O R I G I N A L R E S E A R C H P A P E R
Sulfated polysaccharides isolated from the green seaweed
Caulerpa racemosa
plays antinociceptive and anti-inflammatory
activities in a way dependent on HO-1 pathway activation
Nata´ssia Albuquerque Ribeiro•Ticiana Monteiro Abreu•Hellı´ada Vasconcelos Chaves• Mirna Marques Bezerra•Helena Serra Azul Monteiro•Roberta Jeane Bezerra Jorge• Norma Maria Barros Benevides
Received: 20 September 2013 / Revised: 20 February 2014 / Accepted: 24 February 2014 / Published online: 16 March 2014
ÓSpringer Basel 2014
Abstract
Objective Marine algae are abundant sources of sulfated polysaccharides with various biological activities. Conse-quently, their biomolecules are of great of commercial interest. In this study, we investigated the potential antin-ociceptive activity of a sulfated polysaccharide obtained from the green seaweedCaulerpa racemosa(CrII) and the involvement of the hemoxigenase-1 (HO-1) pathway in its anti-inflammatory effect.
Methods We used a systemic evaluation to verify possi-ble toxic effects of Crll after consecutive treatments.Swiss mice andWistarrats were used for all experiments. Results In Swiss mice, CrII (0.01, 0.1 and 1.0 mg/kg) significantly reduced the number of abdominal contortions and the duration of paw licking in the second phase after treatment with acetic acid and formalin, respectively.
However, CrII was unable to prolong the reaction time of thermally stimulated animals. The anti-inflammatory effect of CrII (0.01, 0.1 and 1.0 mg/kg) was evidenced by a decreased number of leukocytes in the peritoneal cavities of the rats. CrII (0.01, 0.1 and 1.0 mg/kg) also reduced the amount of paw edema induced by carrageenan (Cg) and dextran. The anti-inflammatory effect of CrII was con-firmed by reduced levels of myeloperoxidase in the paw tissue of the Cg groups. After inhibition with ZnPP IX, a specific HO-1 phenotype inhibitor, the anti-inflammatory effect of CrII was no longer observed in Cg-induced paw edema tests. Consecutive Crll (1.0 mg/kg) for 14 days did not change any biochemical or histopathological parame-ters, or cause mortality of mice.
Conclusions CrII did not produce any signs of toxicity and effectively decreased nociception and inflammation. Also, the anti-inflammatory effect of Crll is at least in part dependent on the integrity of the HO-1 pathway.
Keywords Marine alga Sulfated polysaccharide InflammationNociception Caulerpa racemosa
Introduction
Pain transmission occurs through a mechanism that involves a very complex interaction between the peripheral and central structures of the skin surface and the central cerebral cortex [1]. This transmission occurs through nociceptors that can detect a wide variety of stimuli, including those of physical and chemical natures, and convert such stimuli into electrochemical signals [2]. Inadequate treatment of acute pain can lead to persistent and chronic pain, indicating the importance of rapid and effective pain management [3]. The treatment of chronic
Responsible Editor: Mauro Teixeira.
Electronic supplementary material The online version of this article (doi:10.1007/s00011-014-0728-2) contains supplementary material, which is available to authorized users.
N. A. RibeiroT. M. AbreuN. M. B. Benevides (&) Department of Biochemistry and Molecular Biology, Federal University of Ceara´, Fortaleza, CE 60455-760, Brazil e-mail: nmbb@ufc.br
H. V. Chaves
Faculty of Dentistry, Federal University of Ceara´, Sobral, CE, Brazil
M. M. Bezerra
Faculty of Medicine, Federal University of Ceara´, Sobral, CE, Brazil
H. S. A. MonteiroR. J. B. Jorge
Department of Physiology and Pharmacology, Federal University of Ceara´, Fortaleza, CE, Brazil
pain is a serious problem due to the adverse effects of the available medications, which vary according to the class of agent used and include dependence, tolerance, abuse and gastrointestinal effects [4].
The inflammatory process is an important bodily defense mechanism that is composed of complex responses or a complex response of vascularized tissue. The inflam-matory response aims to destroy, immobilize or dilute the harmful agent, isolate the lesion, inactivate toxins and prepare the tissue or organ for healing and repair [5]. However, excessive or inappropriate inflammation is the cause of many diseases [6].
One of the mechanisms involved in the resolution of inflammation is the expression of the enzyme hemoxi-genase-1 (HO-1). In inflammatory conditions, HO-1 becomes the limiting enzyme in the catabolism of free heme groups, which produces equimolar amounts of carbon monoxide (CO), iron (Fe) and biliverdin (BV). These products reduce inflammation and prevent the development of inflammatory diseases [7]. Substances called metalloporphyrins can inhibit HO-1 activity. These compounds were identified as competitive inhibitors of the hemoxygenase pathway [8]. Zinc protoporphyrin (ZnPP) is a metalloporphyrin that competes with heme (natural substrate) by binding to the active site of HO-1 and inactivating the enzyme [9].
In the quest to discover new anti-inflammatory and analgesic effects, scientists continue to use bioproducts as anti-inflammatory agents in the various stages of inflam-mation [10]. According to Cardozo et al. [11] important new anti-inflammatory agents have been isolated from various classes of algae. For example, sulfated polysac-charides have diverse biological characteristics, such as anti-inflammatory properties, and are generally nontoxic [12].
In previous studies, the polysaccharides of Caulerpa racemosa were shown to be selective inhibitors of the reference strains TK—aciclovirus, and resistant strains of herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in Vero cells [13]. Polysaccharide fractions obtained by ion exchange chromatography with DEAE-52 cellulose displayed strong antitumor activity in vitro and in vivo, and inhibited the proliferation of K562 cells in vitro [14].
The objective of this study was to investigate the unexplored antinociceptive and anti-inflammatory activ-ities of a sulfated polysaccharide fraction isolated from the marine green algaC. racemosa (CrII) and perform a systemic evaluation to verify possible toxic effects of Crll after consecutive treatments. We also investigated whether the anti-inflammatory effect of this polysac-charide was related to the integrity of the HO-1 pathway.
Materials and methods
Animals
Male and female Swiss mice (20–25 g) and Wistar rats (males 180–250 g) from the Animal Care Unit of the Federal University of Ceara´ (Fortaleza, Brazil) were used for all experiments. The animals were housed in a tem-perature-controlled room with a 12/12-h light/dark cycle and free access to water and food. For each experiment, groups of six animals were segregated and handled sepa-rately. All procedures and animal treatments were performed at a controlled ambient temperature (20–22 °C),
and special care was taken to avoid environmental distur-bances that might influence the animals’ responses. This study was conducted in accordance with the Committee for Research and Ethical Issues of the International Associa-tion for the Study of Pain (IASPÒ), Institutional Animal Care, and with the approval of the Ethics Committee of the Federal University of Ceara´, Fortaleza, Brazil (CEPA n°.
80/10).
Drugs and reagents
The following drugs and reagents were purchased from Sigma Aldrich (St. Louis, MO, USA): dextran sulfate, k -carrageenan, cetylpyridinium chloride (CPC), 1-9 dime-thyl-methylene blue (DMB), DEAE-cellulose, o-dianisidine dihydrochloride, N-acetyl-N,N,N-trimethylam-monium bromide, hexadecyltrimethylamN-acetyl-N,N,N-trimethylam-monium bromide (HTAB), zinc protoporphyrin IX (ZnPP IX), 37 % form-aldehyde, cystein, papain and bovine serum albumin (BSA). Gelatin was purchased from Oxoid Ltd. (Basing-stoke, UK). Glacial acetic acid (Reagen; Rio de Janeiro, RJ, Brazil), chlorohydrate of morphine (Dimorf, Crista´lia; Itapira, SP, Brazil), dexamethasone (Decadron, Ache´; Campinas, SP, Brazil) and indomethacin (Indocid, Merck Sharp and Dohme; Campinas, SP, Brazil) were obtained from the indicated companies. Ethylenediaminetetraacetic acid (EDTA) and chloral hydrate were purchased from Vetec Quı´mica Fina, Ltda (SP, Brazil). The enzymatic kits used to evaluate the systemic toxicity of Crll were pur-chased from LABTEST (Diagnostic Tests, Brazil). All chemicals were analytical grade.
Isolation of sulfated polysaccharides
(25°C), macerated with liquid nitrogen and stored in glass
jars at room temperature. A voucher specimen (no. 52418) was deposited in the Herbarium Prisco Bezerra in the Department of Biological Sciences (Federal University of Ceara´, Brazil).
A 5-g sample of dry tissue was rehydrated with 250 ml of 0.05 M sodium acetate buffer (pH 6.0) containing 5 mM EDTA and 5 mM cystein, and then incubated for 6 h with papain (1,020 mg) at 60°C before centrifugation
(5,000g; 20 min; 4°C), as previously described by Farias
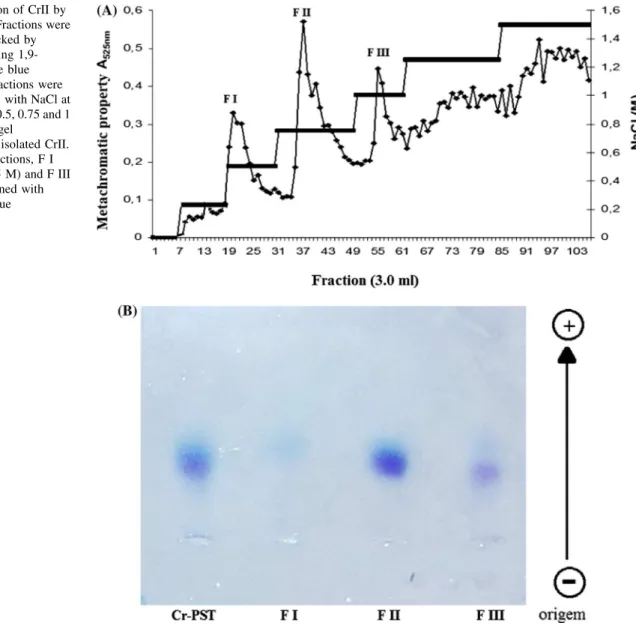
et al. [15]. The total sulfated polysaccharide (Cr-PS) obtained from C. racemosa (100 mg) was dissolved in 50 ml of 50 mM sodium acetate buffer (pH 6.0) and applied to a DEAE-cellulose column (30 cm92 cm) that was equilibrated with the same buffer. The analytes were separated using a step-wise gradient from 0 to 1.5 M NaCl at 0.25 M intervals in the same buffer. The flow rate through the column was 3 ml/min. Fractions (3 ml) were collected and analyzed for sulfated polysaccharides using the metachromatic assay (A525 nm) with DMB, as previously described [16]. The obtained Cr-PS and frac-tions were analyzed by 0.5 % agarose gel electrophoresis [17]. The biological protocols were performed with the fraction that showed the highest yield, which was termed CrII.
Chemical composition
The sulfate content was estimated after acid hydrolysis of the soluble polysaccharides in 1 M HCl at 110°C for 5 h,
in accordance with the previously described gelatin–bar-ium method, using Na2SO4 as the standard [18]. The protein content was measured using Coomassie Brilliant Blue G-250, using BSA as a standard [19] to assess protein contamination.
Antinociceptive activity Writhing test
A chemical model of nociception was used to assess analgesic activity [20]. First, the male mice received CrII (0.01, 0.1 or 1.0 mg/kg; i.v.) or sterile saline (0.9 %, w/v, NaCl). After 30 min, abdominal constrictions were induced by intraperitoneal (i.p.) injection of 0.8 % acetic acid (v/v; 0.1 ml/10 g body weight). Shortly after the acetic acid was administered, the number of abdominal constrictions was counted for consecutive 30 min. The percentage of nociception inhibition was calculated by comparing the mean number of constrictions obtained from the control group (injected with only acetic acid) with that of the experimental group (pre-treated with CrII).
Formalin test
The formalin test was performed following the method described by Dubuisson and Dennis [21], as modified by Hunskaar et al. [22]. This method is characterized by a local tissue injury to the paw, which induces pain and localized inflammation. Male mice were injected with either CrII (0.01, 0.1 or 1.0 mg/kg; i.v.) or sterile saline (0.9 %, w/v, NaCl). After 30 min, 20 ll of 2 % formalin solution was injected into the right paw of male mice, and the duration of licking was recorded during the first 5 min (first phase, which corresponds to the direct application of a chemical stimulus to the nociceptors—the initial phase) and for an additional 5 min after a 20 min time period (second phase, which involves inflammation—late phase). Hot-plate test
Central analgesic activity was evaluated using the hot plate test, according to the method described by Eddy and Leimbach [23]. This test involves recording the time(s) that male mice require to manifest a response when in contact with a heated metal plate (51±1°C), which corresponds
to the act of removing or licking the hind paw and/or jumping. This specific test is used to verify central noci-ception. The animals were first acquainted with the hot plate to observe the control reaction time. Animals with a reaction time exceeding 10 s were discarded from the test. Immediately after the trial (control reaction time), the mice were divided into groups of six. The mice then received an injection of sterile saline (0.9 %, w/v), CrII (0.01, 0.1 or 1.0 mg/kg; i.v.), morphine (5 mg/kg; s.c.) or indomethacin (5 mg/kg; s.c.), and the reaction times were measured at 0 30, 60 and 90 min after drug administration. A cutoff time of 40 s was used to avoid paw lesions.
Anti-inflammatory activity Peritonitis model
Carrageenan-induced rat paw edema
Carrageenan-induced paw edema was achieved according to the method described by Winter et al. [26]. Cg (700 lg/ paw) was administered subcutaneously (s.c.). Rats were pre-treated with CrII at doses of 0.01, 0.1 or 1.0 mg/kg (0.1 ml/100 g body weight) 30 min before the Cg injec-tion. As a reference, dexamethasone (1 mg/kg; s.c.) was administered 1 h before Cg. An additional group received only sterile saline (0.15 M NaCl; s.c.). The volumes of the right hind paw of each animal were measured using a plethysmometer before injection of the inflammatory stimulus (time zero), and at intervals of 1, 2, 3, 4 and 5 h after administration of the inflammatory stimulus. Swelling was calculated as the difference between the volumes of fluid displaced by the paw at time zero and at each time point after the stimulus.
Dextran-induced rat paw edema
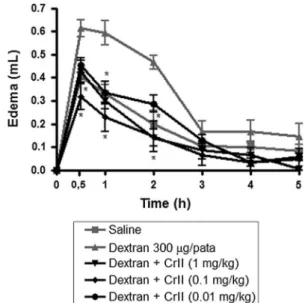
The dextran-induced paw edema model was generated according to the method described by Maity et al. [27]. Dextran (300lg/paw) was administered subcutaneously. The rats were pre-treated with CrII at doses of 0.01, 0.1 or 1.0 mg/kg (0.1 ml/100 g body weight; s.c.) 1 h prior to receiving the stimuli. Control animals received the same volume of sterile saline (0.9 % w/v, NaCl). The volumes of the right hind paw of each animal were measured using a plethysmometer prior to injection of the inflammatory stimulus (time zero) and at intervals of 30 min, 1, 2, 3 and 4 h after administration of the inflammatory stimulus. Swelling was calculated as the difference between the volumes of fluid displaced by the paw at time zero and at each time point after the stimulus.
Determination of myeloperoxidase (MPO) activity
Myeloperoxidase is an enzyme found primarily in azuro-philic granules within neutrophils, and it has been used extensively as a biochemical marker of granulocyte infil-tration in various tissues. Neutrophil accumulation in rats paw tissues was measured using an MPO activity assay, as previously described [28]. Briefly, 50 mg of paw tissue was homogenized in 1 ml of 50 mM potassium phosphate (pH 6.0) containing 0.5 % HTAB using a Polytron homoge-nizer (two cycles of 10 s). After centrifugation at 4,0009g for 12 min at 4°C, samples of the supernatant
(7 ml) were added to phosphate buffer (200 ml) containing dihydrochloride (1 mM), o-dianisidine and 0.0005 % hydrogen peroxide in a 96-well microplate. The absorbance was measured at 450 nm, and two readings were taken at 60 s intervals. MPO activity was determined by measuring
the change in absorbance at 450 nm using o-dianisidine dihydrochloride and 1 % hydrogen peroxide. One unit of MPO activity was defined as the activity required to con-vert 1 mol of hydrogen peroxide to water in 1 min at 22°C. The results are reported as MPO units/mg of tissue.
Analysis of HO-1 pathway involvement in the anti-inflammatory effect of CrII
To analyze the involvement of HO-1 in the anti-inflammatory activity of CrII, rats were pre-treated (s.c.) with ZnPP IX (3 mg/kg) and then injected with Crll (0.1 mg/kg; i.v.) 60 min later. After 30 min, Cg (500lg/ paw) was injected s.c. [29], and the paw volume was measured immediately before (0 h) the stimulus and at selected time intervals (1, 2, 3, 4 and 5 h) using a ple-thysmometer. The results are expressed as the variation in paw volume (ml), which was calculated relative to the basal volume (0 h).
Evaluation of repeated dose toxicity
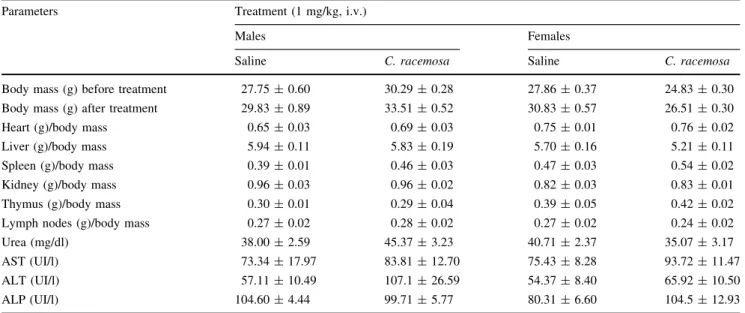
Body mass loss, organ weight alteration and blood levels of the biochemical parameters [alanine amino transferase (AST), aspartate amino transferase (ALT), alkaline phos-phatase (ALP) and urea] were evaluated after once-daily treatment with CrII (1 mg/kg; i.v.) or sterile saline (0.9 % w/v, NaCl) for seven consecutive days. After treatment, the male and female mice were weighed, and peripheral blood was collected for biochemical analysis (determined by enzymatic and colorimetric tests—LABTEST). After the animal was euthanized, the liver, kidney, spleen, thymus, lymph nodes and heart were removed and weighed. Pos-sible ulcerative lesions or hemorrhaging were quantified and macroscopically measured.
Histological analysis
After euthanasia, the liver, kidney, spleen, thymus, lymph nodes and heart were fixed with formalin. The material was then dehydrated using ethanol and processed for embed-ding in paraffin. The resulting blocks were sliced into 5-lm-thick sections, stained with hematoxylin-eosin (HE) and observed under a light microscope.
Statistical analysis
The data are presented as the mean±SEM of six animals per group. Analysis of variance (ANOVA) was performed using Bonferroni’s test and Student’s t test for unpaired values. Values of p\0.05 were considered to be
Results
Isolation of sulfated polysaccharides
Total sulfated polysaccharides yielded low levels of poly-saccharide (2.2 %) and free sulfate (15.17 %) and trace amounts of protein. Cr-PS produced three different fractions (FI, FII and FIII, eluted with 0.5, 0.75 and 1.0 M of NaCl, respectively) when separated in a DEAE-cellulose column (Fig.1a). These fractions had yields of 10.5, 20.5, and 17.6 % and sulfate contents of 5.91, 20.84 and 28.39 %, respectively, with no protein contamination. The Cr-PS and the three obtained fractions exhibited different charge densities when analyzed using 0.5 % agarose gel electrophoresis. The FII fraction was more concentrated than the FIII fraction. The FI fraction was not detected in the gel, most likely because of the low number of sulfated groups in the chemical structure of this polysaccharide fraction (Fig.1b).
Antinociceptive activity
Pretreatment with CrII (0.01, 0.1 or 1.0 mg/kg; i.v.) sig-nificantly reduced the number of abdominal constrictions induced by acetic acid in mice in a dose-dependent manner (32.60, 49.26 and 60.75 % for 0.01, 0.1 and 1.0 mg/kg, respectively). For this experiment, animals that were pre-treated with either morphine or indomethacin (5 mg/kg; s.c.) were used as positive controls. Treatment with mor-phine and indomethacin inhibited 96 and 54 % of the abdominal constrictions, respectively (Fig.2a). To evalu-ate whether CrII exhibited antinociceptive effects in another model of peripheral analgesia, we tested its effi-cacy in the formalin model. Intraplantar injection of a 2 % formalin solution in mice induced a nociceptive response that was characterized by an increased licking duration. No reduction in the licking duration was observed during the first phase for any of the tested Crll doses (Fig. 2b).
Fig. 1 aSeparation of CrII by DEAE-cellulose. Fractions were collected and checked by metachromasia using 1,9-dimethylmethylene blue (diamond). The fractions were eluted ‘‘step wise’’ with NaCl at concentrations of 0.5, 0.75 and 1 (line).bAgarose gel
However, CrII (0.01, 0.1 or 1.0 mg/kg; i.v.) injected 30 min prior to the administration of formalin inhibited the formalin response during the second phase by 97.37, 97.37 and 98.68 %, respectively. Similarly, morphine and indo-methacin inhibited the second phase by 99 and 92.26 %, respectively (Fig.2b).
In the hot plate test, saline (0.15 M, NaCl; i.v.), CrII (0.01, 0.1 or 1.0 mg/kg; i.v.) or indomethacin (5 mg/kg; s.c.) did not increase the average reaction time to thermal stimulus compared to the control (morphine), which sig-nificantly increased the reaction time to thermal stimulation at 30, 60 and 90 min (Fig.2c).
Anti-inflammatory activity
When CrII (0.01, 0.1 or 1.0 mg/kg; i.v.) was administered 30 min before Cg administration in the rats’ peritoneal cavity, 46.41, 56.69 and 33.96 % reductions in the neu-trophil counts were observed, respectively. Likewise, pretreatment with dexamethasone reduced the neutrophil counts by 59.94 % (Fig.3). In the paw edema model in
rats, CrII (0.01, 0.1 or 1.0 mg/kg; i.v.) was also able to diminish the temporal course of edema over the 5 h testing period after Cg administration (700lg/paw; s.c.) (Fig.4a). Particularly during the third hour, CrII (0.01, 0.1 or 1.0 mg/ kg; i.v.) significantly reduced the occurrence of edema by 60.3, 73.0 and 79.3 %, respectively, in response to the stimulus. Dexamethosone pretreatment inhibited edema by 82.5 %. These data were confirmed using MPO activity assays, which revealed that CrII (0.01, 0.1 or 1.0 mg/kg; i.v.) inhibited neutrophil accumulation in the paw by 44.92, 71.56 and 53.78 %, respectively. Dexamethasone also inhibited MPO activity (79.67 %) (Fig.4b). Additionally, dextran (300 lg/paw; s.c.) induced a significant increase in vascular permeability; the maximum effect was observed between 30 main and 1 h after administration, and it decreased over time. Treatment (i.v.) with different doses of CrII (0.01; 0.1 or 1.0 mg/kg) inhibited the paw edema induced by dextran at 1 h by 47.36, 59.64 and 47.36 %, respectively (Fig. 5).
To investigate the role of HO-1 activity in the anti-inflammatory effect of CrII, rats were pretreated (s.c.) with
Fig. 2 Effect of CrII in nociceptive models. Thirty minutes prior to the application of stimuli, the rats were treated with sterile saline (0.15 M NaCl, i.v.), morphine (5 mg/kg, s.c.), indomethacin (5 mg/ kg, s.c.) or CrII (0.01, 0.1 and 1.0 mg/kg, i.v.). Data are expressed as the mean±SEM of six animals for each group (ANOVA; Bonfer-roni’s test).a Effect of CrII on the writhing response induced by acetic acid in mice.Asterisk significantly different from the sterile saline group (p\0.05).bEffect of CrII on the formalin test in mice.
The duration of licking was determined during the first 5 min (first phase) and during the period from 20 to 25 min (second phase) after the injection of 2 % formalin.Asterisksignificantly different from the saline group (p\0.05), and Hash significantly different from the
morphine group (p\0.05). cEffect of CrII on the reaction times
ZnPP IX (3 mg/kg), a specific HO-1 inhibitor. The anti-inflammatory effect of CrII (0.1 mg/kg; i.v.) on the Cg-induced rat paw edema was abolished in the presence of ZnPP-IX (Fig.6).
Repeated dose toxicity
After seven consecutive days of CrII (1.0 mg/kg, i.v.) administration, no mice died and no physical, behavioral or body mass changes were observed (p[0.05). Following
euthanasia and organ removal, there were no significant changes in the wet weights of the liver, kidney, thymus, lymph nodes and heart compared to control animals. The serum levels of AST, ALT, ALP (a measure of hepatic function) and urea (an indicator of renal function) did not differ between CrII treated mice and the saline control group (Table1).
Additionally, histological analyses of the organs were conducted to verify the presence or absence of abnormal cellular or tissue morphology. No significant changes were observed in the kidney, heart, thymus, spleen and lymph nodes in any of the groups, with the exception of the liver, which presented discrete cytoplasmic vacuolation (degen-eration, swelling or edema). This change in the liver was characterized by the accumulation of water in the cyto-plasm, which resulted in a thick and pale nucleus that was normally positioned. In all groups, these changes appeared to be reversible, including the untreated (Fig.7). There-fore, there were no consistent signs of systemic damage in response to Crll treatment.
Discussion
Since antiquity, substances derived from natural products have been used for various purposes, including the treatment of pain and inflammation [4, 12]. The results reported here demonstrate that a polysaccharide fraction (CrII) obtained from the alga C. racemosa by ion exchange chromatography on DEAE-cellulose (eluted with 0.75 M NaCl) has antinociceptive and anti-inflam-matory effects in models of nociception (writhing, formalin and hot-plate tests) and inflammation (peritonitis and paw edema tests).
A writhing test induced by acetic acid is a widely used model of inflammatory pain used to search for new agents with analgesic, peripheral and anti-inflammatory properties [30]. CrII (1.0 mg/kg, i.v.) displayed enhanced behavior in writhing tests compared to sulfated polysaccharides (3.0 mg/kg; i.v.) obtained from the green seaweed Caul-erpa cupressoides, which belongs to the same genus of algae [31]. However, the acetic acid-induced abdominal
Fig. 3 Effect of CrII on neutrophil migration in rats. Before receiving an injection of carrageenan (700lg/cav; i.p.), the rats received sterile saline or CrII (0.01, 0.1 and 1.0 mg/kg, i.v.). Then, dexamethasone (1 mg/kg; s.c.) was injected. Another group received only saline (s.c.), without Cg. Data are expressed as the mean±SEM of six animals for each group. Asterisk indicates a significant difference (p\0.05) from the carrageenan group (ANOVA;
Bon-ferroni’s test) Fig. 4 Effect of CrII using the carrageenan (Cg)-induced paw edema model and myeloperoxidase (MPO) activity in the supernatant of paw section homogenates injected with carrageenan.aRats were treated for 30 min before the injection of carrageenan (700lg/paw, i.pl.), with sterile saline (0.15 M NaCl, i.v.), dexamethasone (1 mg/kg, s.c.) or CrII (0.01, 0.1 and 1.0 mg/kg, i.v.). Paw edema was measured 1, 2, 3, 4 and 5 h after the injection of Cg, and data are expressed as the increase in paw volume (ml).bCrII (0.01, 0.1 and 1.0 mg/kg, i.v.), dexamethasone (Dexa, 1 mg/kg, s.c.) or sterile saline (NaCl 0.15, s.c.) were administered at 30 min, 1 and 1 h, respectively, before the s.c. injection of carrageenan (Cg) (700lg/paw). The results are expressed in activity units of MPO/mg of tissue. Data are expressed as the mean±SEM of six animals for each group (ANOVA; Bonferroni’s test).Asteriskindicates statistically significant difference (p\0.05)
contortion test is also known to be nonspecific [32]. Thus, to determine the best analgesic effect, CrII was assessed using the formalin (2 %) test.
Formalin-induced nociception is widely used as model of persistent pain [33]. This test is specific and is charac-terized by a distinct, biphasic response; the first response is termed neurogenic, and the second is inflammatory. Drugs that act primarily on the central nervous system have been reported to inhibit both phases, while others act peripher-ally, such as anti-inflammatory and nonsteroidal agents and corticosteroids that predominantly inhibit the second phase [22, 34]. Therefore, because CrII is only effective in the second test phase, this compound likely possesses proper-ties similar to those of anti-inflammatory and nonsteroidal agents and corticosteroids, which exert peripheral effects (inflammatory pain) on nociceptors. These results corrob-orate previously published reports that sulfated polysaccharide fractions from the red seaweedSolieria fi-liform (9 mg/kg; i.v.) only affected the duration of paw licking in the second phase of the formalin test. Impor-tantly, the seaweed used in this study produced a similar result at a dose that was 900 times less than the doses used in the work of Arau´jo et al. [35].
The hot plate test is widely used as an experimental method to study nociception in rats and mice [36], and it is considered to be a gold standard test for the evaluation of analgesics that elicit a central effect, such as opioids, which exert their analgesic effects via supra spinal receptors [37, 38]. Therefore, the results obtained using CrII in this model suggest that its antinociceptive activity is not related to central opioid receptors and that it likely acts through a peripheral mechanism. These results are similar to those obtained for the peripheral antinociceptive action of sul-fated polysaccharide fractions from the alga S. filiform [35], but differ from those of Rodrigues et al. [31], who reported that the sulfated polysaccharide fraction of the green alga C. cupressoideswas able to significantly delay the response to thermal stimuli.
Because of the well-established link between the development of pain and inflammatory processes, we investigated the anti-inflammatory activity of CrII using the peritonitis and paw edema tests. The inflammatory effect of Cg involves the action of a series of mediators, characterized by the initial release of histamine, serotonin and bradykinin, followed by increased levels of prosta-glandins, which coincide with leukocyte migration to sufficiently amplify the inflammatory response and trigger the production of other mediators [39]. Peritonitis has been well characterized as an experimental model of acute inflammation that allows for the quantification and corre-lation of cell migration in sets with several inflammatory mediators [40]. In this model, all doses of CrII significantly decreased the leukocyte count in the peritoneal cavities. The anti-inflammatory effect elicited by CrII was similar to the reported effects of the sulfated galactan from the red alga Pophyridium sp., which also showed
anti-Fig. 5 Effect of CrII in the dextran-induced paw edema model. The animals were treated with sterile saline (0.15 M NaCl, i.v.) or CrII (0.01, 0.1 and 1.0 mg/kg, i.v.) 30 min prior to the injection of dextran (300lg/paw, i.pl.). Paw edema was measured 1, 2, 3, 4 and 5 h after dextran injection and is expressed as the increase in paw volume (ml). Data are expressed as the mean±SEM of six animals for each group (ANOVA; Bonferroni’s test).Asteriskindicates a statistically signif-icant difference (p\0.05) compared to the dextran control
Fig. 6 The HO-1 activity in paw edema induced by carrageenan in the rats. Before receiving an injection of carrageenan (700lg/paw, i.v.), the groups of animals received CRII (0.1 mg/kg) with or without ZnPP IX (3 mg/kg, s.c.). Another group received only saline without Cg. Data are expressed as mean±SEM of six animals for each group (ANOVA; Bonferroni’s test).Asteriskindicates statistically signifi-cant difference (p\0.05) when compared to control carrageenan.
Hash indicates statistically significant difference (p\0.05) when
Fig. 7 Four-millimeter-thick photomicrographs of the organs taken from Swiss mice after receiving no treatment, saline or CrII (7 days of CrII, 1.0 mg/kg, i.v.):akidney,bheart,ctimo, dspleen,elymph node and fliver; note the discrete cytoplasmic vacuolization (white arrows). 4009
inflammatory activity [41]. Interestingly, the genus Caul-erpa is known to possess anti-inflammatory activity. According to Rodrigues et al. [31], a sulfated polysaccha-ride fraction (FII) from the green alga C. cupressoides significantly inhibited neutrophil migration in a dose-dependent manner compared to Cg. In another study, alcoholic and metano´icos extracts from the green seaweed Caulepa Mexicanwere used in an inflammatory model of peritonitis induced by Zymosan, and these extracts were able to suppress cell migration to the peritoneal cavity [42]. Due to the anti-inflammatory effect of CrII on the induced peritonitis, this compound was tested in Cg-induced and dextran-Cg-induced models of rat paw edema. Paw edema induced by dextran increases vascular perme-ability by releasing vasoactive amines, such as histamine and serotonin, which cause osmotic edema with low levels of protein and neutrophils [43]. At all of the doses tested, CrII inhibited both Cg-induced and dextran-induced rat paw edema and neutrophil migration, as well as the activity of MPO. Oral administration of total sulfated polysaccha-rides (2.5, 5, 10 or 20 mg/kg) from the brown seaweed Turbinaria ornatealso inhibited the Cg-mediated induction of paw edema in rats [12]. Again, CrII had a more satis-factory effect at a 1,000-fold lower dose compared to the polysaccharides from the brown seaweedT. ornate. This is the first report of a sulfated polysaccharide that is capable of inhibiting dextran-induced paw edema. Therefore, the anti-edematogenic effect of CrII suggests that the inhibi-tion of Cg-induced and dextran-induced paw edema is related to inflammatory events that involve the inhibition of
multiple mediators, including histamine, serotonin, brady-kinin, prostaglandins and vasoactive amines.
Heme oxygenase is an enzyme involved in the degra-dation of heme groups, and its involvement in inflammatory contexts is well established in the literature. HO-1 production is elevated in inflammatory cells during the resolution phase of inflammation [44]. HO-1 induction significantly suppresses inflammation; however, inhibition of this enzyme potentiates the inflammatory response in different inflammation models [45–50]. Additionally, the anti-inflammatory effects of HO-1 have been reported in Cg-induced rat models [44]. Considering these data, we explored the involvement of HO/BVD/CO in the anti-inflammatory effect of CrII using ZnPP IX, a specific HO-1 inhibitor. After the pretreatment with ZnPP IX, the anti-inflammatory efficacy of CrII in Cg-induced paw edema in rats was not observed, suggesting that HO-1 activity is involved in the inhibitory effects of CrII, corroborating other data showing that the inhibition of HO-1 pathway is associated with worsening of inflammatory response [51– 53]. In this regard, we have recently demonstrated that ZnPP IX treatment potentiated the effect of acetic acid by increasing the number of writhes and reducing bilirubin levels [54]. Further, we also showed that some sulfated polysaccharides from seaweeds may exert anti-inflamma-tory activity through the HO-1 pathway [55], corroborating the results observed in this study.
Although the reports regarding the safety of seaweed compounds in animals are still limited, several studies have reported on their possible effects. Araujo et al. [35]
Table 1 Systemic effects of CrII (1.0 mg/kg) in mice
Parameters Treatment (1 mg/kg, i.v.)
Males Females
Saline C. racemosa Saline C. racemosa
evaluated the potential toxic effects of sulfated polysac-charides from the seaweedS. filiformin a 14-day subchronic toxicity test. Their results showed that intraperitoneal administration of sulfated polysaccharides produced no signs of toxicity in mice. The sulfated polysaccharides from the seaweed, Gracilaria cornea, were also administered intraperitoneally in rats for 14 consecutive days, and they also produced no significant signs of toxicity [56]. To evaluate the safety of CrII administration, we assessed the integrity of the liver, kidney, thymus, lymph nodes and heart in mice injected with CrII. Biochemical analyses revealed no changes in the enzymatic activities of trans-aminases and urea in the serum of treated mice. In addition, histological analysis of tissues taken from the organs of Crll-treated animals revealed no damage.
Conclusion
In conclusion, we demonstrated the antinociceptive and anti-inflammatory efficacy of sulfated polysaccharides from the green seaweedC. racemosa(Crll). Additional, our results strongly suggest that the anti-inflammatory CrII efficacy at least in part depends on the integrity of the HO-1 pathway. Further, we verified that CrII is safe in the effective dose. Taking into account the well-demonstrated antinociceptive and anti-inflammatory efficacy of CrII, the designing of alternative compounds to classical anti-inflammatory and analgesic agents is very much encour-aged, to define new pharmacological targets for the treatment of inflammatory pain.
Acknowledgments This work was supported by Conselho Nacional de Desenvolvimento Cientı´fico e Tecnolo´gico (CNPq) and Coorde-naca˜o de Aperfeic¸oamento de Pessoal de Nı´vel Superior (CAPES). H. S. A. Monteiro and N. M. B. Benevides are senior investigators of CNPq/Brazil.
Conflict of interest No conflict of interest is declared.
References
1. Fu¨rst S. Transmitters involved in antinociception in the spinal cord. Brain Res Bull. 1999;48:129–41.
2. Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10.
3. Moore ND. In search of an ideal analgesic for common acute pain. Acute Pain. 2009;11:129–37.
4. McCurdy CR, Scully SS. Analgesic substances derived from natural products (natureceuticals). Life Sci. 2005;78:476–84. 5. Robbins SL, Cotran RS, Kumar V. Pathologic basic of diseases.
5a ed. Philadelphia: W. B. Sauders Co; 1994.
6. Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:232–40.
7. Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50: 323–54.
8. Mitrione SM, Villalon P, Lutton JD, Levere RD, Abraham NG. Inhibition of human adult and fetal heme oxygenase by new synthetic heme analogues. Am J Med Sci. 1988;296:180–6. 9. Blumenthal SB, Kiemer AK, Tiegs G, Seyfried S, Ho¨ltje M,
Brandt B, Ho¨ltje H, Zahler S, Vollmar AM. Metalloporphyrins inactivate caspase-3 and -8. FASEB J. 2005;19:1272–9. 10. Pather N, Viljoenb AM, Kramer B. A biochemical comparison of
the in vivo effects ofBulbine frutescensandBulbine natalensis
on cutaneous wound healing. J Ethnopharmacol. 2011;133: 364–70.
11. Cardozo KHM, Guarantini T, Barros MP, Falca˜o VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, Pinto E. Metabolites from algae with economical impact. Comp Bio-chem Physiol C. 2007;146:60–78.
12. Ananthi S, Raghavendran HRB, Sunil AG, Gayathri V, Rama-krishnan G, Vasanthi HR. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide fromTurbinaria ornata (Marine Brown Alga). Food Chem Toxicol. 2010;48: 187–92.
13. Ghosh P, Adhikari U, Ghosal PK, Pujol CA, Carlucci MJ, Da-monte EB, Ray B. In vitro anti-herpetic activity of sulfated polysaccharide fractions fromCaulerpa racemosa. Phytochem-istry. 2004;65:3151–7.
14. Ji H, Shao H, Zhang C, Hong P, Xiong H. Separation of the polysaccharides inCaulerpa racemosaand their chemical com-position and antitumor activity. J Appl Polym Sci. 2008;110: 1435–40.
15. Farias WRL, Valente AP, Pereira MS, Moura˜o PAS. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red alga Botryocladia occi-dentalisand comparison of its anticoagulant action with that of sulfated galactans. J Bio Chem. 2000;275:29299–307.
16. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dime-thyl-methyleno blue. Biochem Biophys Acta. 1986;883:173–7. 17. Dietrich CP, Dietrich SMC. Electrophoretic behaviour of acidic
mucopolysaccharides in diamine buffers. Anal Biochem. 1966;70:645–7.
18. Dodgson KS, Price RG. Determination of inorganic sulphate in studies on the enzymatic and non-enzymic hydrolysis of carbo-hydrate and other sulphate esters. Biochem J. 1961;78:312–9. 19. Bradford MM. A rapid and sensitive method for the quantitation
of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
20. Koster R, Anderson M, de Beer EJ. Acetic acid for analgesic screening. Federation Proc. 1959;18:412.
21. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic affects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;21:161–74.
22. Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14:69–76.
23. Eddy NB, Leimbach D. Synthetic analgesics. II. dithienylbutenyl and dithienylbutylamines. J Pharmacol Exp Ther. 1995;107: 385–93.
24. Assreuy AMS, Gomes DM, Silva MSJ, Torres VM, Siqueira RCL, Pires AF. Biological effects of a sulfated-polysaccharide isolated from the marine red algae Champia feldmannii. Biol Pharma Bull. 2008;31:691–5.
26. Winter CA, Risley EA, Nuss GW. Carrageenin induced edema in hind paw of rats as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7.
27. Maity TK, Mandal SC, Mukherjee PK, Saha K, Das J, Pal M, Saha BP. Studies on antiinflammatory effect ofCassia toraleaf extract (Fam. Legumirosae). Phytotherapy Res. 1988;12:221–3. 28. Bradley PP, Priebat DA, Christensen RD, Rothstein G.
Mea-surement of cutaneous inflammation: estimation of neutrophils content with an enzyme marker. J Invest Dermatol. 1982;78: 206–9.
29. Freitas A, Alves-Filho JC, Secco DD, Neto AF, Ferreira SH, Barja-Fidalgo C, Cunha FQ. Heme oxygenase/carbon monoxide biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br J Pharmacol. 2006;149: 345–54.
30. le Bars D, Gozariu M, Cdden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652.
31. Rodrigues JAG, Vanderlei ESO, Silva LMCM, Arau´jo IWF, Queiroz INL, Paula GA, Abreu TM, Ribeiro NA, Lima V, Bezerra MM, Chaves HV, Jorge RJB, Monteiro HSA, Leite EL, Benevides NMB. Antinociceptive and anti-inflammatory activities of a sul-fated polysaccharide isolated from the marine green seaweed
Caulerpa cupressoides. Pharmacol Rep. 2012;64:282–92. 32. Hendershot LC, Forsaith J. Antagonism of frequency of
phenyl-quinone induced writing in ten mouse by weak analgesics as non-analgesics. J Pharmacol Exp Ther. 1959;125:237–40.
33. Shields SD, Cavanaugh DJ, Lee H, Anderson DJ, Basbaum AI. Pain behavior in the formalin test persists after ablation of the great majority of C-fiber nociceptors. Pain. 2010;151:422–9. 34. Bitencourt FS, Figueiredo JG, Mota MRL, Bezerra CCR,
Sil-vestre P, Vale MR, Nascimento KS, Sampaio AH, Nagano CS, Saker-Sampaio S, Farias WRL, Cavada BS, Assreuy AMS, A-lencar NMN. Antinociceptive and anti-inflammatory effects of a mucin-binding agglutinin isolated from the red alga Hypnea cervicornis. Naunyn-Schmiedeberg’s Arch Pharmacol. 2008;377: 139–48.
35. Arau´jo IWF, Vanderlei ESO, Rodrigues JAG, Coura CO, Quin-dere´ ALG, Fontes BP, Queiroz INL, Jorge RJB, Bezerra MM, Silva AAR, Chaves HV, Monteiro HAS, Paula RCM, Benevides NMB. Effects of a sulfated polysaccharide isolated from the red seaweed Solieria filiformis on models of nociception and inflammation. Carbohydr Polymers. 2011;86:1207–15.
36. Gunn A, Bobeck EN, Weber C, Morgan MM. The influence of non-nociceptive factors on hot-plate latency in rats. J Pain. 2011;12:222–7.
37. Nemirovsky A, Chen L, Zelma V, Jurna I. The antinociceptive effest of the combination of spinal morphine with systemic morphine or buprenorphine. Anesth Analg. 2001;93:197–203. 38. Yalcin I, Charlet A, Freund-Mercier MJ, Barrot M, Poisbeau P.
Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain. 2009;10(767):773. 39. Nantel F, Dennis D, Gordon R, Northey A, Cirino M, Metters
KM, Chan CC. Distribution and regulation of cyclooxygenases-2 in carrageenan-induced inflammation. Br J Pharmaco. 1999;128: 853–9.
40. Montanher AB, Zucolotto SM, Schenkel EP, Frode TS. Evidence of anti-inflammatory effects of Passiflora edulis in an inflam-mation model. J Ethnopharmacol. 2007;109:281–8.
41. Matsui SM, Muizzudin N, Arad S, Marenus K. Sulfated poly-saccharides from red microalgae have anti-inflammatory in vitro and in vivo. Appl Biochem Biotechnol. 2003;104:13–22. 42. Bitencourt MAO, Dantas GR, Lira DP, Barbosa-Filho JM,
Mir-anda GEC, Santos BVO, Souto JT. Aqueous and Methanolic
extracts ofCaulerpa mexicanasuppress cell migration and ear edema induced by inflammatory agents. Mar Drugs. 2011;9: 1332–45.
43. Lo TN, Almeida AP, Beaven MA. Dextran and carrageenin evoke different inflammatory response in rat with respect to composition of infiltrates and effect of indomethacin. J Pharma-col Exp Ther. 1982;221:261–7.
44. Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxy-genase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90.
45. Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR. Upregulation of heme oxy-genase-1 protects genetically fat zucker rat livers from ischemia/ reperfusion injury. J Clin Invest. 1999;104:1631–9.
46. Willi D, Moore AR, Willoughby DA. Heme oxygenase isoform expression in cellular and antibody-madiates models of acute inflammation in the rats. J Pathol. 2000;190:627–34.
47. Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, Adema G, van Kooyk Y, de Witte T, Figdor CG. Heme is a potent inducer of inflammation in mice and is coun-teracted by heme oxygenase. Blood. 2001;98:1802–11. 48. Song HJ, Shin CY, Oh TY, Sohn UD. The protective effect of
eupatilin on indomethacin-induced cell damage in cultured feline ileal smooth muscle cells: involvement of HO-1 and ERK. J Ethnopharmacol. 2008;118:94–101.
49. Jeonga G, Lee D, Kim D, Jahng Y, Son J, Lee S, Kim Y. Neu-roprotective and anti-inflammatory effects of mollugin via up-regulation of heme oxygenase-1 in mouse hippocampal and mi-croglial cells. Eur J Pharmacol. 2011;654:226–34.
50. Li B, Lee D, Jeong G, Kim G. Involvement of heme oxygenase-1 induction in the cytoprotective and immunomodulatory activities of 6,40-dihydroxy-7-methoxyflavanone in murine hippocampal
and microglia cells. Eur J Pharmacol. 2012;674:153–62. 51. Alcaraz MJ, Fernandez P, Guillen MI. Anti-inflammatory actions
of the heme oxygenase-1 pathway. Curr Pharm Des. 2003;9: 2541–51.
52. Vicente AM, Guillen MI, Habib A, Alcaraz MJ. Beneficial effects of heme oxygenase-1 up-regulation in the development of experimental inflammation induced by zymosan. J Pharmacol Exp Ther. 2003;307:1030–7.
53. Bednarz N, Zawacka-Pankau J, Kowalska A. Protoporphyrin IX induces apoptosis in HeLa cells prior to photodynamic treatment. Pharmacol Rep. 2007;59:474–9.
54. Grangeiro NMG, Aguiar JA, Chaves HV, Silva AAR, Lima V, Benevides NMB, Brito GAC, Grac¸a JRV, Bezerra MM. Heme oxygenase/carbon monoxide-biliverdin pathway may be involved in the antinociceptive activity of etoricoxib, a selective COX-2 inhibitor. Pharmacol Rep. 2011;63:112–9.
55. Vanderlei ESO, Araujo IWF, Quindere ALG, Fontes BP, Eloy YRG, Rodrigues JAG, Silva AAR, Chaves HV, Jorge RJB, Menezes DB, Evangelista JSAM, Bezerra MM, Benevides NMB. The involvement of the HO-1 pathway in the anti-inflammatory action of a sulfated polysaccharide isolated from the red seaweed
Gracilaria birdiae. Inflamm Res. 2011;60:1121–30.