www.cbpv.com.br/rbpv
Prevalence and risk factors associated with anti-Toxoplasma
gondii
antibodies in sheep from Bahia state, Brazil
Prevalência e fatores de risco associados a anticorpos anti-
Toxoplasma gondii
em ovinos da Bahia, Brasil
Luciana Afonso Guimarães1, Rodrigo Alves Bezerra1, Daniele de Santana Rocha1, George Rêgo Albuquerque2*
1Programa de Pós-graduação em Ciência Animal, Universidade Estadual de Santa Cruz – UESC, Ilhéus, BA, Brasil 2Departamento de Ciências Agrárias e Ambientais, Universidade Estadual de Santa Cruz – UESC, Ilhéus, BA, Brasil
Received June 19, 2012 Accepted February 14, 2013
Abstract
In this study, we aimed to determine the prevalence of Toxoplasma gondii antibodies and identify risk factors associated with this infection in sheep from the southern region of Bahia state. Between February and December 2010, 795 sheep from 31 farms located in nine municipalities were tested. We found seroprevalence of 30.2% (240/795), with titers of 64 (38.3%), 256 (34.2%), 1,024 (18.3%), and 4,096 (9.2%) by Indirect Fluorescent Antibody Test (IFAT). Seropositive sheep were detected in all farms sampled. Univariate statistical analysis detected association between T. gondii seropositivity and the variables age, use of fresh food mainly, water source, stocking rate, production system, presence and number of cats on the farm, and transit of cats (p < 0.05). In the logistic regression model, transit of cats (p = 0.001), production system (p = 0.007), and age (p = 0.027) were identified as risk factors associated with
T. gondii infection.
Keywords: Epidemiology, ovine, toxoplasmosis, zoonosis, risk factors.
Resumo
Objetivou-se com este estudo determinar a prevalência de anticorpos anti-Toxoplasma gondii e identificar os fatores de risco associados à infecção em ovinos no sudeste do Estado da Bahia. De fevereiro a dezembro de 2010, 795 ovinos de 31 propriedades localizadas em nove municípios foram analisados. A soroprevalência foi de 30,2% (240/795), com títulos de 64 (38,3%), 256 (34,2%), 1.024 (18,3%) e 4.096 (9,2%) pela Reação de Imunoflorescência Indireta (RIFI). Ovinos positivos foram detectados em todas as fazendas estudadas. Na análise estatística univariada detectou-se associação entre a soropositividade e idade, uso de alimentação fresca, fonte de água, sistema de produção, presença e número de gatos na fazenda e o transito de gatos (p < 0,05). No modelo de regressão logística, transito de gatos (p = 0,001), sistema de produção (p = 0,007) e idade (p = 0,027) foram identificados como fatores de risco associados à infecção por T. gondii.
Palavras-chaves: Epidemiologia, ovino, toxoplasmose, zoonose, fatores de risco.
Introduction
Toxoplasmosis is an important parasitic zoonosis of cosmopolitan distribution caused by the protozoan Toxoplasma gondii. Humans can be infected mainly through the ingestion of cysts in contaminated food (consumption of undercooked or raw meat) or sporulated oocysts in water (FERGUNSON, 2009). Cats play a fundamental role in spreading this agent because they are the definitive hosts (DUBEY; JONES, 2008; DUBEY, 2009).
The sheep industry is an economic activity in operation in every continent with different climates. The current Brazilian sheep flock
is of approximately 16 million animals and 58.4% of these are in the northeastern region of the country. The state of Bahia is the second largest domestic sheep producer with 3,028,507 animals (BRASIL, 2009). Toxoplasmagondii is an important parasite of sheep, causing reproductive disorders such as embryonic death, abortion, stillbirth, and birth of weak animals (MASALA et al., 2003; DUBEY; JONES, 2008).
Antibodies to T. gondii have been found in sheep worldwide and seroprevalence rate ranges from 6.7% to 84.5% (KAMANI et al., 2010; KLUN et al., 2006). The seroprevalence of T. gondii
infection in flocks of sheep in Brazil ranges from 7.0% to 54.6% (MOURA et al., 2007; OGAWA et al., 2003). The only study in Bahia state with sheep was carried out in the metropolitan region of Salvador and Recôncavo, with prevalence of 18.75% (GONDIM et al., 1999).
*Corresponding author: George Rego Albuquerque
Departamento de Ciências Agrárias e Ambientais, Universidade
Estadual de Santa Cruz – UESC, Rod. Ilhéus-Itabuna, Km 16, Salobrinho, CEP 45662-000, Ilhéus, BA, Brasil
e-mail: gralbu@uesc.br
Risk factors such as age, contact with cats, breeding system, and water supply can be highlighted and have been previously identified in ovine toxoplasmosis in Brazil (ROMANELLI et al., 2007; PINHEIRO et al., 2009; LOPES et al., 2010; ROSSI et al., 2011). Considering the importance of toxoplasmosis and the lack of epidemiological information in the state of Bahia, the aims of this study were to determine the prevalence of T. gondii infection in sheep flocks from southern Bahia state and identify possible risk factors associated with the infection.
Materials and Methods
Study population and blood collection
The study was conducted from February to December 2010; it involved 795 animals from 31 farms, which are distributed in nine municipalities and enrolled in the Cooperative of Sheep and Goat Producers of Southern Bahia (COOPERVINO). A sample size of 650 animals was estimated by the statistical program EPI-INFO, version 3.5.1, considering a prevalence prospect of 50%, with sampling error of 5% and confidence level of 99% for a population of 33,000 animals (BRASIL, 2009); however, a sample size larger than required was chosen.
The herd was primarily composed of animals of the Santa Inês breed or crossbreds. The age of the animals was estimated by monitoring dentition, and the animals were categorized in two age groups based on Carneiro et al. (2009): above and below 36 months. No blood samples were collected from lambs under six months of age due to the possible presence of colostral antibodies.
Blood was collected by jugular vein puncture and the serum was separated after centrifugation and stored at –20 °C until use. The proposed study was approved by the ethics committee (CEUA-UESC 031/09).
Questionnaire
In order to determine risk factors, a structured interview with objective questions was conducted regarding the general characteristics of the farm, including the total area (≥ 100 hectares or <100 hectares); breeding system (extensive or intensive); production type (family or commercial); size of the herd (≥100 animals or <100 animals);
stocking rate (≥1 animal or <1 animal); cats owned by farmers (yes or no); transit of stray cats or wild felids (yes or no); number of cats (<3 or ≥3 cats); water source (trough or river/dam); source of water/food common to young and adult animals (yes or no); presence of pen (yes or no); cats with access to the animals’ food and/ or water storage source (yes or no); use of fresh food mainly - grass (yes or no); gender (male or female); age (<36 months or ≥36 months); and animal breed (purebred or crossbred animals).
Detection of antibodies
Indirect fluorescence antibody test (IFAT) was performed to study anti-T. gondii antibodies. In this study, IFAT slides containing antigens of the RH strain of T. gondii were used. Negative and positive control serum (1 × 106 tachyzoites of T. gondii experimentally
inoculated in a six-month-old lamb) were added to each slide and anti-sheep antibody conjugated to fluorescein isothiocyanate (F-7634, Sigma-Chemical, USA) were used. The IFAT cut-off value was 1:64 (FIGLIUOLO et al., 2004) and the positive samples were subjected to sequential dilutions until negative response.
Statistical analysis
To identify risk factors associated with infection by T. gondii, a bivariate analysis was carried out using Chi-square and Fisher’s exact tests with significance level of 5%, using the statistical program EPI-INFO, version 3.5.1. All variables with p ≤ 0.2 on bivariate analysis were subjected to collinearity analysis determined by the Spearman’s rank correlation test according to BioEstat 5.0. Subsequently, multivariate logistic regression analysis was performed using EPIINFO, version 3.5.1.
Results
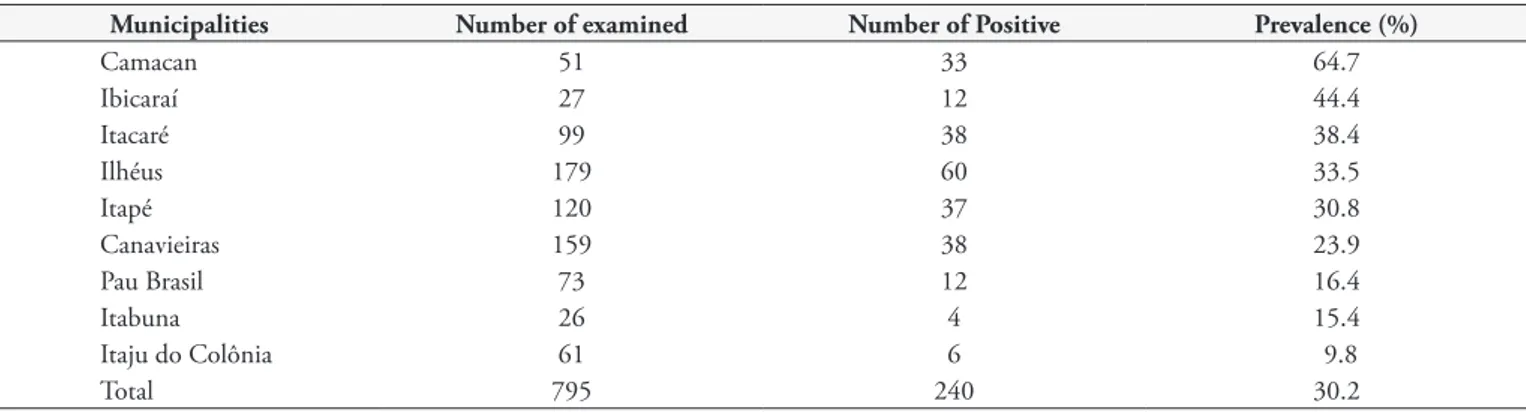
All farms used extensive breeding programs and no artificial breeding methods. Toxoplasma gondii antibodies were found in 240 (30.2%) sheep, with titers of 64 (38.3%), 256 (34.2%), 1,024 (18.3%), and 4,096 (9.2%). All farms presented T. gondii-positive animals, with seropositivity varying from 6.3% to 100%. Among the municipalities analyzed, Camacan presented the highest frequency of seropositive animals (64.7%) and Itaju do Colônia presented the lowest frequency (9.8%) (Table 1).
Table 1. Detection and distribution of anti-Toxoplasma gondii antibodies in sheep in some municipalities of the microregion Ilhéus–Itabuna, State of Bahia, Brazil.
Municipalities Number of examined Number of Positive Prevalence (%)
Camacan 51 33 64.7
Ibicaraí 27 12 44.4
Itacaré 99 38 38.4
Ilhéus 179 60 33.5
Itapé 120 37 30.8
Canavieiras 159 38 23.9
Pau Brasil 73 12 16.4
Itabuna 26 4 15.4
Itaju do Colônia 61 6 9.8
Table 2. Bivariate analysis of risk factors associated with the presence of Toxoplasma gondii in sheep flocks of southern Bahia.
Variables
Animals
χ2 P value OR CI 95%
Positive Total
n % n %
Age group 4.17 0.041 1.43 1.02-2.00
≥03 years 175 72.9 537 67.5
<03 years 65 27.1 258 32.5
Breed 0.28 0.596 0.91 0.67-1.23
Purebred 128 53.3 411 51.7
Crossbred 112 46.7 384 48.3
Gender 0.03 0.870 0.93 0.57-1.51
Male 26 10.8 90 11.3
Female 214 89.2 705 88.7
Farmers have cats 1.68 0.195 1.29 0.90-1.86
Yes 190 79.2 604 76
No 50 20.8 191 24
Quantity of cats 3.52 0.030 1.41 1.00-1.99
≥03 104 54.7 295 48.8
<03 86 45.3 309 51.2
Cats with access to the water source 0.41 0.520 0.86 0.59-1.27
Yes 140 72.5 455 74.5
No 53 27.5 156 25.5
Transit of stray cats or wild felids 0.0001 2.14 1.56-2.94
Yes 104 43.3 250 31.4
No 136 56.7 545 68.6
Production system 5.34 0.020 1.62 1.09-2.42
Familiar 56 23.3 523 65.8
Commercial 184 76.6 272 34.2
Mostly fresh food 1.40 0.235 0.81 0.58-1.12
Yes 239 99.6 781 98.2
No 1 0.4 14 1.8
Presence of Pen 2.30 0.129 0.76 0.55-1.06
Yes 185 77.1 625 78.6
No 55 22.9 170 21.4
Stocking rate 3.61 0.057 0.72 0.52-0.99
<1 140 58.3 404 50.8
≥1 100 41.7 391 49.2
Water source 1.23 0.267 0.83 0.61-1.13
Trough 123 51.3 382 48.1
River, dam 117 48.8 413 51.9
Water source common* 0.01 0.001 1.05 0.58-1.88
Yes 223 92.9 737 92.7
No 17 7.1 58 7.3
Food source common* 0.01 0.001 1.05 0.58-1.88
Yes 223 92.9 737 92.7
No 17 7.1 58 7.3
*young and adult animals; % - percentage; χ2 - Chi-square; OR - Odds ratio; CI - confidence interval.
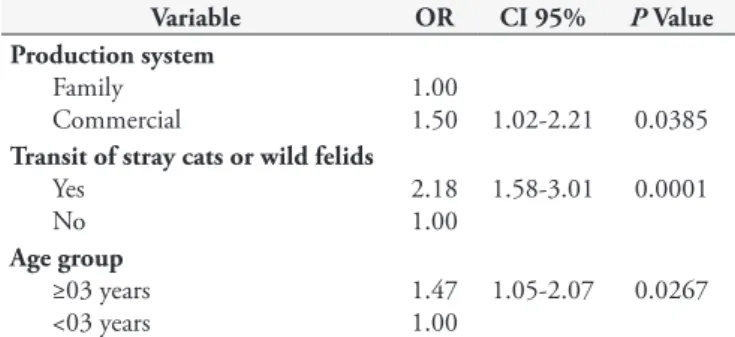
Risk factors identified by bivariate and multivariate analyses are shown in Tables 2 and 3, respectively. In the bivariate analysis, seropositivity was observed in association with age, diet based on fresh food, production system, number of cats, water source, stocking rate, and transit of stray cats or wild felids. In the multivariate analysis, age, production system, and transit of stray cats or wild felids were identified as risk factors in this study.
Regarding age, 32.6% of animals over 3 years old were identified as positive, while 25.2% of animals under 3 years old were positive (OR=1.43).
multivariate analysis, and increased the probability (OR=2.14) of sheep infection.
By the bivariate analysis, it was possible to observe that the commercial production system used in the farms presented higher seropositivity (OR=1.61) when compared to the family production system, which is possibly due to the higher ingestion of oocysts in a contaminated environment. The family production system was characterized by only the family taking care of the animals – subsistence, while the commercial production system has employees and aims at the commercialization of animals.
Discussion
Toxoplasma gondii prevalence found in this study is similar to that described by Ueno et al. (2009), in Federal District (38.2%) and by Pinheiro et al. (2009), in Pernambuco (32.9%); higher than that found by Soares et al. (2009), in Rio Grande do Norte (20.7%) and Langoni et al. (2011), in São Paulo (18.61%); and it lower than that found by Lopes et al. (2010), in São Paulo (52%) - all Brazilian states. All studies aforementioned used the IFAT for serology investigation. The differences observed may be due to climatic variations, age, type of animals and cut-off values used in the different studies.
All farms presented T. gondii-positive animals, showing that
T. gondii is widely disseminated in sheep flocks in the studied region. Among the municipalities studied, Camacan presented the highest frequency of T. gondii-positive animals (64.7%), and Itaju do Colônia (9.8%) showed the lowest frequency. Camacan and Itaju do Colônia belong to the same microregion; however, Camacan municipality has higher relative humidity (above 80% in average) due to the presence of the Atlantic Forest, which is used for cacao farming, thus favoring the maintenance of oocysts in the environment. In contrast, the municipality of Itaju de Colônia has replaced cacao farming by cattle raising and is no longer forested. It is also characterized by dry season (monthly rainfall of less than 60 mm) between the months of June and September, and higher average temperatures, which could explain the lower prevalence of T. gondii.
By multivariate statistical analysis, it was possible to observe that commercial production presented higher seropositivity (p = 0.03) compared to the family system. Commercial systems showed greater density of animals in an environment contaminated
with oocysts and, therefore, more infected animals. Caballero-Ortega et al. (2008) found that flocks with more than 320 animals had more sheep infected with T. gondii compared to flocks with fewer animals.
There was no significant association between T. gondii infection and gender, confirming the data found by Soares et al. (2009) and Pinheiro et al. (2009). This result differs from those reported by Ramzan et al. (2009) and Van Der Puije et al. (2000), who observed that females were more affected than males, which was probably due to the lower immunologic resistance of females in some periods of their lives.
With regard to age, Pinheiro et al. (2009) and Clementino et al. (2007) found results similar to those of this study. We found that seroprevalence increases with age, suggesting that most animals acquire post-natal infection and according to Dubey and Kirkbride (1989) animals over three years of age have longer exposure to sporulated oocysts and increased risk of infection.
Stray cats and wild felids are most commonly found in almost all areas where sheep are kept, thus explaining the higher seroprevalence (p ≤ 0.0001) in farms with these animals. Similar results were found by Vesco et al. (2007), Pinheiro et al. (2009), and Lopes et al. (2010). In the studied region, the presence of stray cats and wild felids is common due to the neighboring forest area for cocoa production.
Ingestion of oocysts from the environment and congenital transmission are the two main routes of T. gondii transmission in sheep. The proportion of prenatal and post-natal infection in these animals is often unknown, but epidemiological data indicate that most animals are infected after birth by horizontal transmission via ingestion of sporulated oocysts (BUXTON et al., 2006). Sporulated oocysts survive for long periods of time in favorable environmental conditions, such as humid soil (DUBEY, 2009), and this is the case of most of the municipalities studied.
The results showed the presence of T. gondii infection in sheep flocks in the microregion of Ilhéus–Itabuna, where mutton is increasingly popular as an animal protein source. As a result, it is a potential source for human toxoplasmosis. Control and prophylactic measures such as avoiding the presence of stray cats or wild felids in areas where sheep are raised, excluding this felids from where products used in sheep feed are stored, and decreasing the stocking rate of pastures should be adopted to improve the production system. Health programs for farmers, along with the dissemination of information about the means of transmission of this parasite, should be implemented.
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support.
References
Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Censo
Agropecuário. IBGE; 2009 [cited 2010 Jan. 10]. Available from: http://
www.sidra.ibge.gov.br/bda/pecua/default.asp?t=2&z=t&o=24&u1=1& u2=1&u3=1&u4=1&u5=1&u6=1&u7=1.
Table 3. Multivariate analysis of risk factors associated with the pres-ence of Toxoplasma gondii in sheep flocks of southern Bahia.
Variable OR CI 95% PValue
Production system
Family Commercial
1.00
1.50 1.02-2.21 0.0385
Transit of stray cats or wild felids
Yes No
2.18 1.00
1.58-3.01 0.0001
Age group
≥03 years <03 years
1.47 1.00
1.05-2.07 0.0267
Buxton D, Rodger SM, Maley SW, Wright SE. Toxoplasmosis: The possibility of vertical transmission. Small Rum Res 2006; 62(1-2): 43-46. http://dx.doi.org/10.1016/j.smallrumres.2005.07.037
Caballero-Ortega H, Palma JM, García-Marquez LJ, Gildo-Cardenas A, Correa D. Frequency and risk factors for toxoplasmosis in ovines of various regions of the State of Colima, Mexico.
Parasitology 2008; 135(12): 1385-1389. PMid:18801208. http://dx.doi.
org/10.1017/S0031182008004873
Carneiro ACAV, Carneiro M, Gouveia AMG, Vilas-Boas LS, Vitor RWA. Seroprevalence and risk factors of sheep toxoplasmosis in Minas Gerais, Brazil. Revue Méd Vét 2009; 160(11): 527-531.
Clementino MM, Souza MF, Andrade Neto VF. Seroprevalence
and Toxoplasmagondii-IgG avidity in sheep from Lajes, Brazil. Vet
Parasitol 2007; 146(3-4): 199-203. PMid:17418950. http://dx.doi.
org/10.1016/j.vetpar.2007.02.036
Dubey JP. Toxoplasmosis in sheep — The last 20 years. Vet
Parasitol 2009; 163(1-2): 1-14. PMid:19395175. http://dx.doi.
org/10.1016/j.vetpar.2009.02.026
Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol 2008; 38(11): 1257-1278. PMid:18508057. http://dx.doi.org/10.1016/j.ijpara.2008.03.007
Dubey JP, Kirkbride CA. Enzootic toxoplasmosis in sheep in North-Central United-States. J Parasitol 1989; 75(5): 673-676. PMid:2795369. http://dx.doi.org/10.2307/3283047
Fergunson DJP. Toxoplasma gondii: 1908-2008, homage to Nicolle, Manceaux and Splendore. Mem Inst Oswaldo Cruz 2009; 104(2): 133-148. http://dx.doi.org/10.1590/S0074-02762009000200003
Figliuolo LPC, Kasai N, Ragozo AMA, Paula, VSO, Dias RA, Souza SLP, et al. Prevalence of anti-Toxoplasma gondii and anti-Neospora
caninum antibodies in ovine from São Paulo State, Brazil. Vet
Parasitol 2004; 123(3-4): 161-166. PMid:15325042. http://dx.doi.
org/10.1016/j.vetpar.2004.06.006
Gondim LFP, Barbosa HV Jr, Ribeiro Filho CHA, Saeki H. Serological survey of antibodies to Toxoplasma gondii in goats, sheep, cattle and water buffaloes in Bahia State, Brazil. Vet Parasitol 1999; 82(4): 273-276. http:// dx.doi.org/10.1016/S0304-4017(99)00033-3
Kamani J, Mani AU, Egwu GO. Seroprevalence of Toxoplasma gondii
infection in domestic sheep and goats in Borno state, Nigeria. Trop Anim
Health Prod 2010; 42(4): 793-797. PMid:19882227. http://dx.doi.
org/10.1007/s11250-009-9488-3
Klun I, Djukovic-Djakovic O, Katic-Radivojevic SK, Nikolic A. Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep and pigs in Serbia: Seroprevalence and risk factors. VetParasitol 2006: 13(2): 121-131. PMid:16188388. http://dx.doi.org/10.1016/j.vetpar.2005.08.010
Langoni H, Greca H Jr, Guimarães FF, Ullmann LS, Gaio FC, Uehara RS, et al. Serological profile of Toxoplasma gondii and Neospora
caninum infection in commercial sheep from São Paulo State, Brazil.
Vet Parasitol 2011; 177(1-2): 50-54. PMid:21256676. http://dx.doi.
org/10.1016/j.vetpar.2010.11.024
Lopes WDZ, Dos Santos TR, Da Silva RS, Rossanese WM, De Souza FA, Rodrigues JDF, et al. Seroprevalence of and risk factors for Toxoplasma
gondii in sheep raised in the Jaboticabal microregion, São Paulo State,
Brazil. Res Vet Sci 2010; 88(1): 104-106. PMid:19589550. http://dx.doi. org/10.1016/j.rvsc.2009.06.006
Masala G, Porcu R, Madau L, Tanda A, Ibba B, Satta G, et al. Survey of ovine and caprine toxoplasmosis by IFAT and PCR assays in Sardinia, Italy. Vet Parasitol 2003; 117(1-2): 15-21. PMid:14597274. http://dx.doi. org/10.1016/j.vetpar.2003.07.012
Moura AB, Osaki SV, Zulpo DL, Marana ERM. Ocorrência de anticorpos contra Toxoplasma gondii em suínos e ovinos abatidos no município de Guarapuava, PR, Brasil. Rev Bras Parasitol Vet 2007; 16(1): 54-56. PMid:17588324.
Ogawa L, Navarro IT, Freire RL, Oliveira RC, Vidotto O. Occurrence of antibodies to Toxoplasma gondii in sheep from the Londrina Region of the Paraná State, Brazil. Semina: Cienc Agrar 2003; 24(1): 57-62.
Pinheiro JW Jr, Mota RA, Oliveira AAF, Faria EB, Gondim LFP, Da Silva AV, et al. Prevalence and risk factors associated to infection by
Toxoplasma gondii in ovine in the State of Alagoas, Brazil. Parasitol
Res 2009; 105(3): 709-715. PMid:19468755. http://dx.doi.org/10.1007/ s00436-009-1472-3
Ramzan M, Akhtar M, Muhammad F, Hussain I, Hiszczyńska-sawicka E, Haq AU, et al. Seroprevalence of Toxoplasmagondii in sheep and goats in Rahim Yar Khan (Punjab), Pakistan. Trop Anim
Health Prod 2009; 41(7): 1225-1229. PMid:19225903. http://dx.doi.
org/10.1007/s11250-009-9304-0
Rossi GF, Cabral DD, Ribeiro DP, Pajuaba ACAM, Corrêa RR, Moreira RQ, et al. Evaluation of Toxoplasma gondii and Neosporacaninum
infections in sheep from Uberlândia, Minas Gerais State, Brazil, by different serological methods. Vet Parasitol 2011; 175(3-4): 252-259. PMid:21075529. http://dx.doi.org/10.1016/j.vetpar.2010.10.017
Romanelli PR, Freire RL, Vidotto O, Marana ERM, Ogawa L, De Paula VSO, et al. Prevalence of Neosporacaninum and Toxoplasmagondii in sheep and dogs from Guarapuava farms, Paraná State, Brazil. Res Vet Sci 2007, 82(2): 202-207. PMid:17266999. http://dx.doi.org/10.1016/j. rvsc.2006.04.001
Soares HS, Ahid SMM, Bezerra ACDS, Pena HFJ, Dias RA, Gennari SM. Prevalence of anti-Toxoplasmagondii and anti-Neosporacaninum
antibodies in sheep from Mossoró, Rio Grande do Norte, Brazil. Vet
Parasitol 2009; 160(3-4): 211-214. PMid:19091473. http://dx.doi.
org/10.1016/j.vetpar.2008.10.102
Ueno TEY, Gonçalves VSP, Heinemann MB, Dilli TLB, Akimoto BM, Souza SLP, et al. Prevalence of Toxoplasma gondii and Neospora caninum
infections in sheep from Federal District, central region of Brazil. Trop
Anim Health Prod 2009; 41(4): 547-552. PMid:18726165. http://dx.doi.
org/10.1007/s11250-008-9220-8
Van Der Puije WNA, Bosompem KM, Canacoo JM, Wastling JM, Akanmori BD. The prevalence of anti-Toxoplasma gondii antibodies in Ghanaian sheep and goats. Acta Trop 2000; 76(1): 21-26. http://dx.doi. org/10.1016/S0001-706X(00)00084-X
Vesco G, Buffolano W, La Chiusa S, Mancusco G, Caracappa S, Chianca A, et al. Toxoplasmagondii infections in sheep in Sicily, southern Italy.
Vet Parasitol 2007; 146(1-2): 3-8. PMid:17383099. http://dx.doi.