The
Operculina macrocarpa
(l.) urb. (jalapa) tincture modulates
human blood platelet aggregation
$
Taiana Magalhães Pierdoná
a, Nathalia Rocha Lima
b, Raony Cássio Millet Rodrigues
b,
Jonas Pires Teixeira
b, Romélia Pinheiro Gonçalves
b, Juvenia Bezerra Fontenele
b,
Silvânia Maria Mendes Vasconcelos
a, Glauce Socorro de Barros Viana
a,
Luzia Kalyne Almeida Moreira Leal
a,b,naPrograma de Pós-Graduação em Farmacologia, Departamento de Fisiologia e Farmacologia, Faculdade de Medicina, Universidade Federal do Ceará, Brazil bCentro de Estudos Farmacêuticos e Cosméticos (CEFAC), Departamento de Farmácia, Faculdade de Farmácia, Odontologia e Enfermagem, Universidade
Federal do Ceará 60431-327, Brazil
a r t i c l e
i n f o
Article history: Received 13 May 2013 Received in revised form 13 September 2013 Accepted 2 October 2013 Available online 4 November 2013 Keywords:
Operculina macrocarpa Convolvulaceae Antiplatelet Anticoagulant
a b s t r a c t
Ethnopharmacological relevance: Operculina macrocarpais an ornamental climbing plant of the North-eastern Brazil extensively used in traditional medicine as depurative of the blood and for the treatment of thrombosis. To investigate the antiplatelet and anticoagulant potential ofOperculina macrocarpaand to determine the possible mechanisms of action.
Material and methods: TheOperculina macrocarpatincture (OMT) was characterized by the polyphenol content and chromatographic profile established by HPLC with detection and quantification of three phenol acids (caffeic, clorogenic and gallic acids). The human platelet aggregation was inducedin vitroby the agonists ADP, collagen, thrombin, epinephrine or arachidonic acid, and the antiplatelet effect of OMT was evaluated in the presence or absence of aspirin (a nonselective inhibitor of cyclooxygenase), pentoxifylline (a phosphodiesterase inhibitor), ticlopidine (a P2Y12 purinoceptor antagonist) or ODQ (a selective inhibitor of guanilate cyclase). The effect of OMT on the partial thromboplastin time, prothrombin time and bleeding time were investigated on human or rat plasma.
Results: The strongest antiplatelet effect of OMT (50–400mg/mL) was observed on the ADP- induced aggregation with inhibitions up to 55%, while among others agonists (epinephrine, collagen, thrombin and arachidonic acid) maximal inhibitions reached by OMT (200mg/mL) were on platelet aggregation induced by collagen (18%) or epinephrine (20%). The antiplatelet effect of OMT (400mg/mL) was comparable to aspirin, a nonspecific inhibitor of cyclooxygenase. The ticlopidine and pentoxifylline increased 5.1 and 3.8 fold the inhibitory effect of OMT on ADP-induced platelet aggregation, respectively. On the other hand,L-arginine, ODQ and aspirin showed a slightly or no effect on antiplatelet effect of OMT. The bleeding time in rats was significantly increased by OMT, but the tincture did not interfere on the activated partial thromboplastin or prothrombin time in human plasma.
Conclusions: This study showed that the tincture ofOperculina macrocarpahas antiplatelet effect that cannot be attributed to a single biochemical mechanism and at least part of it cannot be related to the OMT inhibition of P2Y12purinergic receptors.
&2013 Elsevier Ireland Ltd. All rights reserved.
1. Introduction
The participation of platelets in hemostasis is of fundamental importance to the normal physiology. Circulating platelets in normal conditions must be able to maintain a direct and repeated contact with the vessel wall without premature activation. On the other hand, after a vascular injury, the platelets must recognize the damaged wall,
adhere and form a stable clot, which will remain in place until repair is no more needed (Brass, 2010). When there is an injury to the blood vessel wall or endothelial cell layer, platelets adhere to the site of injury by binding to von Willebrand factor (vWF) and collagenfibrils in the sub-endothelial matrix. Subsequently, platelet are stimulated to secrete various molecules such as adenosine diphosphate (ADP) and thromboxane A2 (TXA2), which activate neighboring platelets, and increase the expression of membrane integrins including glycoprotein IIb/IIIa (GP IIb/IIIa) receptors, which form bridges between adjacent platelets by binding to fibrinogen. These processes lead to the formation of a platelet plug at the injury site (Davi and Patrono, 2007). Thrombosis refers to a pathological formation of a hemostatic plug within the blood vessels in the absence of bleeding. Arterial Contents lists available atScienceDirect
journal homepage:www.elsevier.com/locate/jep
Journal of Ethnopharmacology
0378-8741/$ - see front matter&2013 Elsevier Ireland Ltd. All rights reserved. http://dx.doi.org/10.1016/j.jep.2013.10.008
☆Chemical compounds studied in this article, caffeic acid (PubChem CID:
689043); gallic acid (PubChem CID: 370); chlorogenic acid (PubChem CID: 1794427).
n
thrombosis is an acute complication that develops in association with atherosclerosis and causes cardiovascular diseases such as ischemic heart disease and stroke (Ruggeri, 2002; Davi and Patrono, 2007). Platelets are critical components in thrombosis and may participate in the progression of the atherosclerotic plaque (Ruggeri, 2002; Jennings, 2009). Thrombotic diseases, especially heart disease and cerebral vascular thrombosis became the leading causes of death and its incidence is increasing every year (Bao et al., 2009).
Antiplatelet agents such as aspirin and clopidogrel are widely used clinically and directly inhibit platelet aggregation (Gaglia et al., 2010). However, the continuous use of these agents is limited because they can induce resistance or adverse effects such as head-ache, abdominal cramps, vomiting and gastric ulceration (Angiolillo et al., 2007). For these reasons, a number of studies (Tsai et al., 2000;
Ballabeni et al., 2007;Ryu et al., 2009) have been conducted to search new medicines from synthetic or natural source.
Several authors have shown the antiplatelet effect of extracts or molecules from medicinal plants and their constituents, such as Amburana cearensis(Leal, 1995) and piplartine, an alkamide iso-lated fromPiper tuberculatum(Fontenele et al., 2009).
Operculina macrocarpa(L.) Urb (Convolvulaceae), is an ornamental climbing plant of the Northeastern Brazil popularly known as
“jalapa”or“batata-de-purga”. The roots of this plant are mainly used in traditional medicine as depurative of the blood (Matos, 1982). In addition, a phytomedicine entitled“Aguardente Alemã”an ethanolic solution (tincture) produced from Operculina macrocarpa (OMT) associated with Convolvulus scamnonia is extensively used in folk medicine in the Northeastern Brazil for the treatment of circulatory diseases such as thrombosis (Carvalho et al., 2003). In this sense, the objective of this present study was to investigate the antiplatelet and anticoagulant effects ofOperculina macrocarpa.
2. Materials and methods
2.1. Drugs, chemicals and phytomedicine
Adenosine diphosphate (ADP), dimethylsulfoxide (DMSO), Tween 80, acetylsalicylic acid (ASA), 1H-[1,2,4] oxadiazolol [4,3-a] quinoxalin-1-one (ODQ),L-arginine, caffeic acid, chlorogenic acid and gallic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Arachidonic acid and thrombin were purchased from Chrono-log (USA). Pentoxifyl-line (Trentals) and ticlopidine (Ticlids
) were obtained from Sanofi-Aventis (Brazil). Sodium nitroprusside (Nitrops
) was obtained from Hypofarma (Brazil). Heparin (Liquemines
) was obtained from Roche (Brazil). Collagen and epinephrine were purchased from Helena Laboratories (USA). Trifluoroacetic acid was obtained from VETEC (Brazil), while acetonitrile was originally provided by TEDIA (Brazil). All others reagents were of analytical grade.
2.2. Samples of human blood
Blood samples were obtained from healthy volunteers who had not taken any drugs for at least fifteen days. The study was approved by the Human Research Ethics Committee of the Federal University of Ceará, Brazil, under the number 169/10.
2.3. Animals
Male Swiss mice (25–30 g) were housed in standard environ-mental conditions (23711C, humidity 6075% and a 12 h/12 h dark/light cycle-light on at 7.00 a.m.), with food and water availablead libitumin accordance to the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services, 1985. All experiments were conducted in a quiet room at
a constant temperature of 251C. All drugs were administered orally at a volume of 10 mL/kg. The experiments were approved by the Animal Research Ethics Committee, Federal University of Ceará (registered under No. 63/10).
2.4. Operculina macrocarpa tincture
The tincture from the roots of Operculina macrocarpa (resin content: 1.3870.47 g% w/v) was obtained from the Ravick Che-mical Products and Cosmetics Laboratory (Fortaleza, CE, Brazil). The plant was collected in the city of Parnaíba (Piauí, Brazil) located at coordinates 2154′49″S and 41147′15″W. The botanical material was identified by the Herbarium Prisco Bezerra, Federal University of Ceará (voucher specimen registered under the number 45489).
2.5. Chemical characterization of Operculina macrocarpa
The chromatographic analysis by high-performance liquid chro-matography (HPLC) of OMT was performed according the method described byMichelin (2008). The assays were performed on Alliance
—Waters 2695 (Milford, MA) chromatograph with a binary pump, auto-sampler, and photodiode-array detector (Waters-2996 PDA) at 310 nm. The separations were performed with an analytical reverse-phase column C18 (Varians, 250
4.65 mm) and a guard column
(Phenomenexs
, 435 mm) at 401C in a thermostatic oven. The
mobile phase was made from water/trifluoroacetic acid (mobile phase A) and acetonitrile/trifluoroacetic acid (mobile phase B) in a gradient mode for 31 min (total run time) by varying the mobile phase B from 10 to 100% at 1.0 mL/minflow rate. The solvents were previously degassed under vacuum by sonication during 5 min and
filtered through Phenomenex nylon membrane (0.45
μ
m). Thesamples were dissolved in the initial mobile phase and filtered through Waters PTFE membrane (0.45
μ
m) before injection (20mL). The data was processed by Empowers(Waters, USA) software. The identification of caffeic, chlorogenic and gallic acid in Operculina macrocarpatincture by HPLC experiments were based on the retention time (rt) of external standards. The contents of the three phenol acids in the tincture were calculated using calibration curves. The ranges of calibration curves were 0.01–
0.015 mg/mL for chlorogenic acid and gallic acid, 0.001–0.005 mg/ mL for caffeic acid. The linear relationship was obtained correlat-ing the concentration of phenols acids to the correspondent peak area. The linear regression equations were determined and the correlation coefficients for and caffeic acid, chlorogenic acid and gallic acid were 0.996, 0.998 and 0.993, respectively. The content of phenols acids were expressed as mean of three determinations and coefficient of variation.
2.6. Measurement of total polyphenol content and phytochemical prospecting in OMT
The determination of the total polyphenol contents of OMT was measured by the Folin–Ciocalteu colorimetric method (Singleton and Rossi, 1965). An aliquot (100
μ
L) of the OMT was mixed to 250μ
L of Folin–Ciocalteu reagent (1 N). The mixture was shaken before adding 1250μ
L Na2CO3 (20%) and adjusting with Milli-Q water to afinal volume of 10 mL. After 40 min of standing in dark, the mixture was analyzed by spectrophotometry (Beckman Coulter DU 640, Germany) at 715 nm. The amount of total polyphenols was calculated through calibration curve (linear regression equation: y¼0.077xþ0.0468;r¼0.9985) of gallic acid (4–16μ
g/mL). Resultsreactions based on the chemical group to be determined or by thin layer chromatography.
2.7. Platelet aggregation test
2.7.1. Preparation of platelet-rich and platelet-poor plasma Blood from healthy volunteers was collected by venipuncture into silicon coated glass flasks containing 3.8% sodium citrate (9:1 v/v). Platelet-rich plasma was prepared by centrifugation of blood at 1000 rpm for 10 min at room temperature. Platelet-poor plasma was obtained immediately afterwards by centrifugation of an aliquot of platelet-rich plasma at 1500 rpm for 10 min. The platelets were counted according to the method ofBrecher and Cronkite (1950)and adjusted to a concentration of 300,000/mm3.
2.7.2. Aggregation in platelet-rich plasma
Platelet aggregation was measured with an aggregometer (model 450, Chrono-Log Co., USA) according to the method ofBorn and Cross (1963). Briefly, after stirring at 1200 rpm, platelet aggregation was induced at 371C, by addition of ADP (2mM), AA (1 mM), thrombin (1 U/mL), collagen (2mg/mL) or epinephrine (EPI 40mM) as agonists. Operculina macrocarpatincture—OMT (50–400mg/mL) or 0.08% DMSO in water (vehicle, control) were added 10 min before the addition of the agonist. The resulting aggregation, measured as the change in light transmission, was recorded for 8 min. Values are presented as percentage aggregation compared with the control (100%).
Evaluation of the OMT antiplatelet effect in presence of other antiplatelet agents in human plasmain vitro.
The effect of the OMT (100mg/mL) was evaluated in the presence of drugs as acetylsalicylic acid (ASA 25mM), ticlopidine (TIC 250mM) or pentoxifylline (PTX 120mg/mL), and aggregation was induced by ADP (2
μ
M). The OMT and/or antiplatelet agents were incubated in PRP before the agonist.2.7.3. OMT antiplatelet effect in human plasma and the role of nitric oxide/guanylate cyclase
The antiplatelet effect of OMT (100mg/mL) was investigated in the absence and in the presence ofL-arginine (L-ARG 10mg/mL) or ODQ (2mM). The ADP (2mM) was used as an agonist of platelet aggregation and sodium nitroprusside (SNP 10
μ
M), a nitric oxide donor, as standard drug (Jang et al., 2002).2.8. Anticoagulant tests
2.8.1. Determination of the bleeding time in mice
The bleeding time was determined using a method described by
Huang et al. (2007). Male Swiss mice were divided in groups of ten
and pre-treated with OMT (100, 200, 400 mg/kg), with the tincture vehicle (Tween 80 4% in water, control), with water (normal) or ASA (100 mg/kg) (a standard, COX nonspecific inhibitor), p.o., once a day, for ten days. One hour after the last administration, the mouse tail was transected about 1 cm from the tip using a disposable surgical blade. The bleeding time was measured from the time of transection until it was completely stopped. The blood was absorbed withfilter paper at 20 s intervals during bleeding.
2.8.2. In vitro anticoagulation activity
Anticoagulation activity was evaluated by measuring the plasma clotting times (aPTT and PT) by a modification of the method ofYuk et al. (2000)using a coagulometer (Teco, Coatron M1, Germany) and commercial kits Hemostat thromboplastin SI and Hemostat aPTT (Human, Alemanha). Platelet-poor plasma was incubated with OMT (200 and 400mg/mL), DMSO (0.08%) (vehicle, control) or heparin (0.1 ou 0.5 UI/mL, standard, anticoagulant) for 10 min at 371C. To evaluate the activated partial thromboplastin time (aPTT), in the human plasma (50
μ
L) previously incubated with OMT was added cephalin (50μ
L) and clotting was started by adding CaCl2(50mL). For evaluating the prothrombin time (PT), the human plasma (50μ
L) preincubated with OMT, was mixed with calcium thromboplastin (100μ
L) and then measured the time of clot formation.2.9. Statistical analysis
The statistical analysis was performed using the GraphPad Prism version 5.0 (USA). The results were expressed as mean7 -standard error of mean (SEM). For multiple comparisons the Analysis of Variance (ANOVA) followed by Tukey as thepost hoc test were used. Probability levels of less than 5% were considered statistical significant (n
po0.05).
3. Results
3.1. Chemical characterization of Operculina macrocarpa tincture (OMT)
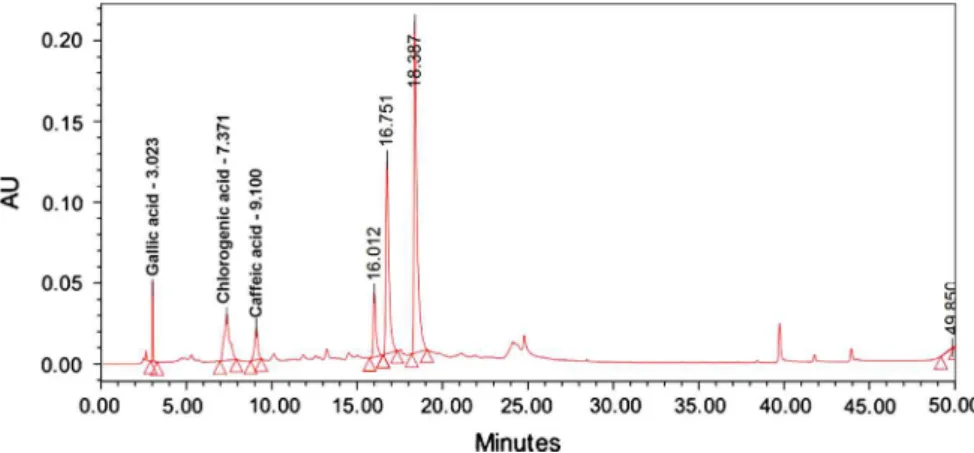
The HPLC analysis permitted to detect, identify and quantify three phenol acids in OMT, such as caffeic acid—CA (rt¼9.1 min), gallic acid —GA (rt¼3.0 min) and chlorogenic acid—CLA (rt¼7.4 min) (Fig. 1).
The content of CA, GA and CLA in OMT were of 0.03 (7.5), 0.14 (1.6) and 0.17 (4.4) mg/mL, respectively. The polyphenol content of OMT was 0.14 g GAE/mL of OMT. In addition, chemical studies carried out with the OMT demonstrated the presence of tannins, phenols and coumarins.
Effects of OMT on platelet aggregation induced by ADP, throm-bin, arachidonic acid, collagen or epinephrine in human plasma.
The addition of OMT (50, 100, 200, 300 and 400mg/mL) on human platelet suspension reduced significantly the platelet aggregation induced by ADP with inhibitions of up to 55% (IC50 value: 363.07mg/mL).Fig. 2shows the original record of this effect of OMT (100, 200 and 400mg/mL) on ADP-induced platelet aggregation in human plasma.
The OMT was investigated for its effect on human platelet aggregation induced by other agonists such as epinephrine, collagen, arachidonic acid and thrombin. The OMT (200
μ
g/mL) inhibited significantly the platelet aggregation induced by epinephrine (20%) or collagen (18%) but no significant effect on thrombin or arachidonic acid-induced platelet aggregation was observed (data not shown).3.1.1. Evaluation of the antiplatelet effect of the OMT in the absence and presence of aspirin, pentoxifylline or ticlopidine in human plasma The addition of OMT (100mg/mL) in the presence of ASA (25
μ
M), on platelet suspension did not induce any significant change in the antiplatelet effect of OMT (aggregation: 64.576.4%) when compared to OMT alone (aggregation: 77.472.8%) on ADP-induced platelet aggregation. The evaluation of the antiplatelet effect of OMT (100μ
g/mL) in the presence of pentoxifylline (PTX, 120mg/mL) or ticlopidine (TIC, 250mM) showed a significant decrease in the percentage of aggregation (OMTþPTX: 72.074.4; OMTþTIC: 18.772.8) as compared to OMT alone, where the percentage of aggregation was around 92 (Fig. 3).3.1.2. OMT antiplatelet effect in human plasma: Role of NO/GC/cGMP In order to verify whether the NO/GC/cGMP system has a role in the antiplatelet effect of OMT,L-arginine (10mg/mL) a substrate for nitric-oxide synthase, was preincubated with OMT (100
μ
g/mL) prior to the percentage of ADP-induced platelet aggregation. The effect of OMT (91.271.9%) was significantly modified byL-arginine (OMTþ L-ARG: 81.673.0%), although it induced an increase of only 9.6% of the antiplatelet effect of OMT (Fig. 3). The combination of OMT with ODQ, an inhibitor of guanylate cyclase, did not reverse the antiplate-let effect of OMT (OMT and ODQþOMT: 84.172.9% and 73.375.9%, respectively). Different results were observed for sodium nitroprus-side (SNP: 37.875.2%), a NO donor, which had its antiplatelet effect significantly reversed by preincubation with ODQ (ODQþSNP:103.674.0%) (data not shown).
3.1.3. Effect of OMT on the bleeding time in mice
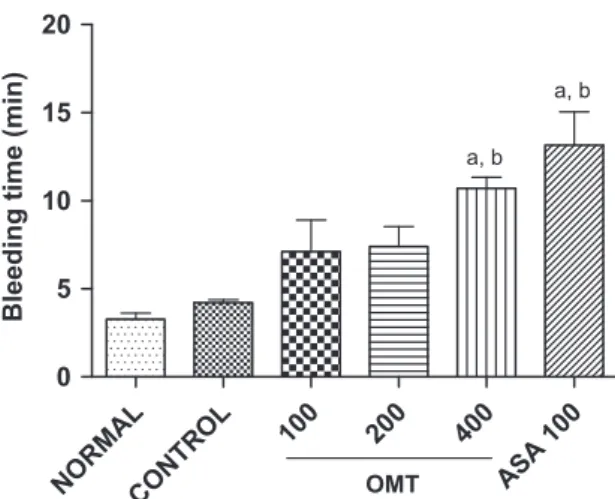
The pretreatment of mice with OMT (400 mg/kg, p.o.) increased significantly the animals bleeding time (10.371.0 min) when related to the control group (3.370.3 min). Similar results were observed in animals pretreated with ASA at concentration of 100 mg/kg, p.o. (13.271.9 min) (Fig. 4).
3.1.4. Effects of the OMT in the activated partial thromboplastin time (aPTT) and prothrombin time (PT) in human plasma
The OMT (200 and 400mg/mL) did not increase the aPTT (46.671.6 and 51.573.8 s, respectively) when related to the control group (40.371.2 s), while heparin (0.1 U/mL), an anticoagulant drug, increased significantly the aPTT (100.977.6 s). The addition of OMT (200 and 400mg/mL) to human plasma did not increase the TP (12.570.2 and 13.970.4 s) as compared to the control group (11.770.2 s).
4. Discussion
The present study showed for the first time the effect of Operculina macrocarpatincture (OMT) on human platelet aggrega-tion, as well as performed a preliminary evaluation of its antic-oagulant potential.
The OMT was chemically characterized by spectrophotometric and HPLC analysis which determined the polyphenol and phenols acids content such as caffeic acid, gallic acid and chlorogenic acid. In addition, coumarins and tannins where also identify in the OMT. In this context, the polyphenols content of OMT determined in the present study was possibly related at least in part with the presence of phenol acids, coumarin and tannins in plant. These data corroborate a previous study (Michelin, 2008), which showed the presence of various phenolic compounds in the ethanolic extract ofOperculina macrocarpa, such as chlorogenic acid, caffeic acid, caffeic acid dimer and protocatechuic acid, besides coumarins, triterpenes and reducing sugars.
The OMT inhibited the ADP-induced platelet aggregation, a primary agonist that induces platelet aggregation by a mechanism independent of the production or release of others mediators by platelets. The activation of platelets by ADP involves at least three receptor types. The P2Y1 purinergic receptor, which is coupled to G protein, causes the mobilization of intracellular calcium, and is responsible for platelet shape change, while the P2Y12, Gi protein coupled receptor, causes the inhibition of adenylate cyclase and the amplification of the final response. A third receptor, the P21
mediates the ionicfluxes and is more sensitive to ATP (Cattaneo and Gachet, 2001).
Several studies (El Haouari et al., 2006;Gadi et al., 2012) have shown the antiplatelet effect of phenolic compounds, including flavonoids and phenols acids. Evidences (Cho et al., 2012) showed that the chlorogenic acid (10–50mM) has antiplatelet activity in human platelets collagen and ADP-induced aggregation. Other studies (Hsiao et al., 2007; Cheng et al., 2007) also demonstrated that a derivative of caffeic acid (6–25mM) is capable of inhibiting platelet aggregation induced by collagen, and that this inhibition appears to be modulated by an increase in cGMP levels. Furthermore, gallic acid (100–1000mM) was shown to be also capable of inhibiting the platelet aggregation induced by ADP, TXA2analog, collagen and arachidonic acid (Chang et al., 2012;Lim et al., 2004). Thus, it is possibly that the chlorogenic, caffeic and gallic acids present in the tincture of Operculina macrocarpa(480, 167 and 823mM, respectively), may be responsible at least in part for the antiplatelet activity of this plant. However, the possible participation of other compounds present in this species, such as coumarin and tannins, cannot be ruled out.
The OMT reduced significantly the platelet aggregation induced by ADP, collagen and epinephrine, but it showed no effect on ADP 2µM
DMSO 0,08%
OMT 100µg/mL
OMT 200µg/mL
OMT 400µg/mL
thrombin or arachidonic acid-induced platelet aggregation. After an injury, the collagen on the subendothelial region, is on the surface of the vascular wall and has an important role in platelet activation, an event that starts the process of lesion repairing (Clemetson, 1999). As the collagen may interact with several proteins, a wide variety of receptors have been described for this agonist (Colman, 1991). However, studies suggest that the glycoprotein Ia/IIa (GPIa/IIa) and glycoprotein VI (GPVI) have a role in modulating platelet aggregation induced by collagen (Ruggeri and Savage, 2007).
Epinephrine may exert its effects on platelet aggregation by various signaling pathways, as the metabolism of AA (with formation of TXA2, increased levels of intracellular Ca2þ
, exposure of the GPIIb/IIIa, release of ADP and others), or by hydrolysis of phosphatidylinositol
(via phospholipase C, with mobilization of cytoplasmic Ca2þ , and previously stimulated adenylate cyclase prostaglandin GI2 or prosta-glandin E1). However, it is important that the effects of epinephrine appear to be mediated through activation of
α
2-adrenergic receptors present in platelets (Yun-Choi et al., 2000), which are of the subtypeα
2A (Mustonen et al., 2000). Taken together, the results of the present study demonstrated that the antiplatelet effect of OMT cannot be attributed to a single biochemical mechanism and it is possibly influenced by several pathways involved with the platelet function.The association of OMT with PTX, ASA or L-ARG induced a slight increase (20, 12.9 and 9.6%, respectively) in the antiplatelet effect of this tincture, but only PTX and L-ARG changed signifi-cantly the effects of OMT. Our data showed that the ODQ, an CONTROL O
MT 100
ASA 25
OMT 10 0 + AAS 25 0
50 100
a a
a
%AGGREGATION
CONTROL O MT
100
PTX 120
OM T 100
+PTX 120
0 50 100
a, b
%AGGREGATION
CONTROL O MT
100 TIC
250
OMT 100 + TI
C 250
0 50 100
a
a, b
a, b
%AGGREGATION
CON TRO
L
OMT 1 00
L- AR G10
OM T 1
00 + L-AR
G10
0 50 100
a, b
%AGGREGATION
DMSO 0,08% OMT 100µg/mL
OMT 100µ g/mL
+ TIC250µM
TIC250µM
ADP 2µM
inhibitor of the soluble guanylate cyclase, did not interfere with the antiplatelet effect of OMT. Together, these data suggest that the COX pathway, NO and cyclic nucleotide levels may play a role in the antiplatelet effect of OMT. However, OMT appears to modulate the levels of cyclic nucleotides without causing a direct stimula-tion of guanylate cyclase.
Studies have shown that the clopidogrel and ticlopidine, plate-let antiaggregant drugs exert their effects by inhibiting the action of ADP in the P2Y12 receptors (Ohlmann et al., 2000;Hollopeter et al., 2001). In the present study it was observed that the inhibitory action of OMT on platelet aggregation was increased by 65.4% in the presence of ticlopidine. Thus, ourfindings suggest that the antiplatelet effect of OMT seems not to be related the participation of this purinergic receptor (P2Y12). In addition, some other possible mechanism unrelated to purinergic receptor also seem to account for the antiplatelet effect of OMT.
Like ASA, the OMT (400 mg/kg) induced a significant increase in the bleeding time in mice. Thesefinding are in accordance with other study (De Gaetano et al. 1977), which showed that the inhibition of ADP-induced but not collagen-induced platelet aggregation determines the prolongation of the bleeding in rats. The OMT did not show anticoagulant effect investigated by aPTT and PT assays in human plasma.
In conclusion, our study demonstrated an unprecedented way that OMT has anti-platelet activity, which appears to result.
5. Conclusion
In conclusion, our study demonstrated an unprecedented way that the tincture from Operculina macrocarpa has antiplatelet effect, which appears to result from several intracellular actions that seems not to be related the participation of P2Y12 receptors. In addition, an independent purinergic receptor mechanism for the OMT action must be account such as COX system, NO and cyclic nucleotides levels. The OMT effect is possibly related at least in part, to the presence of phenols compounds in this product. Finally, the results of the present study agree with the popular use ofOperculina macrocarpafor the treatment of circulatory diseases such as thrombosis. Additional studies to further chemical char-acterization of the OMT and establish the precise pharmacological mechanisms of action of OMT are in development.
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvi-mento Científico e Tecnológico (CNPq, Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil).
References
Angiolillo, D.J., Fernandez-Ortiz, A., Bernardo, E., Alfonso, F., Macaya, C., Bass, T.A., Costa, M.A., 2007. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J. Am. Coll. Cardiol. 49, 1505–1516.
Ballabeni, V., Tognolini, M., Bertoni, S., Bruni, R., Guerrini, A., Rueda, G.M., Barocelli, E., 2007. Antiplatelet and antithrombotic activities of essential oil from wild Ocotea quixos(Lam.) Kosterm. (Lauraceae) calices from Amazonian Ecuador. Pharmacol. Res. 55, 23–30.
Bao, Q.Q., Zhang, L., Li, X.Q., Li, Z.L., Liu, Y.M., 2009. Clinical significance of acute infection in elderly patients with thrombotic disease. Chin. J. Gerontol. 17, 2253–2255.
Born, G.V.R., Cross, M.J., 1963. The aggregation of blood platelets. J. Physiol. 168, 178–195.
Brass, L.F., 2010. Understanding and evaluating platelet function. Hematol. Am. Soc. Hematol. Edu. Program, 387–396.
Brecher, G, Cronkite, E.P., 1950. Morphology and enumeration of human blood platelets. J. Appl. Physiol. 3, 365–377.
Carvalho, D.M.S., Veneranda, A.L.F., Silveira, P.; Francelino, E., Coelho, H.L.L., Fonteles, M.M.F., 2003. Post-marketing surveillance of the Aguardente Alemã product. In: Pharmatech, 7th, João Pessoa. Anais, 87.
Cattaneo, M., Gachet, C., 2001. The platelet ADP receptors. Haematologica 86, 346–348.
Chang, S.S., Lee, V.S., Tseng, Y.L., Chang, K.C., Chen, K.B., Chen, Y.L., Li, C.Y., 2012. Gallic acid attenuates platelet activation and platelet-leukocyte aggregation: involving pathways of Akt and GSK3β. Evid. Based Complement. Alternat. Med., 1–8.
Cheng, T.G., Lee, J.J., Lin, K.H., Shen, C.H., Chou, D.S., Sheu, J.R., 2007. Antiplatelet activity of caffeic acid phenethyl ester is mediated through a cyclic GMP-dependent pathway in human platelets. Chin. J. Physiol. 50, 121–126. Cho, H.J., Kang, H.J., Kim, Y.J., Lee, D.H., Kwon, H.W., Kim, Y.Y., Park, H.J., 2012.
Inhibition of platelet aggregation by chlorogenic acid via cAMP and cGMP-dependent manner. Blood Coagul. Fibrinolysis 23, 629–635.
Clemetson, K.J., 1999. Platelet collagen receptors: a new target for inhibition. Haemostasis 29, 16–26.
Colman, R.W., 1991. Receptors that activate platelets. Proc. Soc. Exp. Biol. Med. 197, 242–248.
Davi, G., Patrono, C., 2007. Platelet activation and atherothrombosis. N. Engl. J. Med. 357, 2482–2494.
De Gaetano, G., Cavenaghi, A.E., Stella, L., 1977. Ditazole and platelets II. Effect of ditazole on in vivo platelet aggregation and bleeding time in rats. Haemostasis 6, 190–196.
El Haouari, M., Bnouham, M., Bendahou, M., Aziz, M., Ziyyat, A., Legssyer, A., Mekhfi, H., 2006. Inhibition of rat platelet aggregation byUrtica dioicaleaves extracts. Phytother. Res. 20, 568–572.
Fontenele, J.B., Leal, L.K.A.M., Silveira, E.R., Felix, F.H., Felipe, C.B., Viana, G.S.B., 2009. Antiplatelets effects of piplartine, an alkamide isolated fromPiper tubercula-tum: possible involvement of cycloxygenase blockade and antioxidant activity. J. Pharm. Pharmacol. (JPP) 61, 1–5.
Gadi, D., Bnouham, M., Aziz, M., Ziyyat, A., Legssyer, A., Bruel, A., Berrabah, M., Legrand, C., Fauvel-Lafeve, F., Mekhfi, H., 2012. Flavonoids purified from parsley inhibit human blood platelet aggregation and adhesion to collagen underflow. J Complement Integr Med. 10.
Gaglia Jr., M.A., Manoukian, S.V., Waksman, P., 2010. Novel antiplatelet therapy. Am. Heart J. 160, 595–604.
Hollopeter, G., Jantzen, H.M., Vincent, D., Li, G., England, L., Ramakrishman, V., Yang, R.B., Nurden, P., Nurden, A.T., Julius, D., 2001. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409, 202–207.
Hsiao, G., Lee, J.J., Lin, K.H., Shen, C.H., Fong, T.H., Chou, D.S., Sheu, J.R., 2007. Characterization of a novel and potent collagen antagonist, caffeic acid phenethyl ester, in human platelets: in vitro and in vivo studies. Cardiovasc. Res. 75, 782–792.
Huang, J., Wang, S., Luo, X., Xie, Y., Shi, X., 2007. Cinnamaldehyde reduction of platelet aggregation and thrombosis in rodents. Thrombosis Res. 119, 337–342. Jang, E.K., Azzam, J.E., Dickinson, N.T., Davidson, L.M.M., Haslam, R.J., 2002. Roles for both cyclic GMP and cyclic AMP in the inhibition of collagen-induced platelet aggregation by nitroprusside. Br. J. Haematol. 117, 664–675.
Jennings, L.K., 2009. Role of platelets in atherothrombosis. Am. J. Cardiol. 103, 4A–10A.
Leal, L.K.A.M. Estudo Farmacológico do extrato hidroalcoólico e constituintes químicos deTorresea cearensisFr. All. (cumaru), 1995. Dissertação (Mestrado em Farmacologia)—Departamento de Fisiologia e Farmacologia, Universidade Federal do Ceará, Fortaleza, Ceará.
Lim, M.Y., Park, Y.H., Kim, M.K., 2004. Antiplatelet activity of gallic acid and methyl gallate. Food Sci. Biotechnol. 13, 806–809.
NO
RMAL
CONT
R
OL
10
0
20
0
400
ASA 100
0
5
10
15
20
a, b a, b
OMT
Bleeding time (min)
Matos, F.J.A., 1982. Aproveitamento de plantas medicinais da região nordeste. Revista Brasileira de Farmácia 63, 132–140.
Matos, F.J.A., 2009. Introdução à Fitoquímica Experimental. Fortaleza: Edições UFC. 150p.
Michelin, D.C., 2008. Estudo químico-farmacológico deOperculina macrocarpaL. Urb. (Convolvulaceae). Tese (Doutorado em Ciências Farmacêuticas)—Centro de Ciências Farmacêuticas, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Araraquara, São Paulo, São Paulo.
Mustonen, P., Savola, J.M., Lassila, R., 2000. Atipamezole, an imidazoline type α2-adrenoceptor inhibitor, binds to human platelets and inhibits their adrenaline-induced aggregation more effectively than yohimbine. Thrombosis Res. 99, 231–237. Ohlmann, P., Eckly, A., Freund, M., Cazenave, J.P., Offermanns, S., Gachet, C., 2000. ADP induces partial platelet aggregation without shape change and potentiates collagen-induced aggregation in the absence of Gαq. Blood 96, 2134–2139. Ruggeri, Z.M., 2002. Platelets in atherothrombosis. Nat. Med. 8, 1227–1234. Ruggeri, Z.M., Savage, B., 2007. Platelet thrombus formation inflowing blood. In:
MICHELSON, A.D. (Ed.), Platelets. Academic Press, California.
Ryu, K.H., Han, H.Y., Lee, S.Y., Jeon, S.D., Im, G.J., Lee, B.Y., Kim, K., Lim, K.M., Chung, J. H., 2009.Ginkgo bilobaextract enhances antiplatelet and antithrombotic effects of cilostazol without prolongation of bleeding time. Thrombosis Res. 124, 328–334.
Singleton, V., Rossi, J.A., 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158.
Tsai, I.L., Lin, W.Y., Teng, C.M., Ishikawa, T., Doong, S.L., Huang, M.W., Chen, Y.C., Chen, I.S., 2000. Coumarins and antiplatelet constituents from the root bark of Zanthoxylum schinifolium. Planta Medica 66, 618–623.
Yuk, D.Y., Ryu, C.K., Hong, J.T., Chung, K.H., Kang, W.S., Kim, Y., Yoo, H.S., Lee, M.K., Lee, C.K., Yun, Y.P., 2000. Antithrombotic and antiplatelet activities of 2-chloro-3-[4-(ethylcarboxy)-phenyl]-amino-1,4-naphthoquinone (NQ12), a newly synthe-sized 1,4-naphthoquinone derivative. Biochem. Pharmacol. 60, 1001–1008. Yun-Choi, H.S., Park, K.M., Pyo, M.K., 2000. Epinephrine induced platelet