ing variousplasmacomponents, suchasthosereportedby Peraramelli et al.,22 Spronk et al.,23 Omarova et al.24 and
Zamolodchikovetal.25Thesestudieshaveexploredtherole
ofPSinTFPIactivity,22providednewinsightsintothrombin
formation23 and reporteda novelmechanism forthe
inhi-bitionofthrombin-mediatedFVactivationbythefibrinogen
␥′ peptide.24 Furthermorestudieshaveinvestigatedtherole
Table1–Potentialclinicalapplicationsofthethrombingenerationtest.
Author Mainobjectivesofthestudy
Kesselsetal.,26Arachchillageetal.27and
Zabczyketal.28
Tomonitortreatmentwithoralanticoagulantsandantiplateletdrugs.
AlDierietal.29 ToassessbleedingriskinpatientswithvonWillebranddisease.
Simionietal.,30Castoldietal.,31Alhenc-Gelas
etal.32andCastoldiatal.33
ToevaluatehypercoagulablestatesinpatientswiththeprothrombinG20210A mutation,factorVLeiden,andantithrombinorproteinSdeficiency. Tripodietal.34andHronetal.35 Tousethethrombingenerationtesttopredicttherecurrenceofvenous
thromboembolism.
Lewisetal.36andMatsumotoetal.37 ToestimatebleedingriskinpatientswithhemophiliausingfactorVIIIorfactorVIIa.
vanHylckamaetal.38 Toassessriskofdeepvenousthrombosis.
Brummel-Ziedinsetal.39 Todiscriminatebetweenacuteandstablecoronaryarterydisease.
Segersetal.40 Tousethethrombingenerationtesttoidentifynovelgeneticriskfactorsforvenous
thromboembolism.
Carcaillonetal.41 Tocorrelatethrombingenerationwithcoronaryheartdiseaseandacuteischemic
strokeintheelderly.
Tchaikovskietal.42 Toinvestigatechangesanddeterminantsofthrombingenerationandactivatedprotein
Cresistanceinthefirst16weeksofgestationinwomenwithahistoryofpreeclampsia. Boschetal.43 Toevaluatepatientsundergoingcardiacsurgery.
Orsietal.44 Toevaluatepossiblepathophysiologicalmechanismsthatmaycontributetothe
bleedingtendencyobservedinpatientswithdenguefever. Kamphuisenetal.45 Toevaluatecardiovascularriskinpatientswithhemophilia.
Gouldetal.46 Toassessprocoagulantpotentialofintactneutrophilextracellulartrapsreleasedfrom
activatedneutrophils.
Picoli-Quainoetal.47 Toinvestigatehypercoagulabilityduringtheveryearlyphasesofthehostresponseto
aninfectionovertheclinicalcourseofsepsisandsepticshock.
Loeffenetal.48 Toevaluatethehypercoagulableprofileofpatientswithstentthrombosis.
Ziaetal.49andGlintborgetal.50 Toevaluatehypercoagulabilityinwomenusingoralcontraceptives.
Dargaudetal.51 Toidentifyanautosomaldominantbleedingdisorderinafamily,causedbya
thrombomodulinmutation.
Barcoetal.52andHonickeletal.53 Toevaluatetheeffectofprothrombincomplexconcentrateinreversingthe
anticoagulanteffectofrivaroxabananddabigatran.
contributedtoourunderstandingofhemostaticmechanisms, howeverthesearebeyondthescopeofthisreport.
Limitations
and
perspectives
AlthoughTGThasmany potentialclinicalapplicationsand considerableadvantagesoveralmostallothertechniques,it alsohassomelimitationsthatneedtobeovercomebeforeits useinclinicallaboratories.Althoughthis techniquecanbe performedusingPPPorPRP,thisinvolvesatime-consuming process,whichpreventsitsuseforrapiddiagnosis.Anideal technique would be performed using whole blood sam-plescontaining all blood cells,whichwould reproduce the
invivoconditionsbetter.18Itshouldbenotedthatthereare
other teststhat can beused forthe overall assessmentof hemostasis, such as thromboelastography (TEG® systems, Haemonetics Corporation,Braintree, MA, USA) and throm-boelastometry (ROTEM®-Analyser, Tem Innovations GmbH, Munich,Germany),bothofwhichareperformedusingwhole blood. Thromboelastography, a global test widely used in the management ofacute hemorrhages, is alsocapable of detecting hypercoagulable states.54 However, according to
Lancé,13thismethoddoesnotfullyreflecttheeffectsofusing
low-molecular-weightheparinorthedirectuseoforal antico-agulants,northeeffectsofinheritedordrug-inducedplatelet dysfunction.TGTpresents awider rangeofpotential clini-calapplicationscomparedtothromboelastography.Thus,the applicationofTGTonwholeblood(containingallbloodcells)
mayoutweightheadvantagesofTGTonplasma,asthisassay maybetterreflecttheinvivoconditions.
ThelackofreferencevaluesforspecificTGTconditions, suchasthetypeandconcentrationofthetriggeringagent, orwhether ornotacontactinhibitorfactor isused,makes theuseofTGTdifficultinclinicallaboratories,asitrequires appropriateinterpretationoftheresults.AsstatedbySpronk etal.,55thereisnoconsensusastowhetheracontact
path-wayactivationinhibitorshouldbeused,forexample,whether corn trypsin inhibitor is required. These reference values shouldideallybeestablishedforeachcenteraccordingtothe adoptedprotocol,andwouldrequireidenticalconditionsfor patientandcontrolsamples,includingbloodcollection, sam-plepreparationandstorage.Anotherimportantlimitationof TGTisthelackofsensitivitytochangesintheendothelium. Althoughitisincreasinglybeingrecognizedasamoreprecise testthatreplicatestheinvivohemostaticconditions,TGTstill lacksofficialstandardization,despiteseveralstudieshaving beenconducted.55–57Therefore,itismandatoryto
characteristics andconditions toensurethereproducibility and accuracyofTGTare required.54 Inline withthis,
Dar-gaudetal.8evaluatedastandardizedprotocolformeasuring
thrombin generation usingthe CATmethod inan interna-tionalmulti-centerstudy.Theseauthorsdemonstratedthat theuseofstandardconditions,suchasidenticalequipment, standardizedreagents,referenceplasmaforthe normaliza-tionofresults,andthesametestprocedure,showedagreat reductionin assay variability comparedto previously pub-lisheddata.Their datademonstrated thatthestandardized TGTmethodologyevaluatedinthisreporteffectivelyreduces thevariabilityofthisassaytoacceptablelimits.
Inshort,weagreewithOthman58ontheneedfora
stan-dardized global test that can reliably detect, predict and monitorhemostaticstatusforcliniciansandresearchers,in bothclinicalandexperimentalstudies.
Conclusions
Basedonthecurrent literatureTGTaimstogloballyassess hemostasis,providinginformationabouttheinitiation, ampli-fication/propagationandresolutionphases.Theresultsfrom thistestare morerepresentativeofthephysiological state, whichmaybetterreflectthehemostaticphenotypecompared toroutinetests,andhasgreatpotentialfortheevaluationof therisksofbleedingandthrombosis.However,TGTisnotyet availableforclinicaluse,asitstillrequiresstandardizationand validation.Theuseofastandardizedmethodmayreducethe variabilityofthisassaytoacceptablelimits.Finally,anideal coagulationtestdoes notexistyet. However,new develop-mentsarecontinuouslyemergingaimedatimprovingexisting testsinordertogloballyassesshemostasis,particularlyTGT usingtheCATmethod.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
WethanktheBrazilianagenciesFAPEMIG/SESandMS/CNPq forfundingthisstudy.
r
e
f
e
r
e
n
c
e
s
1. BerntorpE,SalvagnoGL.Standardizationandclinicalutility ofthrombin-generationassays.SeminThrombHemost. 2008;34(7):670–82.
2. LecutC,PetersP,MassionPB,GothotA.Isthereaplacefor thrombingenerationassayinroutineclinicallaboratory?Ann BiolClin(Paris).2015;73(2):137–49.
3. MannKG,ButenasS,BrummelK.Thedynamicsofthrombin formation.ArteriosclerThrombVascBiol.2003;23(1):17–25. 4. WolbergAS.Thrombingenerationandfibrinclotstructure.
BloodRev.2007;21(3):131–42.
5. LipetsEN,AtaullakhanovFI.Globalassaysofhemostasisin thediagnosticsofhypercoagulationandevaluationof thrombosisrisk.ThrombJ.2015;13(1):4.
6.Brummel-ZiedinsKE,WolbergAS.Globalassaysof hemostasis.CurrOpinHematol.2014;21(5):395–403. 7.vanGeffenM,vanHeerdeWL.Globalhaemostasisassays,
frombenchtobedside.ThrombRes.2012;129(6):681–7. 8.DargaudY,WolbergAS,LuddingtonR,RegnaultV,SpronkH,
BaglinT,etal.Evaluationofastandardizedprotocolfor thrombingenerationmeasurementusingthecalibrated automatedthrombogram:aninternationalmulticenterstudy. ThrombRes.2012;130(6):929–34.
9.MacfarlaneRG,BiggsR.Athrombingenerationtest;the applicationinhaemophiliaandthrombocytopenia.JClin Pathol.1953;6(1):3–8.
10.PitneyWR,DacieJV.Asimplemethodofstudyingthe generationofthrombininrecalcifiedplasma;applicationin theinvestigationofhaemophilia.JClinPathol.1953;6(1):9–14. 11.HemkerHC,WieldersS,KesselsH,BéguinS.Continuous
registrationofthrombingenerationinplasma,itsuseforthe determinationofthethrombinpotential.ThrombHaemost. 1993;70(4):617–24.
12.HemkerHC,GiesenP,AlDieriR,RegnaultV,deSmedtE, WagenvoordR,etal.Calibratedautomatedthrombin generationmeasurementinclottingplasma.Pathophysiol HaemostThromb.2003;33(1):4–15.
13.LancéMD.Ageneralreviewofmajorglobalcoagulation assays:thrombelastography,thrombingenerationtestand clotwaveformanalysis.ThrombJ.2015;13:1.
14.HemkerHC,AlDieriR,DeSmedtE,BéguinS.Thrombin generation,afunctiontestofthehaemostatic–thrombotic system.ThrombHaemost.2006;96(5):553–61.
15.CastoldiE,RosingJ.Thrombingenerationtests.ThrombRes. 2011;127Suppl.3:S21–5.
16.IgnjatovicV,GreenwayA,SummerhayesR,MonagleP. Thrombingeneration:thefunctionalroleof
alpha-2-macroglobulinandinfluenceofdevelopmental haemostasis.BrJHaematol.2007;138(3):366–8.
17.TenCateH.Thrombingenerationinclinicalconditions. ThrombRes.2012;129(3):367–70.
18.NinivaggiM,Apitz-CastroR,DargaudY,deLaatB,Hemker HC,LindhoutT.Whole-bloodthrombingenerationmonitored withacalibratedautomatedthrombogram-basedassay.Clin Chem.2012;58(8):1252–9.
19.KeulartsIM,ZivelinA,SeligsohnU,HemkerHC,BéguinS.The roleoffactorXIinthrombingenerationinducedbylow concentrationsoftissuefactor.ThrombHaemost. 2001;85(6):1060–5.
20.BrinkmanHJ.Globalassaysandthemanagementoforal anticoagulation.ThrombJ.2015;13:9.
21.DielisAW,CastoldiE,SpronkHM,vanOerleR,HamulyákK, TenCateH,etal.CoagulationfactorsandtheproteinC systemasdeterminantsofthrombingenerationinanormal population.JThrombHaemost.2008;6(1):125–31.
22.PeraramelliS,RosingJ,HackengTM.TFPI-dependent activitiesofproteinS.ThrombRes.2012;129(2): S23–6.
23.SpronkHM,BorissoffJI,tenCateH.Newinsightsinto modulationofthrombinformation.CurrAtherosclerRep. 2013;15(11):363.
24.OmarovaF,UitteDeWilligeS,AriënsRA,RosingJ,BertinaRM, CastoldiE.Inhibitionofthrombin-mediatedfactorV
activationcontributestotheanticoagulantactivityof fibrinogen␥′.JThrombHaemost.2013;11(9):1669–78.
25.ZamolodchikovD,RennéT,StricklandS.TheAlzheimer’s diseasepeptide-amyloidpromotesthrombingeneration throughactivationofcoagulationfactorXII.JThromb Haemost.2016;14(5):995–1007.
27.ArachchillageDR,EfthymiouM,MackieIJ,LawrieAS,Machin SJ,CohenH.Rivaroxabanandwarfarinachieveeffective anticoagulation,asassessedbyinhibitionofTGandin-vivo markersofcoagulationactivation,inpatientswithvenous thromboembolism.ThrombRes.2015;135(2):388–93.
28.Z ˛abczykM,MajewskiJ,KarkowskiG,MalinowskiKP,UndasA. VitaminKantagonistsfavourablymodulatefibrinclot propertiesinpatientswithatrialfibrillationasearlyasafter3 daysoftreatment:relationtocoagulationfactorsand thrombingeneration.ThrombRes.2015;136(4):832–8. 29.AlDieriR,PeyvandiF,SantagostinoE,GiansilyM,Mannucci
PM,SchvedJF,etal.Thethrombograminrareinherited coagulationdisorders:itsrelationtoclinicalbleeding. ThrombHaemost.2002;88(4):576–82.
30.SimioniP,CastoldiE,LunghiB,TormeneD,RosingJ,Bernardi F.Anunderestimatedcombinationofoppositesresultingin enhancedthrombotictendency.Blood.2005;106(7):2363–5. 31.CastoldiE,SimioniP,TormeneD,ThomassenMC,SpieziaL,
GavassoS,etal.Differentialeffectsofhighprothrombin levelsonthrombingenerationdependingonthecauseofthe hyperprothrombinemia.JThrombHaemost.2007;5(5):971–9. 32.Alhenc-GelasM,CanonicoM,PicardV.Influenceofnatural
SERPINC1mutationsonexvivothrombingeneration.J ThrombHaemost.2010;8(4):845–8.
33.CastoldiE,MaurissenLF,TormeneD,SpieziaL,GavassoS, RaduC,etal.Similarhypercoagulablestateandthrombosis riskintypeIandtypeIIIproteinS-deficientindividualsfrom familieswithmixedtypeI/IIIproteinSdeficiency.
Haematologica.2010;95(9):1563–71.
34.TripodiA,MartinelliI,ChantarangkulV,BattaglioliT,Clerici M,MannucciPM.Theendogenousthrombinpotentialand theriskofvenousthromboembolism.ThrombRes. 2007;121(3):353–9.
35.HronG,KollarsM,BinderBR,EichingerS,KyrlePA. Identificationofpatientsatlowriskforrecurrentvenous thromboembolismbymeasuringthrombingeneration.JAMA. 2006;296(4):397–402.
36.LewisSJ,StephensE,FlorouG,MacartneyNJ,HathawayLS, KnippingJ,etal.Measurementofglobalhaemostasisin severehaemophilia:afollowingfactorVIIIinfusion.BrJ Haematol.2007;138(6):775–82.
37.MatsumotoT,ShimaM,TakeyamaM,YoshidaK,TanakaI, SakuraiY,etal.ThemeasurementoflowlevelsoffactorVIII orfactorIXinhemophiliaAandhemophiliaBplasmabyclot waveformanalysisandthrombingenerationassay.JThromb Haemost.2006;4(2):377–84.
38.vanHylckamaVA,ChristiansenSC,LuddingtonR, CannegieterSC,RosendaalFR,BaglinTP.Elevated endogenousthrombinpotentialisassociatedwithan increasedriskofafirstdeepvenousthrombosisbutnotwith theriskofrecurrence.BrJHaematol.2007;138(6):769–74. 39.Brummel-ZiedinsK,UndasA,OrfeoT,GisselM,ButenasS,
ZmudkaK,etal.Thrombingenerationinacutecoronary syndromeandstablecoronaryarterydisease:dependenceon plasmafactorcomposition.JThrombHaemost.
2008;6(1):104–10.
40.SegersO,vanOerleRV,tenCateHT,RosingJ,CastoldiE. Thrombingenerationasanintermediatephenotypefor venousthrombosis.ThrombHaemost.2010;103(1):114–22. 41.CarcaillonL,Alhenc-GelasM,BejotY,SpaftC,DucimetièreP,
RitchieK,etal.Increasedthrombingenerationisassociated withacuteischemicstrokebutnotwithcoronaryheart diseaseintheelderly:theThree-Citycohortstudy. ArteriosclerThrombVascBiol.2011;31(6):1445–51.
42.TchaikovskiSN,ThomassenMC,CostaSD,PeetersLL,Rosing J.RoleofproteinSandtissuefactorpathwayinhibitorinthe developmentofactivatedproteinCresistanceearlyin
pregnancyinwomenwithahistoryofpreeclampsia.Thromb Haemost.2011;106(5):914–21.
43.BoschY,AlDieriR,tenCateH,NelemansP,BloemenS, HemkerC,etal.Preoperativethrombingenerationis predictivefortheriskofbloodlossaftercardiacsurgery:a researcharticle.JCardiothoracSurg.2013;8:154.
44.OrsiFA,AngeramiRN,MazettoBM,QuainoSK, Santiago-BassoraF,CastroV,etal.Reducedthrombin formationandexcessivefibrinolysisareassociatedwith bleedingcomplicationsinpatientswithdenguefever:a case–controlstudycomparingdenguefeverpatientswithand withoutbleedingmanifestations.BMCInfectDis.2013;13:350. 45.KamphuisenPW,tenCateH.Cardiovascularriskinpatients
withhemophilia.Blood.2014;123(9):1297–301.
46.GouldTJ,VuTT,SwystunLL,DwivediDJ,MaiSH,WeitzJI, etal.Neutrophilextracellulartrapspromotethrombin generationthroughplatelet-dependentand
platelet-independentmechanisms.ArteriosclerThrombVasc Biol.2014;34(9):1977–84.
47.Picoli-QuainoSK,AlvesBE,FaiottoVB,MontalvaoSA,De SouzaCA,Annichino-BizzacchiJM,etal.Impairmentof thrombingenerationintheearlyphasesofthehostresponse ofsepsis.JCritCare.2014;29(1):31–6.
48.LoeffenR,GodschalkTC,vanOerleR,SpronkHM,Hackeng CM,tenBergJM,etal.Thehypercoagulableprofileofpatients withstentthrombosis.Heart.2015;101(14):1126–32.
49.ZiaA,CallaghanMU,CallaghanJH,SawniA,BartlettH, BackosA,etal.Hypercoagulabilityinadolescentgirlsonoral contraceptives–globalcoagulationprofileandestrogen receptorpolymorphisms.AmJHematol.2015;90(8):725–31. 50.GlintborgD,SidelmannJJ,AltinokML,MummH,AndersenM.
Increasedthrombingenerationinwomenwithpolycystic ovarysyndrome:apilotstudyontheeffectofmetforminand oralcontraceptives.Metabolism.2015;64(10):1272–8.
51.DargaudY,ScoazecJY,WieldersSJ,TrzeciakC,HackengTM, NégrierC,etal.Characterizationofanautosomaldominant bleedingdisordercausedbyathrombomodulinmutation. Blood.2015;125(9):1497–501.
52.BarcoS,WhitneyCheungY,CoppensM,HuttenBA,Meijers JC,MiddeldorpS.Invivoreversaloftheanticoagulanteffect ofrivaroxabanwithfour-factorprothrombincomplex concentrate.BrJHaematol.2016;172(2):255–61.
53.HonickelM,MaronB,vanRynJ,BraunschweigT,tenCateH, SpronkHM,etal.Therapywithactivatedprothrombin complexconcentrateiseffectiveinreducing
dabigatran-associatedbloodlossinaporcinepolytrauma model.ThrombHaemost.2016;115(2):271–84.
54.KashukJL,MooreEE,SabelA,BarnettC,HaenelJ,LeT,etal. Rapidthrombelastography(r-TEG)identifies
hypercoagulabilityandpredictsthromboemboliceventsin surgicalpatients.Surgery.2009;146(4):764–72.
55.SpronkHM,DielisAW,DeSmedtE,vanOerleR,FensD,Prins MH,etal.AssessmentofthrombingenerationII:validationof thecalibratedautomatedthrombograminplatelet-poor plasmainaclinicallaboratory.ThrombHaemost. 2008;100(2):362–4.
56.DargaudY,LuddingtonR,GrayE,LecompteT,SiegemundT, BaglinT,etal.Standardisationofthrombingenerationtest– whichreferenceplasmaforTGT?Aninternational
multicentrestudy.ThrombRes.2010;125(4):353–6. 57.PerrinJ,DepassecF,LecompteT.Largeexternalquality
assessmentsurveyonthrombingenerationwithCAT:further evidencefortheusefulnessofnormalisationwithanexternal referenceplasma.ThrombRes.2015;136:125–30.