Involvement of the GABAergic system in the anxiolytic
effect of sulfated polysaccharides from the red seaweed
Gracilaria cornea
Valdécio Silvano Monteiro1&Felipe Barros Teles1&Chistiane Oliveira Coura1&
Ricardo Basto Souza1&Camila Nayane de Carvalho Lima2&Deiziane Viana da Silva Costa2& Eduardo Ribeiro Honório Junior2&Sarah de Souza Escudeiro2&
Edna Maria Camelo Chaves2&Silvânia Maria Mendes Vasconcelos2& Norma Maria Barros Benevídes1
#Springer Science+Business Media Dordrecht 2015
Abstract Sulfated polysaccharides (SP) are found in various marine organisms and their biological activities have aroused great interest in the medical sciences. This work presents the behavioral effects of SP isolated from the red marine alga
Gracilaria cornea (total sulfated polysaccharides from
G. cornea(TSP-Gc)) in animal models, such as the elevated plus maze (EPM), hole board, open field, and rotarod. The TSP-Gc was administered intraperitoneally to male mice at
single doses of 0.1, 1, and 10 mg kg−1, while diazepam 1 or
2 mg kg−1 was used as a standard drug and flumazenil
2 mg kg−1 was used to evaluate the participation of
benzodiazepinic receptors. The results showed that, similar
to diazepam (1 mg kg−1), TSP-Gc 10 mg kg−1significantly
modified all the observed parameters in the EPM test, without altering the general motor activity in the open field and rotarod. Flumazenil reversed not only the diazepam effect
but also the TSP-Gc 10 mg kg−1effect. In the same way, the
dose of TSP-Gc 10 mg kg−1increased the number of head dips
in the hole-board test. An increased expression of
α2-gamma-aminobutyric acid type A (α2-GABAA) receptor in the hip-pocampus (HC) was observed in the group treatment with diazepam (DZP) or TSP-Gc. However, the pretreatment with flumazenil (flumazenil (FLU)+DZP; FLU+TSP-Gc 10) re-versed this effect. The results showed that sulfated
polysac-charides fromG. cornea(10 mg kg−1) presented an anxiolytic
effect, disproving sedative effects, through GABAAreceptor
α2 subunit.
Keywords Gracilaria cornea. Rhodophyta . Natural
product . Anxiolytic effect .α2-GABAAreceptor
Introduction
Neuroprotection is defined as a therapeutic intervention for the prevention or treatment of neurodegenerative disorders and
neu-robehavioral symptoms such as anxiety (Djaldetti et al.2003).
Anxiety disorders have affected many people throughout the world, and they are among the leading psychiatric disorders that
diminish the quality of life (Lakhan et al.2010). In the last
45 years, the pharmacological treatment of anxiety disorders has extensively used the benzodiazepine, a class of drugs that modulate allosterically the gamma-aminobutyric acid (GABA) type A ionotropic receptors (Olson et al.2002).
GABAAreceptors (GABAAR) are the primary mediators of
fast inhibitory neurotransmission in the CNS and modulate levels of anxiety, insomnia, and memory (Mohler et al.1995) in various brain areas, mainly the hippocampus (Rudolph and Möhler
2014). This ligand-gated ion channel is composed of several
subunits, where the anxiolytic activity is related byα2 subunit (Löw et al.2000; Morris et al.2006; Smith et al.2012).
*
Norma Maria Barros Benevídes nmbb@ufc.br
1
Laboratory of Carbohydrates and Lectins, Department of Biochemistry and Molecular Biology, Federal University of Ceará, Humberto Monte Avenue, s/n, Campus do Pici, CEP
60.451-970 Fortaleza, Ceará, Brazil
2 Laboratory of Neuropsychopharmacology, Department of
Physiology and Pharmacology, Federal University of Ceará, Cel. Nunes de Melo Street, 1127, Rodolfo Teófilo, CEP
60.430-270 Fortaleza, Ceará, Brazil DOI 10.1007/s10811-015-0724-0
Several agents known to mediate GABA neurotransmis-sion have shown great potential for use in the management of anxiety. The benzodiazepines, as a result of their selective mechanism of action targeting the GABAAR, have been
wide-ly used to manage anxiety disorders (Uhlenhuth et al.1999).
However, some adverse effects have been reported, including sedation, muscle relaxation, anterograde amnesia, and
physi-cal dependence (Zarrindast et al.2008; Barbosa et al.2008;
Rabbani et al.2008). Thus, there is a growing interest in the
development of new pharmacological agents based on biolog-ically active natural products as alternative therapeutic for the
management of this disorder (Teixeiras et al.2011).
In the search for new neuroprotective strategies, the high molecular weight molecules such as polysaccharides have been neglected due to their complex structures. However, studies have shown various types of sulfated polysaccharides of animal and vegetable origin with neuroprotective effects,
such as proliferation of neural cells (Lee et al.2007; Sheng
et al.2011; Zhang et al.2012), antineurotoxic (Luo et al.2009; Gao et al.2012), and antioxidant (Yang et al.2011; Pangestuti
and Kim2011) activity.
Sulfated polysaccharides are complex macromolecules that can interact with a wide variety of matrix and cell proteins due to their chemical structure which is rich in polyanions (Arfors
and Ley 1993). Red seaweeds have mainly galactants
(Fonseca et al. 2008), showing enantiomeric configuration
of theα-galactose which classifies the various galactans into
two major groups, the carrageenans and the agars (Stortz and
Cerezo2000).
Agar is a complex mixture of polysaccharides obtained from red algae known as agarophytes (Marino-Soriano and
Bourret 2003). The chemical structure of agar from
Gracilaria corneawas previously characterized with FTIR and NMR spectroscopic analysis. The structural components
of this polysaccharide are mainly 3,6-anhydro-α-L-galactose
(3,6 AG). The minor components such as 6-О
-methyl-galac-tose, glucose, xylose, and sulfated groups were detected as
previously described (Melo et al.2002).
Since the sulfated polysaccharides from the seaweed
G. corneashowed that there are antinociceptive effect and ac-tion on the central nervous system (Coura et al.2012), this led us to investigate the sulfated polysaccharide anxiolytic-like ac-tivity in animal models of anxiety and expression of GABAA α2 subunit receptors (α2-GABAAR) in the hippocampus (HC) of mice to check a possible mechanism of action.
Materials and methods
The specimens ofGracilaria cornea were collected along
Flecheiras Beach, at the city of Trairí, Ceará, Brazil, and they were taken to the Carbohydrates and Lectins Laboratory (CarboLec, Fortaleza, Ceará, Brazil), Department of
Biochemistry and Molecular Biology, Federal University of Ceará, and cleaned of epiphytes, washed with distilled water,
and stored at −20 °C until use. A voucher specimen (no.
34739) was deposited in the Herbarium Prisco Bezerra in the Department of Biological Sciences, Federal University of Ceará, Brazil.
Animals
Male Swiss mice (20–30 g) were used in each experiment, and
animals were maintained at a controlled temperature (25± 1 °C) with a 12-h dark/light cycle with free access to water and food. For the complete study, a total of 367 mice were used. Animals were treated in compliance with ethical stan-dards. The study was performed under the consent and sur-veillance of the Committee of Ethics in Animal Research un-der protocol number CEPA 45/13, Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Ceará, Brazil.
Drugs and doses
Total sulfated polysaccharides fromG. cornea(TSP-Gc) was
solubilized in 0.9 % sterile NaCl (saline). Animals were
treat-ed with the substance at doses of 0.1, 1, and 10 mg kg−1,
intraperitoneally (i.p.), 30 min before the experiments. Controls received vehicle (saline with 0.9 %) at the same
volume (10 mL kg−1) administered by the same route as the
treated groups.
Diazepam (DZP) 1 or 2 mg kg−1(União Química/Brazil),
used as standard, was injected i.p. after dissolution in dis-tilled water. It is well known that benzodiazepines act as anxiolytics (at low doses) and also produce sedation and
myorelaxant effect at higher doses (Novas et al. 1988).
Thereby, our group has used diazepam at 1 mg kg−1 in
elevated plus maze test and hole-board tests as standard drug
for anxiolytic effect, as well as 2 mg kg−1diazepam in open
field and rotarod tests as standard drug for sedative and myorelaxant effects, respectively. Flumazenil (FLU), a rec-ognized competitive antagonist of the central benzodiazepine receptor, was injected i.p. after dissolution in distilled water 15 min before the treatment with TSP-Gc to elucidate a
possible action mechanism which is GABAA–benzodiazepine
related.
Preparation of sulfated polysaccharides
Approximately 5 g of the dried algal tissue was submitted to papain digestion (6 h, 60 °C) in 100 mM sodium acetate buffer (pH 5.0) containing cysteine and EDTA (5 mM) for the ex-traction of total sulfated polysaccharides (TSP) as previously
to Coura et al. (2012). The biological protocols were per-formed with TSP, called TSP-Gc.
Pharmacological tests
Elevated plus maze testThe elevated plus maze (EPM) test for
mice (Lister1987) consisted of two perpendicular open arms
(30×5 cm) and two closed arms (30×5×25 cm) also in perpen-dicular position. The open and closed arms were connected by a central platform (5×5 cm). The platform and the lateral walls of the closed arms were made of transparent acrylic and the floor of black acrylic. The maze was 45 cm above the floor.
After treatment, the animal was placed at the center of the plus maze with its nose in the direction of one of the closed arms and observed for 5 min, according to the following pa-rameters: number of entries in the open and closed arms and time of permanence in each of them. The time of permanence measures the time spent by the animal in the open and closed
arms. Anxiolytic compounds reduce the animal’s aversion to
the open arms and promote the exploration thereof. The pa-rameters observed were percentages of entries into open arms (PEOA), number of entries in the open arms (NEOA), time of permanence in open arms (TPOA), and percentage of time of permanence in the open arms (PTOA).
For this test, the animals were divided into eight groups of
10–15 animals each. The different groups were treated with
saline (control), TSP-Gc (0.1, 1, and 10 mg kg−1), DZP
(1 mg kg−1), FLU (2 mg kg−1)+TSP-Gc (10 mg kg−1), FLU
(2 mg kg−1)+DZP (1 mg kg−1), and FLU (2 mg kg−1).
Open-field testThe open-field area was made of acrylic (trans-parent walls and black floor, 30×30×15 cm) divided into nine squares of equal area. The open-field area was used to evaluate the exploratory activity of the animal for 5 min of the 6-min
testing period (Archer1973). The observed parameter was as
follows: number of squares crossed (with the four paws). The
animals were divided into five groups of 10–15 animals each.
The different groups were treated with saline (control), TSP-Gc (0.1, 1, and 10 mg kg−1), and DZP (2 mg kg−1).
Hole-board testThe hole-board test for exploratory behavior in mice was used as described previously by Clark et al. (1971). The apparatus used was an Ugo Basile of 60×30 cm with 16 evenly spaced holes with built-in infrared sensors. In brief, adult male mice were randomly divided into five groups with eight mice per group: group treated with doses TSP-Gc (0.1, 1, and 10 mg kg−1),
positive control group treated with DZP (1 mg kg−1), and negative
control group (0.9 % saline). Thirty minutes after the i.p. admin-istration of DZP or TSP-Gc, the number of head dips into the holes was counted for each animal for 5 min.
RotarodAnimals were selected for the rotarod test before the pharmacological test. Mice, eight per group, were divided into
five groups and treated with saline (control), TSP-Gc (0.1, 1, and 10 mg kg−1), and DZP (2 mg kg−1). Thirty minutes after
i.p. administration of treatments, mice were placed with the four paws on a 2.5-cm-diameter bar, 25 cm above the floor, and the time of permanence on the bar was measured for 1 min, for each animal. The rotating speed was 12 rpm
(Dunham and Miya1957).
Western blotting analysis
The animals were sacrificed 1 h after the beginning of the experiments by decapitation. The skulls were removed, and
hippocampi were dissected and stored in a freezer at−80 °C
for posterior analysis of the expression levels of anxiolytic-like effects of relevant protein.
Hippocampi were homogenized in RIPA lysis buffer
(25 mM Tris–HCl, pH 7.6; 150 mM NaCl; 5 mM EDTA;
1 % NP40; 1 % Triton X-100; 1 % sodium deoxycholate;
0.1 % SDS) and protease inhibitor (1μL inhibitor: 100μL
RIPA). For protein extraction, HC samples were centrifuged (17 min, 4 °C, 13,000 rpm) and supernatant was collected. Protein concentrations were determined by the method of
Bradford according to the manufacturer’s protocol.
SDS-polyacrylamide gel electrophoresis (10 %) was performed
using 20 μg of protein (previously prepared with Laemmli
sample buffer and heated at 95 °C for 5 min). The proteins were transferred to PVDF membrane, blocked with BSA 5 %
for 1 h, and incubated overnight with mouse anti-GABAAIgG
primary antibody (1:200; Abcam, USA) or mouse anti-α-tubulin IgG primary antibody (1:4000; Sigma, USA). After washing, the blots were incubated with horseradish peroxi-dase conjugated goat anti-mouse IgG secondary antibody (1:500; Thermo Scientific, USA) for 90 min at room temper-ature. Signal was detected using the ECL system (Bio-RAD,
USA) according to the manufacturer’s instructions, and then
the bands were captured with a CCD camera using the ChemiDoc system (Bio-Rad, USA). Densitometric quantifi-cation of bands was done with NIH ImageJ software.
Statistical analysis
All results are presented as mean±S.E.M. Data were analyzed
by ANOVA followed by Student–Newman–Keuls’post hoc
test. Results were considered significant atp<0.05.
Results
Elevated plus maze test
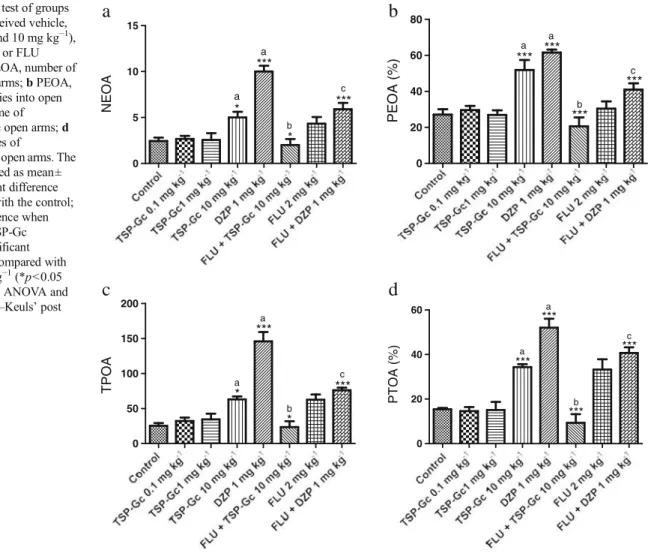
Figure1shows the effect of TSP-Gc in the EPM test in all doses. The results demonstrated that the i.p. treatment of 10 mg kg−1
analyzed, NEOA, PEOA, TPOA, and PTOA, when compared with the respective controls: NEOA [control, 2.44±0.37 (9);
TSP-Gc 10 mg kg−1, 5.00±0.61 (8); DZP 1 mg kg−1, 10.00±
0.63 (8)], PEOA [control, 27.13±2.97 (8); TSP-Gc 10 mg kg−1,
51.88±5.57 (8); DZP 1 mg kg−1, 61.59±1.62 (8)], TPOA
[con-trol, 25.43±13.85 (8); TSP-Gc 50 mg kg−1, 63.00±4.46 (8);
DZP 1 mg kg−1, 146.1±13.16 (8)], and PTOA [control, 15.41
±0.59 (8); TSP-Gc 10 mg kg−1, 34.31±1.36 (8); DZP 1 mg kg−1,
52.10±3.95 (8)].
The results showed that the 10 mg kg−1TSP-Gc group
pretreated with flumazenil (2 mg kg−1) decreased all
parame-ters analyzed when compared with the TSP-Gc 10 mg kg−1
group: NEOA [TSP-Gc 10 mg kg−1, 5.00±0.61 (8); FLU
2 mg kg−1+ TSP-Gc 10 mg kg−1, 2.00 ± 0.65 (8)], PEOA
[TSP-Gc 10 mg kg−1, 51.88 ±5.57 (8); FLU 2 mg kg−1+
TSP-Gc 10 mg kg−1, 20.60 ± 4.93 (8)], TPOA [TSP-Gc
50 mg kg−1, 63.00 ± 4.46 (8); FLU 2 mg kg−1+ TSP-Gc
10 mg kg−1, 23.50 ± 8.44 (8)], and PTOA [TSP-Gc
10 mg kg−1, 34.31 ± 1.36 (8); FLU 2 mg kg−1+ TSP-Gc
10 mg kg−1, 9.32±3.83 (8)].
The diazepam group pretreated with flumazenil also de-creased all parameters analyzed when compared with the
diazepam group: NEOA [DZP 1 mg kg−1, 10.00±0.63 (8);
FLU 2 mg kg−1+DZP 1 mg kg−1, 5.90±0.69 (8)], PEOA
[DZP 1 mg kg−1, 61.59±1.62 (8); FLU 2 mg kg−1+DZP
1 mg kg−1, 41.01±3.47 (8)], TPOA [DZP 1 mg kg−1, 146.1±
13.16 (8); FLU 2 mg kg−1+DZP 1 mg kg−1, 76.22±3.61 (8)],
and PTOA [DZP 1 mg kg−1, 52.10±3.95 (8); FLU 2 mg kg−1+
DZP 1 mg kg−1, 40.73±2.47 (8)].
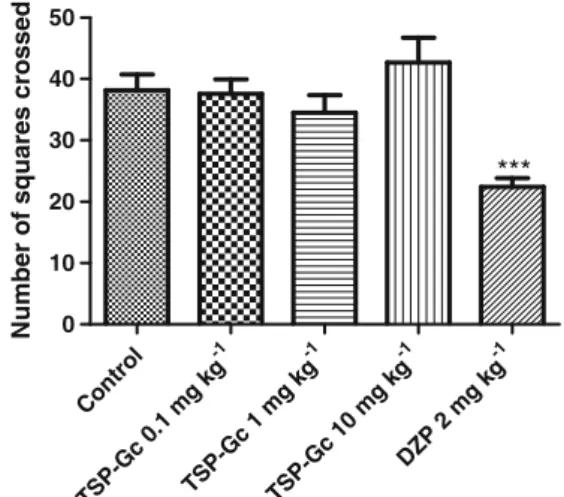
Open-field test
Figure2shows that TSP-Gc (0.1, 1, and 10 mg kg−1) did not
alter the number of crossings as compared to the control
group. The animals treated with diazepam (2 mg kg−1) had a
decreased number of crossings [control, 36.75±2.65 (8); DZP 2 mg kg−1, 22.38±1.45 (12)].
Hole-board test
Similar to diazepam 1 mg kg−1, TSP-Gc 10 mg kg−1(Fig.3)
increased significantly the number of head dips [control,
26.25 ± 2.12 (8); TSP-Gc 10 mg kg−1, 43.29 ± 2.84; DZP
1 mg kg−1, 41.13±1.93 (8)], as compared to the control.
0 5 10 15
* ***
*
*** a
a
b
c
0 50 100 150 200
* ***
*
***
a a
b
c
a
c
NEOA
TPOA
0 20 40 60
*** ***
***
***
a a
b
c
0 20 40 60 80
*** ***
***
***
a a
b
c
b
d
PTOA (%)
PEOA (%)
Fig. 1 Plus maze test of groups
of mice which received vehicle, TSP-Gc (0.1, 1, and 10 mg kg−1), DZP (1 mg kg−1), or FLU (2 mg kg−1).aNEOA, number of entries into open arms;bPEOA, percentage of entries into open arms;cTPOA, time of permanence in the open arms;d PTOA, percentages of
permanence in the open arms. The results are presented as mean± S.E.M.aSignificant difference when compared with the control; b
Rotarod test
The absolute values of the number of falls and the time of
permanence are presented in Fig.4. No alteration was
ob-served in both parameters after treatment with TSP-Gc (0.1,
1, and 10 mg kg−1) compared with the control, while
diaze-pam (2 mg kg−1) in a muscle relaxant dose, as an example,
increased the number of falls [control, 0.37±0.26 (8); DZP
2 mg kg−1, 2.87±0.22 (8)] and decreased the time of
perma-nence on the bar [control, 58.86±0.85 (8); DZP 2 mg kg−1,
40.88±3.57 (8)].
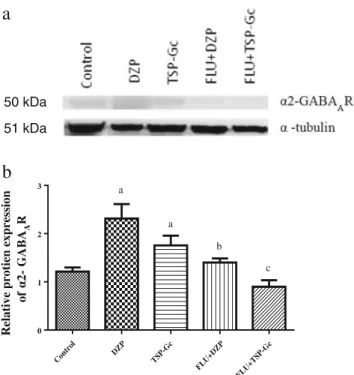
Expression ofα2-GABAAreceptors
An increased expression ofα2-GABAAR (Fig.5) was
ob-served in the groups treated with DZP (90.9 %) or TSP-Gc (44.6 %) as compared to control. However, the pretreatment with flumazenil (FLU+DZP; FLU+TSP-Gc10) reversed this increase induced by DZP and TSP-Gc.
Discussion
In the present work, the behavior effects of TSP-Gc were studied in several classic animal models, such as EPM, hole-board, open-field, and rotarod tests, in order to investigate the possible anxiolytic-like activity of sulfated polysaccharides
found in the red seaweedG. cornea. The effect of TSP-Gc
was comparable to that of diazepam, a benzodiazepine anxi-olytic. It is known that benzodiazepines act as anxiolytics (in low doses) and anticonvulsants and also produce sedation and
myorelaxant effects at higher doses (Melo et al. 2006).
Therefore, diazepam 1 mg kg−1 was used in the EPM and
hole-board test and 2 mg kg−1in open-field and rotarod tests,
as standard drug.
The EPM test is considered one of the most widely vali-dated tests for assaying new benzodiazepine anxiolytic-like agents (Pellow et al.1985; Mizushige et al.2013); it is based on the natural aversion of rodents for open spaces (Almeida et al.2012). The larger the exploratory capacity of the animals and the longer it remains in the open arms, the lower is their
level of anxiety (Casarrubea et al.2013). Benzodiazepines are
the most widely prescribed central nervous system depres-sants, with selective activity at the inhibitory GABAAR com-plex. By enhancing the frequency of the chloride channel opening and thus the chloride flux through GABAAR, benzo-diazepines potentiate the inhibitory effect of GABA (Lilly and Tietz2000; Ishola et al.2012).
Our results showed that TSP-Gc at a dose of 10 mg kg−1was
able to increase significantly all the parameters (PEOA, NEOA, PTOA, and TPOA) in the EPM test, as compared to the control group. Similar results were also observed with the
diazepam-treated group at a recognized anxiolytic dose (1 mg kg−1)
(Gomes et al.2008) suggesting an anxiolytic effect of the
sul-fated polysaccharides from the red seaweed G. cornea.
Flumazenil is a recognized competitive antagonist at the central benzodiazepine receptor and was used to elucidate the possible mechanism by which the TSP-Gc is actuating in this model. The results showed that flumazenil reversed not only the diaz-epam effect but also the TSP-Gc (10 mg kg−1) effect, indicating
that both drugs might present a similar mechanism of action. In order to corroborate the anxiolytic activity observed in the EPM test, we used the hole-board test, in which it is also ob-served that the exploration is gradually inhibited by anxiety
(Crawley1985). In this way, similar to EPM, this test is also
Control
TSP-Gc 10 mg kg
-1
TSP-Gc 1 mg kg
-1
TSP-Gc 0.1 mg kg
-1
DZP 2 mg kg
-1
0 10 20 30 40 50
***
Number of squares crossed
Fig. 2 Open-field test of groups of mice which received vehicle, TSP-Gc
(0.1, 1, and 10 mg kg−1), and diazepam (DZP 2 mg kg−1). The parameter analyzed was number of squares crossed. The results are presented as mean ± S.E.M. Significant difference compared with control (***p<0.01). ANOVA and Student–Newman–Keuls’post hoc test
Control
TSP-Gc 0.1 mg kg
-1
DZP 1 mg kg
-1
TSP-Gc 10 mg kg
-1
TSP-Gc 1 mg kg
-1
0 10 20 30 40 50
** **
Number of head dips
Fig. 3 Hole-board test of groups of mice which received vehicle,
useful for modeling anxiety, and anxiolytic agents have been shown to increase the number of head dips (Takeda et al.1998).
Our results showed that TSP-Gc (10 mg kg−1) significantly
increased the number of head dips, indicating anxiolytic effect. Drugs that increase general motor activity may provide false-positive/negative results in the number of entries into
the open arms and number of head dips in the EPM and hole-board tests, respectively. Therefore, it was decided to use the open-field test, a classical animal model used to eval-uate autonomic effects of drugs and general activity of animals
(Novas et al.1988). Our findings showed that the animals
treated with TSP-Gc (10 mg kg−1) dose, which produced
anxiolytic-like effects, did not induce changes in locomotion of mice in the open-field arena, whereas diazepam (2 mg kg−1)
decreased this parameter, showing a sedative effect. Therefore, it is unlikely that the effects produced by TSP-Gc observed in the plus maze and hole-board tests are based on the stimulation of general motor activity.
A deficit in motor coordination very likely would affect the performance in the EPM, hole-board test, and open-field tests
(Venâncio et al.2011). Therefore, we aimed to investigate the
effects of TSP-Gc in the rotarod test, a classic animal model used to evaluate peripheral neuromuscular blockage. The
find-ings showed that TSP-Gc (0.1, 1, and 10 mg kg−1), different
from diazepam (2 mg kg−1), had no significant effect on the
motor coordination of the animals on rotarod test, suggesting that the anxiolytic-like effect might not be involved in periph-eral neuromuscular blockage, but rather by neurons that act on
the central nervous system (Amos et al.2001).
To confirm the mechanism of action of anxiolytic effect observed in the elevated plus maze test, and the relation to the GABAergic pathway, we decided to study changes of
GABAAprotein expression in the presence of flumazenil by
Western blotting. As expected, FLU reversed the increase in α2-GABAAR expression by the DZP. Similar action was
ob-served in the FLU+TSP-Gc group (Fig.5a, b). According
Low et al. (2000), the α2 subunit mediates the anxiolytic
effect of benzodiazepines. This result strengthens that
anxio-lytic action induced by the TSP-Gc is modulated by
α2-GABAAR which is responsible for the anxiolytic effect on
the GABAAreceptor.
In conclusion, our results suggest that acute treatment with
sulfated polysaccharides from the red seaweedG. corneaat
a b
0 1 2 3 4
***
Number of falls
Control
TSP-Gc 10 mg kg
-1
TSP-Gc 1 mg kg
-1
TSP-Gc 0.1 mg kg
-1
DZP 2 mg kg
-1
Control
TSP-Gc 10 mg kg
-1
TSP-Gc 1 mg kg
-1
TSP-Gc 0.1 mg kg
-1
DZP 2 mg kg
-1
0 20 40 60 80
***
Time of permanence on the bar
Fig. 4 Rotarod test of groups of
mice which received vehicle, TSP-Gc (0.1, 1, and 10 mg kg−1), DZP (2 mg kg−1). The parameters analyzed wereanumber of falls andbtime on the bar. The results are presented as mean±S.E.M. Significant difference compared with control (***p<0.001). ANOVA and Student–Newman– Keuls’post hoc test
50 kDa
51 kDa
Control DZP TSP-Gc
FLU+DZP FLU+TSP-Gc
0 1 2
3 a
a
b
c
Relative protien expression
of 2
-GABA
A
R
b
a
Fig. 5 Western blotting analysis ofα2-GABAAR in the HC of mice.a
Representative Western blotting showing tissue expression of GABAAR
the dose of 10 mg kg−1 presented anxiolytic effects,
disproving sedative effects, through GABAA receptor α2
subunit.
Acknowledgments The authors are thankful to the FUNCAP, CNPq,
and CAPES for the financial support.
References
Almeida AAC, Costa JP, Carvalho RBF, Sousa DP, Freitas RM (2012) Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res 1448:56–62 Amos S, Kolawole E, Akah P, Wambebe C, Gamaniel K (2001)
Behavioral effects of the aqueous extract ofGuiera senegalensisin mice and rats. Phytomedicine 8:356–361
Archer J (1973) Tests for emotionality in rats and mice: a review. Anim Behav 21:205–235
Arfors KE, Ley K (1993) Sulfated polysaccharides in inflammation. J Lab Clin Med 121:201–202
Barbosa PR, Valvassori SS, Bordignon CLJ, Kappel VD, Martins MR, Gavioli EC, Quevedo J, Reginatto FH (2008) The aqueous extracts ofPassiflora alataandPassiflora edulisreduce anxiety-related be-haviors without affecting memory process in rats. J Med Food 11: 282–288
Casarrubea M, Roy V, Sorbera F, Magnusson M, Santangelo A, Arabo A, Crescimanno G (2013) Temporal structure of the rat’s behavior in elevated plus maze test. Behav Brain Res 237:290–299
Clark G, Koster AG, Person DW (1971) Exploratory behavior in chronic disulfoton poisoning in mice. Psychopharmacology 20:169–171 Coura CO, Araújo IWF, Vanderlei ESO, Rodrigues JAG, Quinderé ALG,
Fontes BP, Queiroz INL, Menezes DB, Bezerra MM, Silva AAR, Chaves HV, Jorge RJB, Evangelista JSAM, Benevides NMB (2012) Antinociceptive and anti-inflammatory activities of sulphated poly-saccharides from the red seaweedGracilaria cornea. Basic Clin Pharmacol Toxicol 110:335–341
Crawley JN (1985) Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev 9:37–44
Djaldetti R, Lev N, Melamed E (2003) Neuroprotection in progressive brain disorders. Isr Med Assoc J 5:576–580
Dunham NW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficits in rats and mice. J Am Pharm Assoc 46:208– 209
Farias WRL, Valente AP, Pereira MS, Mourão PAS (2000) Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red algaBotryocladia occidentalisand comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J Biologic Chem 75:29299–29307
Fonseca RJC, Oliveira SNMCG, Melo FR, Pereira MG, Benevides NMB, Mourão PAS (2008) Slight differences in sulfation of algal galactans account for differences in their anticoagulant and venous antithrombotic activities. Thromb Haemost 99:539–545
Gao Y, Li C, Yin J, Shen J, Wang H, Wu Y, Jin H (2012) Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive im-pairment induced by infusion of AB peptide in rats. Environ Toxicol Pharmacol 33:304–311
Gomes PB, Noronha EC, Melo CTV, Bezerra JN, Neto MA, Lino CS, Vasconcelos SMM, Viana GS, Sousa FC (2008) Central effects of isolated fractions from the root ofPetiveria alliaceaL. (tipi) in mice. J Ethnopharmacol 120:209–214
Ishola IO, Chatterjee M, Tota S, Tadigopulla N, Adeyemi OO, Palit G, Shukla R (2012) Antidepressant and anxiolytic effects of
amentoflavone isolated from Cnestis ferruginea in mice. Pharmacol Biochem Behav 103:322–331
Lakhan SE, Vieira KF (2010) Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. J Nutr 9: 42–58
Le H, Do H, Lee S, Sohn E, Pyo S, Son E (2007) Effects of fucoidan on neuronal cell proliferation: association with NO production though the iNOS pathway. J Food Sci Nutr 12:74–78
Lilly SM, Tietz EI (2000) Chronic cocaine differentially affects diaze-pam’s anxiolytic and anticonvulsant actions. relationship to GABAA receptor subunit expression. Brain Res 882:139–148
Lister RG (1987) The use of a plus-maze to measure anxiety in the mouse. Psycopharmacology 92:180–185
Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U (2000) Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290:131–134
Luo D, Zhang Q, Wang H, Cui Y, Sun Z, Yang J, Zheng Y, Jia J, Yu F, Wang X (2009) Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur J Pharmacol 617:33–40
Marino-Soriano E, Bourret E (2003) Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae, Rhodophyta). Bioresour Technol 90:329–333
Melo MRS, Feitosa JPA, Freitas ALP, Paula RCM (2002) Isolation and characterization of soluble of sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr Polym 49:491–498 Melo CTV, Monteiro AP, Leite CP, Araújo FLO, Lima VT, Barbosa-Filho
JM (2006) Anxiolytic-like effects of (O-methyl)-N-2,6 dihydroxybenzoyl-tyramine (riparin III) fromAniba riparia(Nees) Mez (Lauraceae) in mice. Biol Pharm Bull 29:451–454
Mizushige T, Kanegawa N, Yamada A, Ota A, Kanamoto R, Ohinata K (2013) Aromatic amino acid-leucine dipeptides exhibit anxiolytic-like activity in young mice. Neurosci Lett 543:26–129
Mohler H, Benke D, Benson J, Luscher B, Fritschy JM (1995) GABAA-receptor subtypes in vivo: cellular localization, pharmacology and regulation. Adv Biochem Psychopharmacol 48:41–56
Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN (2006) Bothα2 andα3 GABAAreceptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emo-tional response paradigm. Eur J Neurosci 23:2495–2504
Novas ML, Wolfman C, Medina JH, De Robertis E (1988) Proconvulsant and anxiogenic effects of n-butyl-β-carboline-3-carboxylate on en-dogenous benzodiazepine binding inhibitor from brain. Pharmacol Biochem Behav 30:331–336
Olson R, Davis K, Charney D, Coyle J, Nemeroff C (eds) (2002) Neuropsychopharmacology: the fifth generation of progress. Lippincott, Williams & Wilkins, Philadelphia
Pangestuti R, Kim S (2011) Neuroprotective effects of marine algae. Mar Drugs 9:803–818
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Rabbani M, Sajjadi SE, Mohammadi A (2008) Evaluation of the anxio-lytic effect of Nepeta persica Boiss in mice. Evid Based Complement Alternat Med 5:181–186
Rudolph U, Möhler H (2014) GABAAreceptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol 54:483–507
Sheng X, Zhang N, Song S, Li M, Liang H, Zhang Y, Wang Y, Ji A (2011) Morphological transformation and proliferation of rat astrocytes as induced by sulfated polysaccharides from the sea cucumber Stichopus japonicus. Neurosci Lett 503:37–42
Stortz CA, Cerezo AS (2000) Novel findings in carrageenans, agaroids and“hybrid”red seaweed galactans. Curr Topics Phytochem 4:121– 134
Takeda H, Tsuji M, Matsumiya T (1998) Changes in head-dipping be-havior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 350:21–29
Teixeira CP, Melo CT, Araújo FL, Carvalho AM, Silva MI, Barbosa-Fi lho JM, Macedo DS, Viana GSB, Sousa FC (2011) Antidepressant-like effect of riparin II fromAniba ripariain mice: evidence for the involvement of the monoaminergic system. Fundam Clin Pharmacol 27:129–137
Uhlenhuth EH, Balter MB, Ban TA, Yang K (1999) International study of expert judgment on therapeutic use of benzodiazepines and other psychotherapeutic medications: VI. Trends in recommendations for the pharmacotherapy of anxiety disorders, 1992–1997. Dep Anxiet 9:107–116
Venâncio ET, Rocha NFM, Rios ERV, Feitosa ML, Linhares MI, Melo FHC, Matias MS, Fonseca FN, Sousa FCF, Leal LKAM, Fonteles MMF (2011) Anxiolytic-like effects of standardized extract of J u s t i c i a p e c t o r a l i s ( S E J P ) i n m i c e : i n v o l v e m e n t o f GABA/Benzodiazepine in receptor. Phytother Res 25:444–450 Yang Y, Liu D, Chen Y, Wang S (2011) In vitro antioxidant activities of
sulfated polysaccharide fractions extracted fromCorallina officinalis. Inter J Biol Macromolec 49:1031–1037
Zarrindast MR, Babapoor-Farrokhran S, Rezayof A (2008) Involvement of opioidergic system of the ventral hippocampus, the nucleus ac-cumbens or the central amygdala in anxiety-related behavior. Life Sci 82:1175–1181