The association of two single nucleotide polymorphisms (SNPs) in growth
hormone (
GH
) gene with litter size and superovulation response in
goat-breeds
Chunyan Zhang
1, Yun Liu
1, Kunkun Huang
1, Wenbing Zeng
1, Deqing Xu
2, Qunying Wen
3and Liguo Yang
11
College of Animal Science and Technology, Huazhong Agricultural University, Wuhan, China.
2Boer Goat Breeding Station, Yidu, China.
3
Shiyan Municipal Bureau of Animal Husbandry of Hubei Province, Shiyan, China.
Abstract
Two active mutations (A 781 G and A 1575 G) in growth hormone (GH) gene, and their associations with litter size (LS), were investigated in both a high prolificacy (Matou, n = 182) and a low prolificacy breed (Boer, n = 352) by using the PCR-RFLP method. Superovulation experiments were designed in 57 dams, in order to evaluate the effect of dif-ferent genotypes of theGH gene on superovulation response. Two genotypes (AA and AB, CC and CD) in each mu-tation were detected in these two goat breeds. Neither BB nor DD homozygous genotypes were observed. The genotypic frequencies of AB and CC were significantly higher than those of AA and CD. In the third parity, Matou dams with AB or CC genotypes had significantly larger litter sizes than those with AA and CD (p < 0.05). On combin-ing the two loci, both Matou and Boer dams with ABCD genotype had the largest litter sizes when compared to the other genotypes (p < 0.05). When undergoing like superovulation treatments, a significantly higher number of cor-pora lutea and ova, with a lower incidence of ovarian cysts, were harvested in the AB and CC genotypes than in AA and CD. These results show that the two loci ofGH gene are highly associated with abundant prolificacy and superovulation response in goat breeds.
Key words:DNA polymorphism, growth hormone (GH) gene, litter size, superovulation response, goat. Received: April 1, 2010; Accepted: October 13, 2010.
Introduction
It has been reported that metabolic hormones, such as growth hormone (GH), are directly involved in mediating nutritionally-induced changes in follicular development (Downing and Scaramuzzi, 1991; Prunier and Quesnel, 2000; Armstrong et al., 2003). The growth hormone of mammals plays an important role in the control of repro-duction, in those aspects involving cell division, ovarian folliculogenesis, oogenesis and secretory activity (Schams
et al., 1999; Gong, 2002; Hull and Harvey, 2002; Olaet al., 2008). By acting through specific receptors within the ovary, GH is expedient in controlling proliferation and apoptosis, oocyte maturation, and the expression and syn-thesis of receptors to hormones and related substances (Sirotkinet al., 1998; Schamset al., 1999; Hull and Har-vey, 2000; Sirotkinet al., 2003). The addition of bovine GH during in vitro maturation (IVM) of bovine oocytes has been found to induce cumulus expansion and accelerate nu-clear maturation, besides promoting subsequent fertiliza-tion, cleavage and early embryonic development, as shown
by enhancing the number of resultant blastocysts (Izadyar
et al., 1996, 1998; Joudreyet al., 2003). Further studies have revealed that the effect of GH on ovary function is mainly through inducing the development of small antral follicles in the gonadotrophin-dependent stages and stimu-lating oocyte maturation (Silvaet al., 2009).
As abundantly illustrated in the literature, numerous attempts have been made to investigate the effects of the
GHgene on mammal growth and milk production (Wanget al., 2003; Guptaet al., 2007; Huaet al., 2008; Baloghet al., 2009; McCormack et al., 2009), with only few reported studies of this in oogenesis and spermiogenesis (Kmieet al., 2007; Murphyet al., 2008), and no mention of the ef-fects on litter size and superovulation response. To date, lit-tle has been divulged on the major gene associated with litter size in goats, these few studies involving the inhibin alpha-subunit gene (INHA) (Huaet al., 2008; Wuet al., 2009), the gonadotrophin releasing hormone receptor gene (GnRHR)(Anet al., 2009), the bone morphogenetic protein receptor-IB gene (BMPRIB) in the prolific Indian Black Bengal goat (Polleyet al., 2009), and the bone morpho-genetic protein 15 gene (BMP15) in Jining Grey goats (Chu
et al., 2007).
www.sbg.org.br
Send correspondence to Liguo Yang. College of Animal Science and Technology, Huazhong Agricultural University, 430070 Wuhan, China E-mail: yangliguo2006@yahoo.com.cn.
Growth and reproduction, two crucial economic traits in production, are co-ordinated during normal puberty and the adult stages. There is evidence that normal growth-hormone secretion is required for the correct timing of the onset of puberty (Frankset al., 1998). As already confirmed in our previous research, there is a significantly positive as-sociation of two polymorphisms (A 781 G and A 1575 G) ofGHgene with growth in Boer bucks (Huaet al., 2008). Consistent with the essential function of GH in puberty and ovary activity, there is the need for further validation re-garding its effects on dam reproduction.
Hence, in this study, the relatively hyper-prolific Ma-tou breed and the low-prolific Boer were used to investigate the frequency distribution of the two GHgene polymor-phisms (A 781 G and A 1575 G), and evaluate their effects on litter size. We contemplated factors affecting litter size, including year, season and parity, besides the age of the dam, with due account also being given to their mutual in-teractions. Furthermore, specific experiments were de-signed to evaluate the superovulation response in dams with differentGHgenotypes.
Materials and Methods
Data collection from experimental goat breeds
All procedures involving animals were approved and authorized by the Chinese Ministry of Agriculture, through the Animal Care and Use Committee at the respective insti-tution where the experiment was undertaken.
A total of 534 adult females from two goat breeds dif-fering in prolificacy, viz., 352 Boer dams with records of 1188 parities and 182 Matou dams with records of 583 pari-ties, were analyzed in the present study. Those from the Boer breed, the low-prolific line (LS = 1.42) obtained from the Yidu Boer-Goat Breeding Station, had four depth levels of generation in the pedigree consisting of 129 sires and 552 dams, whereas the Matou breed, the native hyper-prolific line (LS = 2.14), collected from farms in Shiyan county, presented three generation depth levels in the pedi-gree.
Blood collection and genomic DNA preparations were carried out according to Huaet al.(2008). The data re-garding litter size (LS) were collected in consecutive pari-ties, considering repeated measurements in the same indi-vidual. Due to the significant effect of parity on litter size in goats, and its stability following the third parity (Moaeen-ud-Dinet al., 2008; Wuet al., 2009; Zhanget al., 2009), the data were calculated separately for primiparity, third parity and all the parities together.
Superovulation dams and sampling
A total of 57 adult native, fertile females in healthy conditions were used in superovulation experiments. The dams, selected under similar and uniform conditions, tak-ing into account age (3 to 5 years) and body weight (35 to
40 kg), were raised for about 2 weeks prior to the outset of superovulation. All the animals were barn housed and un-der controlled nutrition. The diet was a mixture of cured hay and grains, with a daily vitamin supplement. Fresh wa-ter and a mineral supplement were availablead libitum.
Superovulation procedure and response
The synchronization of estrus was induced by the ad-ministration of a single minuscule injection of Cloprostenol Sodium (PG-Cl), in a dose of 0.2 mg, on the day prior to ini-tiating the experiment. Beginning on the 2ndday, Pituitary Follitropin for Capra (cFSH) was given twice daily at inter-vals of 12 h in eight decreasing doses (40, 40, 30, 30, 20, 20, 20, and 20 IU). The last cFSH dose was given concurrently with a single 25 ug dose of Luteinizing Hormone Releasing Hormone A3 (LHRH-A3). All the experimental dams were slaughtered on the 7thday, whereupon the ovaries and ovi-ducts were collected and kept in incubation casks (37 °C) containing PBS, and brought to the laboratory within 1 h. The whole experiment, which took place in November, was undertaken with hormones provided by the Ningbo San-sheng Pharmaceutical Co., Ltd., Ningbo, China, all from single batches.
The ova were recovered by aspiration from the ovi-ducts, and searched immediately after filtering the aspi-rated follicular fluid medium. The numbers of follicles of different sizes on ovarian surfaces were evaluated, based on a classification of follicle diameters as small (< 2.0 mm), medium (2.0 ~ 4.0 mm), large (4.0 ~ 8.0 mm) and ovarian cyst (> 8.0 mm) (Valasiet al., 2007). The total number of ova, corpora lutea and ovarian cysts, per dam and in each class, were recorded. All the observations were done by one and the same person using the same methodology through-out.
Primer synthesis and polymerase chain
reaction-restriction fragment length polymorphism (PCR-RFLP) analysis
Our previous report (Hua et al., 2008) formed the base for designing primer and setting PCR conditions. Two amplicons of 422 bp and 116 bp were yielded by the PCR reaction using the following primers synthesized by Shang-hai Sangon Biotech Co., Ltd.:
GH1: 5’-CTCTGCCTGCCCTGGACT-3’ and 5’-GGAGAAGCAGAAGGCAACC-3’
GH2: 5’-TCAGCAGAGTCTTCACCAAC-3’ and 5’-CAACAACGCCATCCTCAC-3’
silver staining, were used for detecting the reaction prod-ucts. PBR322/MspI (HuaMei Bioengineering Co., Ltd., China) was employed as a size marker for defining restric-tion-fragment sizes.
Statistical analysis
The allelic and genotypic frequencies in theGHgene in both goat breeds were analyzed. A Bonferroni correction (derivative-free restricted maximum likelihood, DFREML) was used to analyze the relationship between
GHgene and litter size with animal models (Meyer and Kirkpatrick, 2005). Pedigrees of base population animals were traced back three (Matou) or four (Boer) generations, in order to create the numerator relationship matrix.
All the analyses were carried out in two steps, first us-ing a full animal model and then usus-ing a reduced animal model. The full-animal model included all the factors, viz., genotypes, parity (1, 2, 3, 4 and³5), age of dam (1, 2, 3 and ³4 years), kidding year (2002-2009), four kidding seasons – Spring (March and April), Summer (May to September), Autumn (October and November), and Winter (December to February), as well as the interactions of year-season, year-parity, parity-season and parity-age. Data of litter size from consecutive parities in the same individual were con-sidered as repeated measures, and included in the statistical model. The reduced-model included only those fixed ef-fects (genotype, kidding year, parity and age of dam) that exerted a significant influence on LS (p < 0.05), and was only used in the final analysis. The reduced-animal model was:
y = Xb+Za+Zp+e
whereyis a vector of observations for litter size,ba vector of fixed effects for genotype, kidding year, parity and age of the dam,aa vector of animal genetic effects,pa vector of permanent environmental effects for parities of each dam, andea vector of random residual effects. When analyzing the phenotypic data of primiparity and the third parity, the parity effect was excluded.
Data on superovulation response were analyzed sta-tistically by applying the one-way ANOVA of SAS proce-dures (SAS Institute, Cary, NC, USA). The significance of the difference was determined by F-test at the significance level of 0.05.
Results
PCR amplification and RFLP analysis
As expected, two fragments of theGHgene (422 and 116 bp) were amplified from caprine genomic DNA by the
GH1 andGH2 primers. Both fragments, when digested by endonuclease HaeIII, resulted in four genotypes named: AA (366 and 56 bp) and AB (422, 366 and 56 bp) forGH1, and CC (88 and 28 bp) and CD (116, 88 and 28 bp) forGH2 (Figure 1). Thus, in the 534 individuals from the 2 differ-ently prolific breeds, four genotypes of the two fragments of theGHgene were detected in both the Matou and Boer breeds, but no homozygotes of either BB or DD individuals were found (Figure 1).
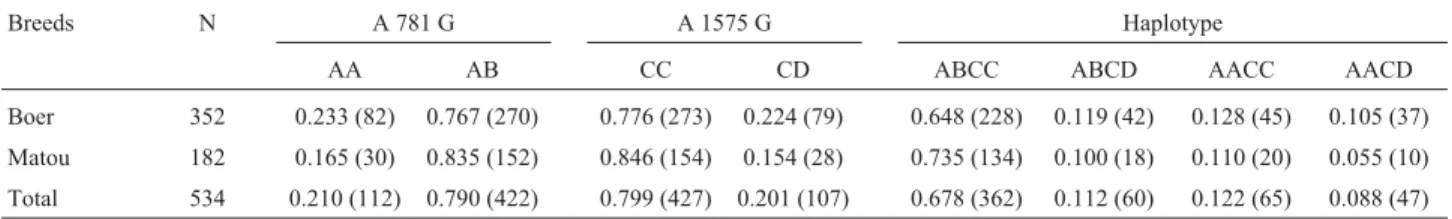
Allele genotypic and haplotypic frequencies of GH gene in the two goat breeds
The genotypic and haplotypic frequencies of se-quence polymorphisms are given in Table 1. The frequen-cies of AB and CC genotypes were much higher than those of AA and CD in both goat breeds. On comparing the same genotypic frequency between the two breeds, that of AB and CC genotypes in the Matou breed was much higher than in the Boer. When combining the two loci, the fre-quency of the ABCC haplotype proved to be the highest and that of the AACD the lowest.
Influence of genotype on litter size
The association of independent genotypes inGHgene with litter size, in Boer and Matou dams, is given in Table 2. In various parity groups, Matou dams with AB or CC genotypes had larger litter sizes than those with AA or CD (p < 0.05), although no significant difference appeared at
primiparity. At third parity, the performance in Matou dams with either genotype AB or CC was significantly su-perior to those with BB or CD by 0.58 and 0.63 kids born (p < 0.05). The same tendency was observed in Boer dams, although the difference was not significant.
The effects of combined genotypes inGHgenes on litter size in Boer and Matou dams are given in Table 3. Combined genotype analysis of the two loci showed that LS was the lowest in the AACD genotype and the highest in ABCD. A significant difference was observed in various parity groups of the Matou breed, whereas, in the Boer breed this only appeared in the third parity (p < 0.05).
Influence of the genotype on superovulation response
The effects of the differentGHgenotypes on super-ovulation response are given in Table 4. The two SNPs of theGHgene had significant effects on the superovulation response. On undergoing identical treatments, corpora
lutea and harvested ova were significantly more numerous in dams with AB and CC genotypes than in those with AA and CD. The numbers of small, medium and large follicles in dams with genotypes AB and CC were also higher than those carrying the genotypes AA and CD, although these differences were not significant. The incidence of ovarian cysts in dams with AB and CC genotypes was lower than in those with AA and CD.
Discussion
Alleles, genotypes and haplotypes diversity
In the present study, two polymorphisms of the goat
GHgene at the loci A 781 G and A 1575 G were detected in 534 dams of the Boer and Matou breeds. The frequencies of AB and CC genotypes were higher in the relatively highly prolific Matou breed than in the relatively lowly prolific Boer breed. On the other hand, neither BB nor DD
homozy-Table 1- Sample size, and genotypic and haplotypic frequencies ofGHpolymorphisms in Boer and Matou goat breeds.
Breeds N A 781 G A 1575 G Haplotype
AA AB CC CD ABCC ABCD AACC AACD
Boer 352 0.233 (82) 0.767 (270) 0.776 (273) 0.224 (79) 0.648 (228) 0.119 (42) 0.128 (45) 0.105 (37)
Matou 182 0.165 (30) 0.835 (152) 0.846 (154) 0.154 (28) 0.735 (134) 0.100 (18) 0.110 (20) 0.055 (10)
Total 534 0.210 (112) 0.790 (422) 0.799 (427) 0.201 (107) 0.678 (362) 0.112 (60) 0.122 (65) 0.088 (47)
Numbers in parentheses indicate sample size.
Table 2- Effects of separateGHgene genotypes on litter size in primiparity, the third parity and all the parities in Boer and Matou dams (means±SD).
Parity groups Breeds A 781 G A 1575 G
AA AB p-value CC CD p-value
Primiparity Boer (352) 1.42±0.51 (82) 1.54±0.71 (270) 0.6160 1.58±0.69 (273) 1.51±0.75 (79) 0.6646
Matou (182) 1.69±0.60 (30) 1.88±0.77 (152) 0.6029 1.75±0.74 (154) 1.73±0.65 (28) 0.9632
Third parity Boer (290) 1.81±0.81 (62) 1.87±0.63 (228) 0.8196 1.94±0.64 (235) 1.77±0.71 (55) 0.5227
Matou (156) 2.04±0.76 (25) 2.62±0.89 (131) 0.0158 2.65±0.87 (133) 2.02±0.83 (23) 0.0294
All parities Boer (352) 1.80±0.73 (82) 1.81±0.65 (270) 0.9341 1.89±0.65 (273) 1.65±0.74 (79) 0.2394
Matou (182) 1.93±0.65 (30) 2.37±0.91 (152) 0.0036 2.32±0.86 (154) 1.80±0.76 (28) 0.0135
Numbers in parentheses indicate sample size.
Table 3- Effects of combinedGHgene genotypes on litter size in primiparity, third parity and all the parities in Boer and Matou dams (means±SD).
Parity groups Breeds AACD AACC ABCC ABCD
Primiparity Boer (352) 1.33±0.57 (37) 1.44±0.53 (45) 1.53±0.70 (228) 1.61±0.78 (42)
Matou (182) 1.50±0.55a(10) 1.62±0.57ab(20) 1.80±0.79ab(134) 2.00±0.71b(18)
Third parity Boer (290) 1.50±1.00a(23) 1.88±0.78ab(39) 1.84±0.62ab(196) 2.04±0.65b(32)
Matou (156) 2.00±0.81a(8) 2.17
±0.00ab(17) 2.25
±0.87ab(116) 2.55
±1.03b(15)
All parities Boer (352) 1.45±0.71 (37) 1.76±0.73 (45) 1.80±0.64 (228) 1.88±0.74 (42)
Matou (182) 1.73±0.47a(10) 1.97±0.68ab(20) 2.02±0.90ab(134) 2.37±0.90b(18)
Numbers in parentheses indicate sample size.
gous were observed in any individual whatsoever. Our pre-vious research on theGHgene also revealed this to be the case in 154 Boer bucks (Huaet al., 2008), as was also re-ported in Chengdu-Ma (n = 37) and Boer (n = 29) goats (Baiet al., 2005), and in a Gannan Yak population (n = 202) (Baiet al., 2009). The A 781 G polymorphism was also de-tected in other goat flocks, such as the LuBei white goat (n = 50), and in the first filial generation of LuBei white and Boer (n = 105) goats (Liet al., 2004).
All told, BB and DD genotypes were absent in both females and males in seven goat breeds, this including purebreds and crossbreds, as well as in Yaks, as mentioned above. It has been amply confirmed in the literature that the
GHgene is essential for normal reproductive functions in-cluding oogenesis, follicular development and embryoge-nesis (Sirotkinet al., 1998, 2003; Schamset al., 1999; Hull and Harvey, 2000; Ola et al., 2008; Silva et al., 2009). Thus, it can be presumed that A 781 G (BB) and A 1575 G (DD) homozygous mutations in theGHgene may give rise to reproductive disturbance, even to the point of infertility.
Influence of the genotype on litter size and superovulation response
The association analysis showed that the different ge-notypes or haplotypes have significant effects on litter size and superovulation response. This is the first time that the effects ofGH gene polymorphism on goat reproduction have been studied. To date, more than 10 goatGHvariants have been detected, most of which involving growth traits (Guptaet al., 2007; Huaet al., 2008), and a few milk pro-duction (Malveiroet al., 2001; Marqueset al., 2003). Nev-ertheless, no report has focused on reproduction traits. Our previous research has already confirmed that these two mu-tations of theGHgene exerted a highly additive effect on growth traits in Boer bucks (Huaet al., 2008). The crucial role ofGH in oogenesis and follicular development has been amply confirmed (Schamset al., 1999; Sirotkinet al., 2003; Silvaet al., 2009). Hence, the present study was de-signed to be a continuing step in evaluating the effect of the
GHgene on litter-size and superovulation, with a mind to
eventually providing further useful and detailed information for molecular marker-associated selection (MAS) programs.
The separation of AB and CC genotypes has been as-sociated with larger litter sizes and a higher superovulation response. This effect is most likely due to allelic interaction and the important biological effects ofGHon reproduction processes, this including oogenesis, follicular development and embryogenesis. It has been reported that, on initiating cattle superstimulation protocols, pre-treatment with growth hormone can increase the population of antral folli-cles (Bolset al., 1998; Gong, 2002). Knockout experiments have demonstrated thatGHenhances the development of small antral follicles up to the gonadotrophin-dependent stage, besides stimulating oocyte maturation (Silvaet al., 2009). The addition of bGH during in vitro maturation (IVM) of bovine oocytes has been found to induce cumulus expansion, besides accelerating nuclear maturation re-flected by the accelerated extrusion of the 1stpolar body, and promoting subsequent fertilization, cleavage and early embryonic development (Izadyaret al., 1996, 1998). Kölle
et al.(2001) reported that theGHgene is also involved in activating cellular functions in blastocysts, thereby stimu-lating glucose uptake and protein synthesis. Joudreyet al.
(2003) reported that during bovine embryogenesis, bovine growth hormone contributes to proliferation, differentia-tion, and modulation of embryonic metabolism. The ab-sence of BB and DD genotypes, as observed in the present and previous research, further confirmed the significance ofGHin goat reproduction and growth.
When combined, the two SNPs displayed more pro-found impacts on LS than separately in both goat breeds. Both Boer and Matou dams with the ABCD genotype had the largest litter sizes compared to the others, and the com-bined genotype AACD was associated with the lowest litter sizes. This is consistent with previous research on goat growth traits by Huaet al.(2008), who reported that body weight and growth rate from birth to weaning was the lowest in Boer bucks bearing the AACD genotype,
Table 4- Effects ofGHgene genotypes on the numbers of corpora lutea (NCL), follicles of different size and ova harvested, as well as the incidence of ovarian cysts after superovulation (means±SD).
Genotype Follicle size in diameter (mm) NCL Ova harvested Incidence of
ovarian cysts (%)
Small (< 2.0) Medium (2.0~4.0) Large (4.0~8.0)
AA (20) 8.3±2.9 5.1±1.8 5.7±3.4 6.6±2.6 4.4±2.1 50.0 (10/20)
AB (37) 12.4±2.3 8.4±2.9 6.5±2.5 11.7±3.1 10.6±3.3 27.0 (10/37)
P-value 0.2069 0.1105 0.4798 0.0379 0.0488
-CC(49) 12.1±3.8 9.3±2.1 6.4±2.6 11.7±3.9 9.8±3.4 22.4 (11/49)
CD(8) 9.0±2.5 5.8±2.3 5.9±3.5 5.0±2.1 5.5±2.0 37.5 (3/8)
p-value 0.5611 0.4508 0.4191 0.0349 0.0782
whereby it can be concluded that goats with this genotype should be avoided in selection programs.
Acknowledgments
The authors wish to thank all the staff from the Boer Goat-Breeding Station and Shiyan Animal Husbandry for their inestimable work in data collection. This work was fi-nancially supported by grants from both the “Chinese Foundation Technique Extension in Agriculture (05EFN214200186)” and the Chinese Transgenic Specific Foundation for Goat Breeding Resistance to Disease (2008ZX08008-005).
References
An XP, Han D, Hou JX, Li G, Wang JG, Yang MM, Song YX, Zhou GQ, Wang YN and Ling L (2009) GnRHR gene polymorphisms and their effects on reproductive perfor-mance in Chinese goats. Small Rumin Res 85:130-134. Armstrong DG, Gong JG and Webb R (2003) Interactions
be-tween nutrition and ovarian activity in cattle: Physiological, cellular and molecular mechanisms. Reproduction 61:403-414.
Bai WL, Wang J and Yin RY (2005) Study on genetic polymor-phism ofHaeIII site ofGHgene in Chengdu-Ma goat and Boer goat. Heilongjiang Anim Sci and Vet Med 8:13-14. Bai JJ, Wang JQ, Hu J and Luo YZ (2009) SNPs detection of
growth hormone gene in Gannan Yak population. China Cattle Sci 25:1-5.
Balogh O, Kovács K, Kulcsár M, Gáspárdy A, Zsolnai A, Kátai L, Pécsi A, Fésüs L, Butler WR and Huszenicza GY (2009)
AluI polymorphism of the bovine growth hormone (GH) gene, resumption of ovarian cyclicity, milk production and loss of body condition at the onset of lactation in dairy cows. Theriogenology 71:553-559.
Bols PE, Ysebaert MT, Lein A, Coryn M, Van Soom A and de Kruif A (1998) Effects of long-term treatment with bovine somatotropin on follicular dynamics and subsequent oocyte and blastocyst yield in an OPU-IVF program. Theriogeno-logy 49:983-995.
Chu MX, Jiao CL, He YQ, Wang JY, Liu ZH and Chen GH (2007) Association between PCR-SSCP of bone morphogenetic protein 15 gene and prolificacy in Jining Grey goats. Anim Biotechnol 18:263-274.
Downing JA and Scaramuzzi RJ (1991) Nutrient effects on ovula-tion rate, ovarian funcovula-tion and the secreovula-tion of gonadotro-phic and metabolic hormones. J Reprod Fertil Suppl 43:209-227.
Franks S, Frcp MD and Horz MD (1998) Growth hormone and ovarian function. Baillieres Clin Endocrinol Metab 12:331-340.
Gong JG (2002) Influence of metabolic hormones and nutrition on ovarian follicle development in cattle: Practical implica-tions. Domest Anim Endocrinol 23:229-241.
Gupta N, Ahlawat SPS, Kumar D, Gupta SC, Pandey A and Malik G (2007) Single nucleotide polymorphism in growth hor-mone gene exon-4 and exon-5 using PCR-SSCP in Black Bengal goats - A prolific meat breed of India. Meat Sci 76:658-665.
Hua GH, Chen SL, Yu JN, Cai KL, Wu CJ, Li QL, Zhang CY, Liang AX, Han L and Geng LY (2008) Polymorphism of the growth hormone gene and its association with growth traits in Boer goat bucks. Meat Sci 81:391-395.
Hull KL and Harvey S (2002) GH as a co-gonadotropin: The rele-vance of correlative changes in GH secretion and reproduc-tive state. J Endocrinol 172:1-19.
Izadyar F, Colenbrander B and Bevers MM (1996)In vitro matu-ration of bovine oocytes in the presence of growth hormone accelerates nuclear maturation and promotes subsequent embryonic development. Mol Reprod Dev 45:372-377. Izadyar F, Hage WJ, Colenbrander B and Bevers MM (1998) The
promotory effect of growth hormone on the developmental competence ofin vitromatured bovine oocytes is due to im-proved cytoplasmic maturation. Mol Reprod Dev 49:444-453.
Joudrey EM, Lechniak D, Petrik J and King WA (2003) Expres-sion of growth hormone and its transcription factor, Pit-1, in early bovine development. Mol Reprod Dev 64:275-283. Kmie M, Terman A, Wierzbicki H and Zych S (2007) Association
ofGHgene polymorphism with semen parameters of boars. Acta Vet Brno 76:41-46.
Kölle S, Stojkovic M, Prelle K, Waters M, Wolf E and Sinowatz F (2001) Growth hormone (GH)/GHreceptor expression and GH-mediated effects during early bovine embryogenesis. Biol Reprod 64:1826-1834.
Li M, Pan Y, Pan QH, Shen YC, Min W and Ren LJ (2004) Poly-morphism analysis of goat growth hormone (GH) gene by PCR-RFLP. J Laiyang Agr Res 21:6-9.
Malveiro E, Pereira M, Marques PX, Santos IC, Belo C, Renaville M and Cravador A (2001) Polymorphisms at the five exons of the growth hormone gene in the algarvia goat: Possible association with milk traits. Small Rumin Res 41:163-170. Marques PX, Pereira M, Marques MR, Santos IC, Belo CC,
Renaville R and Cravador A (2003) Association of milk traits with SSCP polymorphisms at the growth hormone gene in the Serrana goat. Small Rumin Res 50:177-185. McCormack BL, Chase Jr CC, Olson TA, Elsasser TH, Hammond
AC, Welsh Jr TH, Jiang H, Randel RD, Okamura CA and Lucy MC (2009) A miniature condition in Brahman cattle is associated with a single nucleotide mutation within the growth hormone gene. Domest Anim Endocrinol 37:104-111.
Meyer K and Kirkpatrick M (2005) Restricted maximum likeli-hood estimation of genetic principal components and smo-othed covariance matrices. Genet Sel Evol 37:1-30. Moaeen-ud-Din M, Yang LG, Chen SL, Zhang ZR, Xiao JZ, Wen
QY and Dai M (2008) Reproductive performance of Matou goat under sub-tropical monsoonal climate of Central China. Trop Anim Health Prod 40:17-23.
Murphy AM, Meade KG, Hayes PA, Park SDE, Evans ACO, Lonergan P and MacHugh DE (2008) Transmission ratio distortion at the growth hormone gene (GH1) in bovine preimplantation embryos: Anin vitroculture-induced phe-nomenon? Mol Reprod Dev 75:715-722.
Polley S, De S, Batabyal S, Kaushik R, Yadav P, Arora JS, Chattopadhyay S, Pan S, Brahma B and Datta TK (2009) Polymorphism of fecundity genes (BMPR1B,BMP15and
GDF9) in the Indian prolific Black Bengal goat. Small Rumin Res 85:122-129.
Prunier A and Quesnel H (2000) Influence of the nutritional status on ovarian development in female pigs. Anim Reprod Sci 61:185-197.
Schams D, Berisha B, Kosmann M, Einspanier R and Amsel-gruber WM (1999) Possible role of growth hormone, IGFs, and IGF binding proteins in the regulation of ovarian func-tion in large farm animals. Domest Anim Endocrinol 17:279-285.
Silva JRV, Figueiredo JR and van den Hurk R (2009) Review: In-volvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Therioge-nology 71:1193-1208.
Sirotkin AV, Makarevich AV, Kotwica J, Marnet PG, Kwon HB and Hetenyi L (1998) Isolated porcine ovarian follicles as a model for the study of hormone and growth factor action on ovarian secretory activity. J Endocrinol 159:313-323.
Sirotkin AV, Mertin D, Süvegová K, Makarevich AV and Miku-lová E (2003) Effect of GH and IGF-I treatment on repro-duction, growth, and plasma hormone concentrations in do-mestic nutria (Myocastor coypus). Gen Comp Endocrinol 131:296-301.
Valasi I, Leontides L, Menegatos I and Amiridis GS (2007) Oestradiol concentration as a predictor of ovarian response in FSH stimulated ewe-lambs. Anim Reprod Sci 102:145-151.
Wang WJ, Huang LS, Gao J, Ding NS, Chen KF, Ren J and Luo M (2003) Polymorphism of growth hormone gene in 12 pig breeds and its relationship with pig growth and carcass traits. Asian Austral J Anim 16:161-164.
Wu WS, Hua GH, Yang LG, Wen QY, Zhang CY, Khairy MZ and Chen SL (2009) Association analysis of theINHAgene with litter size in Boer goats. Small Rumin Res 82:139-143. Zhang CY, Chen SL, Li X, Xu DQ, Zhang Y and Yang LG (2009)
Genetic and phenotypic parameter estimates for reproduc-tion traits in the Boer dam. Livest Sci 125:60-65.
Associate Editor: Bertram Brenig