www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
Translation
and
cross-cultural
adaptation
into
Brazilian
Portuguese
of
the
Vanderbilt
Head
and
Neck
Symptom
Survey
version
2.0
(VHNSS
2.0)
for
the
assessment
of
oral
symptoms
in
head
and
neck
cancer
patients
submitted
to
radiotherapy
夽
Eliane
Marc
¸on
Barroso
a,∗,
André
Lopes
Carvalho
a,b,c,
Carlos
Eduardo
Paiva
a,c,d,
João
Soares
Nunes
d,
Bianca
Sakamoto
Ribeiro
Paiva
a,caPostgraduatePrograminOncology,HospitaldeCâncerdeBarretos,Barretos,SP,Brazil bHeadandNeckDepartment,HospitaldeCâncerdeBarretos,Barretos,SP,Brazil
cGrupodePesquisaemQualidadedeVidaRelacionadaàSaúde(GPQual)---CNPq,HospitaldeCâncerdeBarretos,Barretos,SP,
Brazil
dDepartmentofClinicalOncology,BreastandGynecologyDivision,HospitaldeCâncerdeBarretos,Barretos,SP,Brazil
Received5May2014;accepted1November2014 Availableonline8September2015
KEYWORDS Headandneck neoplasms; Qualityoflife; Translating; Oralhealth
Abstract
Introduction:Patientssubmittedtoradiotherapyforthetreatmentofheadandneckcancer haveseveralsymptoms,predominantlyoral.TheVanderbilt HeadandNeckSymptom Survey version2.0isanAmericantooldevelopedtoevaluateoralsymptomsinheadandneckcancer patientssubmittedtoradiotherapy.
Objective:TheaimofthepresentstudywastotranslatetheVanderbiltHeadandNeckSymptom Surveyversion2.0intoBrazilianPortugueseandcross-culturallyadaptthistoolforsubsequent validationandapplicationinBrazil.
Methods:Amethodusedforthetranslationandculturaladaptationoftools,whichincluded independenttranslations,synthesisofthetranslations,back-translations,expertcommittee, andpre-test,wasused.Thepre-testwasperformedwith37headandneckcancerpatients, whoweredividedintofourgroups,toassesstherelevanceandunderstandingoftheassessed items.Dataweresubmittedtodescriptivestatisticalanalysis.
夽 Pleasecitethisarticleas:BarrosoEM,CarvalhoAL,PaivaCE,NunesJS,PaivaBSR.Translationandcross-culturaladaptationintoBrazilian PortugueseoftheVanderbiltHeadandNeckSymptomSurveyversion2.0(VHNSS2.0)fortheassessmentoforalsymptomsinheadandneck cancerpatientssubmittedtoradiotherapy.BrazJOtorhinolaryngol.2015;81:622---9.
∗Correspondingauthor.
E-mail:embarroso@uol.com.br(E.M.Barroso).
http://dx.doi.org/10.1016/j.bjorl.2015.08.014
Results:Theoverallmeanofthecontentvalidityindexwas0.79forsemanticandidiomatic equivalence,anditwashigherthan0.8forculturalandconceptualequivalence.The cogni-tiveinterviewshowedthatpatientswereabletoparaphrasetheitems,andconsideredthem relevantandeasilyunderstood.
Conclusion: Thetool was translated andcross-culturally adaptedto beused in Brazil.The authorsbelievethistranslationissuitedforvalidation.
© 2015Associac¸ãoBrasileira de Otorrinolaringologiae CirurgiaCérvico-Facial. Publishedby ElsevierEditoraLtda.Allrightsreserved.
PALAVRAS-CHAVE Neoplasiasdecabec¸a epescoc¸o;
Qualidadedevida; Traduc¸ão;
Saúdebucal
Traduc¸ãoeadaptac¸ãoculturalparaoportuguês(Brasil)doinstrumentoVanderbilt HeadandNeckSymptomSurveyversion2.0(VHNSS2.0)paraavaliac¸ãodesintomas oraisempacientescomcâncerdecabec¸aepescoc¸osubmetidosàradioterapia
Resumo
Introduc¸ão: Pacientes submetidos à radioterapia para tratamento de câncer de cabec¸a e pescoc¸o apresentam diversossintomas,compredominância desintomas orais.OVanderbilt HeadandNeckSymptomSurveyversion2.0éuminstrumentoamericanoquefoidesenvolvido paraavaliarsintomasoraisempacientescomcâncerdecabec¸aepescoc¸osubmetidosà radioter-apia.
Objetivo: TraduziroVanderbiltHeadandNeckSymptomSurveyversion2.0eadaptá-lo cul-turalmenteparasubsequentevalidac¸ãoeaplicac¸ãonoBrasil.
Método: Ummétododetraduc¸ãoeadaptac¸ãocultural deinstrumentosfoiutilizado,oqual incluitraduc¸õesindependentes,síntesedastraduc¸ões,retrotraduc¸ões,comitêdeespecialistas epré-teste.Opré-testefoirealizadoem37pacientescomcâncerdecabec¸aepescoc¸odivididos emquatrogruposparaavaliararelevânciaeentendimentodositens.Dadosforamsubmetidos àanáliseestatísticadescritiva.
Resultados: A média geral do índice de validade de conteúdo foi 0,79 para as equivalên-ciassemânticas eidiomáticas;emaiorque0,8 paraasequivalências culturaleconceitual. A entrevistacognitivamostrou queospacientesforamcapazesdeparafrasear ositenseos consideravamrelevanteedefácilentendimento.
Conclusão:OinstrumentofoitraduzidoeadaptadoculturalmenteaoBrasil.Nósacreditamos queestatraduc¸ãoestáaptaparaavalidac¸ão.
©2015Associac¸ãoBrasileiradeOtorrinolaringologiaeCirurgiaCérvico-Facial.Publicado por ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Head and neck cancer (HNC) includes tumors that affect thelips,oralcavity,oropharynx,nasopharynx,hypopharynx, larynx, nasal cavity and paranasal sinuses, thyroid gland, and salivary glands.1 These cancers account for
approxi-mately 10% of malignant tumors.2 In Brazil, for the years
2012/2013,approximately20,000caseswereestimatedonly
fortumorsintheoralcavityandlarynx.3
Therapeutic options for HNC, including radiotherapy,
contribute to significant adverse symptoms and loss of
function.4 Such symptoms may occur immediately, soon
afterthetreatment, ormayappearlater.5 Theoral
alter-ations are prominent, and include mucositis, dysphagia,
tasteandmucosalsensitivityalterations,xerostomia,teeth
alterations, and excess mucus.5---9 With the exception of
xerostomia, mucositis, and dysphagia, these alterations
havebeenlittlediscussedintheliteratureandarebelieved
tobeunderreported.9Halfof survivingHNCpatientshave
problems and complications five years after the primary
treatment, which include pain, problems with teeth,
problems with chewing and swallowing,10 or high scores
for symptomssuch asxerostomia, mucusproduction, and
swallowingalterationsrelatedtotreatment.11
The xerostomia resulting from HNC treatment causes
damage to oral health12 and has a negative impact on
thequalityof life(QoL)of thesepatients.6 In additionto
hyposalivation,whichcontributestotheonsetofmucositis,
some patients may have an excessiveamount of mucus11
thatobstructs theairway,resultinginalterationsin
sleep-ing,coughing, andchoking.9 Anothercommonsymptomin
patientstreatedforHNCisthealterationoftaste,8adirect
result of the effect of radiation on the taste buds, and
alterationsinsaliva.13 HNCtreatmentalsocontributestoa
worseningof dentalhealth.14 Patientssubmittedto
radio-therapyhad worsedentalstatus whencomparedtothose
submittedtochemotherapy.15IntheBrazilianpopulation,a
longitudinalstudyshowedthatinpatientswithoralcancer,
the most common problems were related to difficulties
in chewing, swallowing, pain, and reduced salivary flow,
suggestingtheimportanceofdentalmonitoringofpatients
Some of the toxicities associated with treatment for
patients with HNC can be minimized, but are inevitable,
highlighting the importance of identifying andcontrolling
adverseeffectsrelatedtotreatmentbythehealthteam.8
Thetoolstoidentifyandevaluatethesealterationsresulting
fromthetreatmentcanserveasdiagnostictools,helpingto
establishthemostappropriateconductforthecareplanof
thesepatients.
Symptom assessment is often considered within the
physicalandfunctionaldomainsofQoLevaluation
question-naires,sothereissomedifficultyindifferentiatingsymptom
researchtoolsfromQoLassessment.17InpatientswithHNC,
themost usedand validatedtools in Brazilto assessQOL
aretheUniversityofWashingtonQOL(UW-QOL),18the
FACIT-HN,19andtheEORTC-HN35,20whereasforsymptomresearch
inHNCitistheMDASI-HN.21Overall,thetoolsusedforQOL
assessment comprise problems such as dysphagia,
xeros-tomia, and mucositis. However, they fail to report oral
symptomssuchasmucosalsensitivity,excessmucus,dental
problems,andtheirfunctionalimplications.TheVanderbilt
HeadandNeckSymptomSurvey(VHNSS)2.09,22,23isan
Amer-icansymptomassessment toolspecificallyusedinpatients
withHNCwhosetreatmentincludesradiotherapythatmore
broadly assesses oral health components, with a specific
domain for dental health and its functional implications.
Itcontains50itemsdistributedinto13domains:nutrition,
swallowing/foodintake,xerostomia,mucositis,pain,excess
mucus, speech/communication, hearing, taste and smell
alterations, dental health, mucosal sensitivity, and range
of motion. The answer options are graded on a scale of
0---10,sothatzero(0)signifiesnoproblemandten(10)is
themaximumpresenceofaspecificproblem.Thereliability
measuredbyCronbach’s˛issuitable,rangingfrom0.70to
0.94.22 Therefore,theVHNSS2.0wasselectedfor
transla-tionandcross-culturaladaptationintoBrazilianPortuguese.
Methods
Thiswasadescriptive,cross-sectionalstudy,usingamethod
oftranslation and cross-culturaladaptation of the
assess-menttool,performedinthefollowingfivesteps:
Translationand cross-cultural adaptation process: The
process of translation and cross-cultural adaptation of
the VHNSS 2.0 into Brazilian Portuguese was performed
according tothe internationalguidelines.24,25 Consentand
authorization in writing wasobtained via email from the
authoroftheoriginaltool,Dr.BarbaraA.Murphy,andthe
studywasapprovedbytheResearchEthicsCommittee
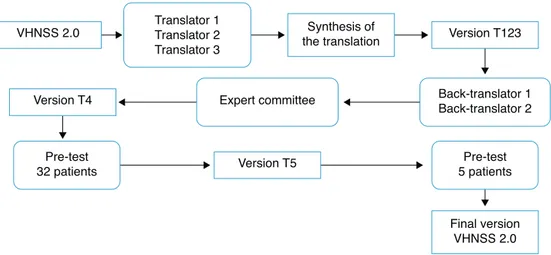
(pro-tocol No. 644/2012). Fig. 1 summarizes the steps of the
translationandcross-culturaladaptationprocess.
Translation:CarriedoutbythreenativeBrazilian
individ-ualsfluentinEnglish,ofwhomtwowerefromthehealthcare
area(physicians),whereasthethirdtranslatorwasnot.
Synthesisoftranslations:Thethreetranslatedversions
weresynthesizedintoasingleversion(T123),inaconsensus
meeting attendedby threeofthe authors (EMB,CEP, and
BSRP).
Back translation: Performed by American Journal
Experts, a company specialized in translations, which
received and complied with the guidelines that the two
back-translatorsshouldbe nativeAmericanEnglish
speak-ersandshouldbeunawareoftheoriginalversion.Theback
translationswereevaluatedandforwardedtotheauthorof
theoriginaltool.
ExpertCommittee: The versions were evaluatedbyan
expert committee in thefieldof health assessment tools,
consisting ofa head-and-neckoncologysurgeon,two
clin-ical oncologists, one nurse, one dentist, and a university
professor of linguistics. The aim of the committee was
to assess the translated version and compare it to the
original one, regarding semantic, idiomatic, cultural, and
conceptual equivalence, scoring ‘‘1’’(one) for the
equiv-alent items,‘‘0’’ (zero)for the items theydidnot know,
and‘‘−1’’(minusone)forthenon-equivalentitems.They
alsohadautonomytosuggestculturalchangestheydeemed
important.Theequivalencecalculationwasmadeusingthe
mean of theitems of the contentvalidity index(CVI)for
eachoneofthem,consideredequivalentwhen>0.8.26
Addi-tionally,thesuggestionsmadebythecommitteeweretaken
intoconsideration,discussedatameetingattendedbyfour
oftheauthors(EMB,CEP,BSRP,JSN),whichyieldedanew
version(T4).
Translator 1 Translator 2 Translator 3
VHNSS 2.0 Synthesis of
the translation Version T123
Back-translator 1 Back-translator 2
Pre-test 5 patients Pre-test
32 patients
Version T4 Expert committee
Version T5
Final version VHNSS 2.0
Table1 ModificationssuggestedforVHNSS2.0itemsbytheexpertcommitteeandtranslators.
Item Originalversion Adaptedversion Justification
Title Survey(pesquisa) Questionnaire(questionário) Moreadequatewordforthe Brazilianculture
1stInstruction AppropriateBox(caixa apropriada)
Chosenoption(opc¸ãoescolhida) Clearerandmoreinformal
1 Icurrentlyhaveafeedingtube inplace
(Euatualmentetenhoumtubo dealimentac¸ãocolocado)
Areyouusingatubetobefed?
(Vocêestáusandoumasonda parasealimentar?)
Clearerandmoreinformal
2ndInstruction ...Ingeneral,a‘‘0’’indicates theleastamountofproblems withaparticularsymptomand ‘‘10’’indicatesthemost problems.(Emgeral‘‘0’’indica amenorquantidadedeproblema comsintomaparticulare‘‘10’’ indicaomaiordosproblemas)
Ingeneral‘‘0’’indicatesthe absenceofasymptom(problem) and‘‘10’’indicatesthe
maximumpresenceofsymptom (problem)(Emgeral,‘‘0’’indica aausênciadeumsintoma (problema)e‘‘10’’indicaa presenc¸amáximadeumsintoma (problema)
Thesubstitutionoftheterm ‘‘amount’’by‘‘presence’’ makesthesentencemost objective
3 ‘‘likeEnsureTMorBoostTM’’ (comoEnsureTM orBoostTM’’)e LiquidSupplement
(suplementolíquido)
‘‘likeEnsureTM,NutrenTM, SustagemTM’’(comoEnsureTM, NutrenTM,SustagemTM) Dietaryliquidsupplement
(suplementoalimentarliquido)
ProductsavailableinBrazil. Dietaryliquidsupplementwas added,asitisknowninBrazil
5 Trouble(problema) Difficulty(dificuldade) Adaptationtoitem4 6 Trouble(problema) Difficulty(dificuldade).
Removalofthewordthin(ralos) andEnsure,substitutedbyjuice (sucos)
Removalofthewordthin,which isnotcommonlyusedinBrazil andsubstitutionofEnsureby juice,atypicalBraziliandrink 15 Swallowing(deglutic¸ão) Swallow(engolir) Moreinformaltranslationof
deglutition 16 Abilitytosleep(capacidadede
dormir)
Sleep(sono) Makestheitemmoreobjective
17 Abilitytotalk
(capacidadedefalar)
Talk(falar) Makestheitemmoreobjective
18,19,20,21 Mucous(muco) Secretion(secrec¸ão) Moreinformalword 27 Average(média) Remoc¸ãodapalavraaverage
(média)
Makestheitemmoreobjective
32 Hearing(audic¸ão) Hear(ouvir) Moreinformalword,culturally
appropriate 34 Ihavelessdesiretoeat(Eu
tenhomenosdesejodecomer)
Youfeellesslikeeating(Você sentemenosvontadedecomer)
Moreinformalword,culturally appropriate
37,38 Anexplanatoryparenthesison
abilitytosmell(capacidadede sentircheiro)wasadded
Facilitatesunderstanding
39,40,41,42 Nonapplicable(nãoaplicável) Cannotbeapplied(nãose aplica).
Facilitatesunderstanding
44,45,46,47,48 Remoc¸ãodapalavralining
(mucosa)
Technicalwordofdifficult understanding,culturally appropriate
48 Theexpression‘‘Cannotbe
applied’’(nãoseaplica)was added.
Becauseeventuallysomeone mightnothaveanyteethand theitemdoesnotapply 49 Limitationsintheability
(limitac¸ãonacapacidade)and jaw(mandíbula)
Difficuly(dificuldade)and
mouth(boca)
Wordofdifficultunderstanding, culturallyappropriate
50 Limitationsintheability
(limitac¸ãonacapacidade)
Difficuly(dificuldade) Culturallyadequate
Table2 ModificationssuggestedfortheanswerstoVHNSS2.0bythecommitteeofexpertsandtranslators.
Answerstoitems Originalversion Adaptedversion Justification
22 Nopain/severe
pain(Nehuma dor/Dorintensa)
Never/Always(Nunca/Sempre) Theitemreferstothefactofhavingornot lesionsandnottheintensityofpain.Theanswer wasadequatetothequestion
32,39 None/Severe
(Nenhum/Grave)
None/Alot(Nenhum/Muito) Theword‘‘grave’’wouldnotbeappropriatein thiscontext,culturallyadequate
42 Notatall/Severe
(Nenhum/Grave)
None/Alot(Nenhum/Muito) Theword‘‘grave’’wouldnotbeappropriatein thiscontext,culturallyadequate
14,49,50 Never/Severe
(Nunca/Grave)
Never/Always(Nunca/Sempre) Answersmixedtheconceptsoffrequencyand intensity.Itwasdecidedtomaintainonlythe frequencyconcept
VHNSS2.0,VanderbiltHeadandNeckCancerSymptomSurveyversion2.0.
Pre-test
In order to assess the clarity and understanding of the T4 version, a pilot test was performed, which included patientswithHNC(oral cavity,larynx,oropharynx,and/or hypopharynx)hadbeensubmittedtooncologictreatment, including radiotherapy, for more than six months previ-ouslyandwhoagreedtoparticipatebysigningtheinformed consent. Patients with cognitive or mental impairment that prevented them from providing correct information wereexcluded.Sociodemographicinformationwasobtained throughaninterviewandclinicaldatafrommedicalrecords. The32patientswhomettheinclusioncriteriaweredivided intofour groups based ondomains,27 with the firstgroup
evaluating items 1---17, the second group items 18---31,
the third group items 32---43, andthe fourth group items
44---50.Patientsanswered astructuredinterview
individu-allytoassesstheimportanceandunderstandingofitemsand
answers,whethertheywouldaskthequestion differently,
andthemeaningoftheitem,27thusensuringthateachitem
wasadequateandunderstoodbythepatients.Furthermore,
fivepatientswereenrolledatthisstagetotestthecultural
adaptationsofsixitems(5,10,14,27,38,43),asthe
ques-tionrelatedtothemeaningshowedtheywerenotclearfor
thesepatients.
Statisticalanalysis
Dataareshownthroughdescriptivestatisticsfor
sociodemo-graphicandclinical characteristics,aswellasfortheCVI,
usingSPSSv.20software.
Results
Theprocessoftranslationandcross-culturaladaptationof
theVHNSS2.0toolintotheBrazilianPortugueselanguageis
describedbelow.Itisnoteworthythattheback-translation
processshowedthattheinitialversioninPortuguese
corre-spondedtotheEnglishversion.Thesuggestionsgivenbythe
expertcommitteewerediscussedandincorporatedintothe
toolinordertoadaptittoBrazilianculture.
Tables 1 and2 describe themain cultural adjustments
withinthe items and tool responses. The main alteration
wastheperson:intheoriginaltool,allitemsareinthe1st
personsingular,whereasthetranslatedversionwasadapted
Table3 MeanoftheCVIoftheitems.
Equivalence CVIofitems CVIofanswers toitems
Semantic/idiomatic 0.79 0.96
Cultural 0.86 0.98
Conceptual 0.89 0.98
CVI,contentvalidityindex.
tothe2ndpersonsingulartoprovidemoreclarityandallow thetooltobebothself-applied,aswellastobeappliedby aninterviewer.
TheCVI,whichwascalculatedasthemeanoftheitems for each equivalence given by the raters, was 0.79 for idiomatic andsemanticequivalenceof theitemsand>0.8 fortheotherequivalences(Table3).
Pre-testinterview
A total of 37 patients participated in this stage, with a
median ageof 60 years,ofwhom 32(86.5%) weremales,
21 (56.8%)Caucasians, 27 (73%)married, 26(70.3%) were
barely literate or had not finished elementary school, 23
(62.2%) were fromthe state of São Paulo, 31 (84%) were
professionally inactive, eight (21.6%) were current
smok-ersand27(73%)ex-smokers,two(5.4%)consumedalcohol
and 21were ex-alcohol drinkers(56.8%), 29(78.5%) were
Catholics,and24(65%)hadnoassociatedcomorbidity.The
clinicalcharacteristicsaredescribedinTable4.
At the first moment, 32 patients participated in the
pre-test and were divided by domain and age,
answer-ing questions related to the understanding of the items,
witheachgroupcontainingeightpatients,equallydivided
betweenthoseagedupto60yearsoldorolder.Thepatients
considered the items important, easily understood, and
wereable tounderstandthe answers.In sixitems(5, 10,
14, 27, 38, 43) with the question ‘‘Could you tell me in
your ownwordswhat itmeans foryou?’’, 25%of patients
understoodincorrectly.Therefore,thesewerediscussedat
aconsensusmeetingandbasedonthecomments,theywere
reformulatedasfollows:
Items5and10:removaloftheword‘‘solid’’,aspatients
Table4 Clinicalcharacteristicsofpatientsparticipatingin thepre-test.
Variable Frequency(n) Percentage(%)
Histologicaltype
SCC 37 100.0
TNM
I 3 8.1
II 2 5.4
III 15 40.5
IV 16 43.2
‘‘Missing’’ 1 2.7
Location
Oralcavity 6 16.2
Hypopharynx 4 10.8
Oropharynx 13 35.1
Larynx 13 35.1
Oralcavityandlarynx 1 2.7
Surgery
Yes 21 56.8
No 16 43.2
Lymphadenectomy
No 6 31.6
Yes 13 68.4
Chemotherapy
No 11 29.7
Yes 26 70.3
ECOG
0 26 70.3
1 11 29.7
TNM,classificationofmalignanttumors;ECOG,Eastern Cooper-ativeOncologyGroup.
Item14:substitutingtheword‘‘problem’’with‘‘feeling,’’ astheyconsideredthedrymouthafeelingandnota prob-lem.
Item38:changeoftheorderofwordsinthesentenceto makeitmoredirect.
Items 27 (‘‘The relief of your pain with analgesics had been:Notapplicable,sinceIdonotuseanalgesics’’)and43 (‘‘Haveyouhadproblemswithyourdentures?Not applica-ble,becauseIdonotweardentures’’)werenotchanged, because the authors that participated in the consensus meeting(EMB,CEP,BSRP)consideredthatthesuggestions madebypatientstothesetwoitemsdidnotaddrelevant information.Theseitemswerereappliedtofivepatientsto confirmthatthechangeswereappropriate.Therefore,the processoftranslationandcross-culturaladaptationended, resultingintheBrazilianPortugueseversionoftheVHNSS 2.0tool,whichinBrazilisknownasthe‘‘Questionáriode sintomasem CâncerdeCabec¸aePescoc¸odeVanderbilt’’ (VHNSS2.0).
Discussion
Themethodusedinthisstudyallowedthetranslationand cross-culturaladaptationoftheVHNSS2.0tooltothe Brazil-iancultureandwillallowitsuseintheassessmentoforal
symptomsrelatedtotreatment thatincludesradiotherapy ofpatientswithHNCandtheirfunctionalimplications.Itis worthmentioningthatthisisthefirsttoolinBrazilian Por-tuguesethat includes adomain that evaluates the dental statusinthispopulation.
During the process of cross-cultural adaptation of the tool,the change from the first tothe second person sin-gularresultedinatoolthatcanbeself-appliedor applied byan interviewer, asa previous validationstudy in Brazil showed that 77% of Brazilians prefer assessment tools to beapplied by interviewers,with the given reasons being mainlypersonal preference anddifficulty reading.28
Addi-tionally,the influence of social and educationallevels on
HNCincidence mustbe considered.A meta-analysisstudy
of41articlesevaluatedtheassociation betweenoral
can-cerandsocioeconomicstatus,andfoundthatindividualsof
lowsocioeconomicstatus,includingloweducationallevel,
lowerincome,andloweroccupationalclass,aremorelikely
tohavethedisease.29
InBrazil,theincidenceofHNCis2.5higherinindividuals
whohaveloweducationallevel.30AstudycarriedoutinSão
Paulofoundthat45.4%and43.6%ofpatientswithHNCwere
illiterateorhadnotfinishedelementaryschoolintheyears
2000and2006,respectively.31Therefore,atoolthatcanalso
beappliedbyaninterviewermeetsthispopulation’sneeds,
whichinthisstudycomprisedthe70.3%ofpatientswhowere
barelyliterateorhadnotfinishedelementaryschool,with
amedianincomeofoneminimumwage.
Thereasonthatledtothetranslationandcross-cultural
adaptation of a new toolwas primarily the fact that the
available tools in Brazil that evaluate symptoms related
to treatment in patients with HNC are associated with
toolsthatassesshealth-relatedQoL,anddonotincludea
complete assessment of dental and oral health and their
functional implications. It is noteworthy that a detailed
oralhealthassessmentisimportant,sincepatientstreated
forHNC,includingradiationtherapy,oftenhaveoral
alter-ations.Additionally, itmustbeconsideredthatdeveloping
newtoolstakestimeandcostsmoney.32
Symptomssuchasdysphagiaandxerostomiahavea
nega-tiveimpactonhealth-relatedQoL.6Xerostomiaisafrequent
andimportantsymptom,reportedby52%ofpatientstreated
fororalandoropharyngealcancer;moreover,thefunctional
outcomemeasuredbytheMandibularFunctionImpairment
Questionnaire(MFIQ)isinfluencedbytheincapacitytowear
dentalprostheses.33 Itisnoteworthythat40.7%of
individ-ualsreportedchewingdifficultiesattributedtotheirteeth
ordentures,50%reportedthattheirteetharesensitive to
heat,cold,orsweets,and36%saidtheyhadfrailorchipped
teeth.9
Murphy et al. reported that 76% of patients
undergo-ingchemoradiotherapyorradiotherapyforHNChadsevere
painin themouthandthroat, resultingin lossoffunction
andincreaseduseof opioidsfor painreductionassociated
withmucosites.34 Dental problems affect a large number
ofpatientsand occurduetothe reductioninthesalivary
flowdirectlyassociatedwithalterationsindentalstructures
(enamel,dentin)causedbyradiation.35
The translation and cross-cultural adaptation method
usedin thisstudy hasbeen consolidated inliterature.24,25
Thetranslationprocessinvolvedindividualsfromthehealth
translatedtofacilitate the understanding by the patients
whowillanswerthetool.The choiceofusingtheservices
ofaspecializedcompanyfortheback-translationwasmade
tooptimizethe processwithprofessionalswhoarenative
English speakers and are also fluent in Portuguese. The
assessmentofequivalencebytheexpertcommitteeshowed
thatthePortugueseversionoftheVHNSS2.0isequivalent
totheEnglishversion.
One limitation of this study is the small sample size
used in the pre-test and the division by domains of the
appliedstructuredinterviews.Theloweducationallevelof
thepatientswithHNCinBrazilmayhindertheunderstanding
ofthequestionnaire,limitingitsself-application,although
thetoolalsohasbeenadaptedtoallowitsapplicationbyan
interviewer.
Conclusion
Translationand cultural adaptationof the VHNSS 2.0 tool
intoBrazilianPortuguesehasbeenperformed,providingan
important tool to assess oral symptoms in patients with
HNCsubmittedtotreatmentthatincludesradiotherapy.The
resultsdemonstratedthattheBrazilianversionoftheVHNSS
2.0toolis equivalenttotheoriginalin English,waseasily
understoodbypatients, andwasalsoadaptedtoBrazilian
culture.Therefore,thetoolisconsideredadequateforthe
validationstep,aprocessthatisunderway.
Authors’
contributions
EMB contributed to the study design, data collection,
and writing of the manuscript; ALC contributed to the
study design, analysis of equivalence, and reviewing of
themanuscript;CEPcontributedtothestudydesign,
par-ticipated in the discussions in the consensus meetings,
data analysis, and study review; JSN participated in the
committeeand discussionsat the consensusmeetingsand
contributedwithmanuscriptreview;andBSRPcontributed
to the study design, participated in the discussions in
theconsensus meetings, and contributedto dataanalysis
andstudy review.Allauthorsread andapproved thefinal
manuscriptversion.
Funding
This study was supported by Fundac¸ão de Amparo à
PesquisadoEstadodeSãoPaulo (FAPESP,Brazil)Case No.
2012/16768-2.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorswouldliketothankDr.LucianodeSouzaViana,
JoséHumbertoTavaresGuerreiroFregnani,Dr.Juliana
Bal-binot Hilgert, Dr.OtoValley Araújo,and Dr.Namie Okino
Sawada,fortheircontributiontothetranslationprocessof
thetoolfromEnglishintoPortugueseandtheirparticipation
intheexpertcommittee.
References
1.Instituto Nacional de Câncer. TNM: classificac¸ão de tumores malignos/traduzidopor AnaLúcia AmaralEisenberg.6thed. RiodeJaneiro:INCA;2004.
2.WHOrganization. WorldCancer Report,2008. Lyon: Interna-tionalAgencyforResearchonCancer;2009.
3.INCA.Estimativa2012:incidênciadecâncernoBrasil/Instituto Nacional de Câncer José Alencar da Silva. Rio de Janeiro: Coordenac¸ão Geral de Ac¸ões estratégicas, Coordenac¸ão de Prevenc¸ãoe Vigilância; 2012. Available from: http://portal. saude.sp.gov.br/resources/ses/perfil/gestor/homepage/ estimativas-de-incidencia-de-cancer-2012/estimativas incidenciacancer2012.pdf[cited12.02.12].
4.MurphyBA,GilbertJ.Oralcancers:supportivecareissues. Peri-odontol2000.2011;57:118---31.
5.LangendijkJA,DoornaertP,Verdonck-deLeeuwIM,Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-relatedtoxicityon quality oflife amongpatients withhead and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770---6.
6.RamaekersBL, JooreMA, GruttersJP, vandenEnde P,Jong J,HoubenR,etal.Theimpactoflatetreatment-toxicityon generichealth-relatedqualityoflifeinheadandneckcancer patientsafterradiotherapy.OralOncol.2011;47:768---74.
7.Epstein JB, Murphy BA. Late effects of cancer and cancer therapyon oralhealth and qualityof life. JMass DentSoc. 2010;59:22---7.
8.EpsteinJB,Thariat J,Bensadoun RJ,Barasch A,Murphy BA, KolnickL,etal.Oralcomplicationsofcancerandcancer ther-apy:fromcancertreatmenttosurvivorship.CACancerJClin. 2012;62:400---22.
9.CoopersteinE,GilbertJ,EpsteinJB,DietrichMS,BondSM, Rid-nerSH,etal.VanderbiltHeadandNeckSymptomSurveyversion 2.0:reportofthedevelopmentandinitialtestingofasubscale forassessmentoforalhealth.HeadNeck.2012;34:797---804.
10.PayakachatN,OunpraseuthS,SuenJY.Latecomplicationsand long-termqualityoflifeforsurvivors(>5years)withhistoryof headandneckcancer.HeadNeck.2013;35:819---25.
11.BjordalK,KaasaS, MastekaasaA. Qualityof lifeinpatients treated for head and neck cancer: a follow-up study 7 to 11 years after radiotherapy. Int J Radiat Oncol Biol Phys. 1994;28:847---56.
12.LogemannJA,SmithCH,PauloskiBR,RademakerAW,Lazarus CL,ColangeloLA,etal.Effectsofxerostomiaonperceptionand performanceofswallowfunction.HeadNeck.2001;23:317---21.
13.Mossman KL. Gustatory tissueinjury in man: radiation dose responserelationshipsandmechanismsoftasteloss.BrJCancer Suppl.1986;7:9---11.
14.Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-MooreP.Qualityoflifeand oralfunctioninpatientstreated withradiationtherapyforheadand neckcancer.HeadNeck. 2001;23:389---98.
15.Hong CH, Nape˜nas JJ, Hodgson BD, Stokman MA, Mathers-StaufferV,EltingLS,etal.Asystematicreviewofdentaldisease inpatientsundergoing cancertherapy. Support CareCancer. 2010;18:1007---21.
16.AndradeFP,AntunesJL,DurazzoMD.Evaluationofthe qual-ityoflifeofpatientswithoralcancerinBrazil.BrazOralRes. 2006;20:290---6.
18.HassanSJ,WeymullerEAJr.Assessmentofqualityoflifeinhead andneckcancerpatients.HeadNeck.1993;15:485---96.
19.D’AntonioLL, Zimmerman GJ, CellaDF, Long SA. Quality of life and functional status measures in patients with head andneckcancer.ArchOtolaryngolHeadNeckSurg.1996;122: 482---7.
20.BjordalK,HammerlidE,Ahlner-ElmqvistM,deGraeffA,Boysen M,EvensenJF,etal.Quality oflifeinheadandneckcancer patients:validationoftheEuropeanOrganizationforResearch andTreatmentofCancerQualityofLifeQuestionnaire-H&N35. JClinOncol.1999;17:1008---19.
21.RosenthalDI,MendozaTR,ChambersMS,AsperJA,GningI,Kies MS,etal.Measuringheadandneckcancersymptom burden: thedevelopmentand validationoftheM.D.Anderson symp-tominventory, head and neckmodule.Head Neck. 2007;29: 923---31.
22.Niermann KJ, Dietrich MS, Ridner SH, Kolnick L, Zatarain LA, Gilbert J, et al. Validation of the Vanderbilt Head and Neck Symptom Survey Version2.0. Proceedings of the 2013 ASCO annual meeting. J Clin Oncol. 2013;31 Suppl.; abstr 6049.
23.KolnickL,DengJ,EpsteinJB,MiglioratiCA,RezkJ,DietrichMS, etal.AssociationsoforalhealthitemsoftheVanderbiltHead andNeckSymptomSurveywithadentalhealthassessment.Oral Oncol.2014;50:135---40.
24.BeatonDE,BombardierC,GuilleminF,FerrazMB.Guidelines fortheprocessofcross-culturaladaptationofself-report meas-ures.Spine(PhilaPa1976).2000;25:3186---91.
25.GuilleminF,BombardierC,BeatonD.Cross-culturaladaptation ofhealth-relatedqualityoflifemeasures:literaturereviewand proposedguidelines.JClinEpidemiol.1993;46:1417---32.
26.RubioDM,Berg-WegerM,TebbSS,LeeES,RauchS.Objectifying contentvalidity:conductingacontentvaliditystudyinsocial workresearch.SocWorkRes.2003;27:94---104.
27.Correia FR [Dissertation] Traduc¸ão, adaptac¸ão cultural e validac¸ãoinicialnoBrasildaPalliativeOutcomeScale(POS). RibeirãoPreto:UniversidadedeSãoPaulo;2012.
28.BraboEP,PaschoalME,BiasoliI,NogueiraFE,GomesMC,Gomes IP,etal.BrazilianversionoftheQLQ-LC13lungcancer mod-uleoftheEuropeanOrganizationforResearchandTreatment ofCancer:preliminaryreliabilityandvalidityreport.QualLife Res.2006;15:1519---24.
29.ConwayDI,PetticrewM,MarlboroughH,BerthillerJ,Hashibe M,Macpherson LM.Socioeconomicinequalities and oral can-cerrisk:asystematicreviewandmeta-analysisofcase---control studies.IntJCancer.2008;122:2811---9.
30.Boing AF [Thesis] Condic¸ões socioeconômicas e câncer de cabec¸aepescoc¸o.SãoPaulo:UniversidadedeSãoPaulo;2007.
31.BergamascoVDB,Marta GN, Kowalski LP,CarvalhoAL. Perfil epidemiológicodocâncerde cabec¸aepescoc¸onoEstadode SãoPaulo[Epidemiologicalprofileoftheheadandneck can-cerintheStateofSãoPaulo].RevBrasCirCabec¸a Pescoc¸o. 2008;37:15---9.
32.Fayers PM,Machin D.Introduction.In: FayersPM,MachinD, editors.Qualityoflife:theassessment,analysisand interpreta-tionofpatient-reportedoutcomes.England:JohnWiley&Sons Ltd;2007.p.3---30.
33.KamstraJI,Jager-WittenaarH,DijkstraPU,HuismanPM,van OortRP,vanderLaanBF,etal.Oralsymptomsandfunctional outcomerelatedtooralandoropharyngealcancer.SupportCare Cancer.2011;19:1327---33.
34.MurphyBA,BeaumontJL,IsittJ,GardenAS,GwedeCK,Trotti AM,etal.Mucositis-relatedmorbidityandresourceutilization in head and neck cancer patients receiving radiation ther-apywithorwithoutchemotherapy. JPainSymptom Manage. 2009;38:522---32.