CENTRO DE CIÊNCIAS
DEPARTAMENTO DE BIOQUÍMICA E BIOLOGIA MOLECULAR PROGRAMA DE PÓS-GRADUAÇÃO EM BIOQUÍMICA
RACHEL HELLEN VIEIRA DE SOUSA LIMA
PHOTOSYNTHETIC AND PHOTORESPIRATORY RESPONSES TO HIGH H2O2 ACCUMULATION TRIGGERED BY PEROXISOMAL ASCORBATE
PEROXIDASE KNOCKDOWN AND CATALASE INHIBITION IN RICE PLANTS: PHYSIOLOGICAL AND PROTEOMIC APPROACHES
PHOTOSYNTHETIC AND PHOTORESPIRATORY RESPONSES TO HIGH H2O2 ACCUMULATION TRIGGERED BY PEROXISOMAL ASCORBATE
PEROXIDASE KNOCKDOWN AND CATALASE INHIBITION IN RICE PLANTS: PHYSIOLOGICAL AND PROTEOMIC APPROACHES
Tese apresentada ao programa de pós-graduação em Bioquímica da Universidade Federal do Ceará, como requisito parcial à obtenção do título de Doutor em Bioquímica. Área de concentração: Bioquímica vegetal. Orientador: Prof. Dr. Joaquim Albenísio Gomes da Silveira (UFC).
PHOTOSYNTHETIC AND PHOTORESPIRATORY RESPONSES TO HIGH H2O2 ACCUMULATION TRIGGERED BY PEROXISOMAL ASCORBATE
PEROXIDASE KNOCKDOWN AND CATALASE INHIBITION IN RICE PLANTS: PHYSIOLOGICAL AND PROTEOMIC APPROACHES
Tese apresentada ao programa de pós-graduação em Bioquímica da Universidade Federal do Ceará, como requisito parcial à obtenção do título de Doutor em Bioquímica. Área de concentração: Bioquímica vegetal.
Aprovada em 15/02/2018.
BANCA EXAMINADORA
__________________________________________ Joaquim Albenísio Gomes da Silveira
Universidade Federal do Ceará (UFC)
__________________________________________ Fabrício Eulálio Leite Carvalho
Universidade Federal do Ceará (UFC)
__________________________________________ Claudivan Feitosa de Lacerda
Universidade Federal do Ceará (UFC)
__________________________________________ Danilo de Menezes Daloso
Universidade Federal do Ceará (UFC)
__________________________________________ Evandro Nascimento da Silva
A Deus, por cuidar de mim e guiar meus passos;
A meu esposo, Cristiano, por ser tão compreensivo, por sempre me apoiar, por torcer por mim e vibrar com minhas conquistas, por me aconselhar e me motivar quando me deixei levar pelo desânimo. Sua ajuda foi essencial para esta conquista que não é só minha, é nossa;
Aos meu pais, por tudo o que me ensinaram, por terem me dado a melhor educação que poderia receber e sempre terem me mostrado o caminho certo a seguir;
Ao meu irmão, Rafael, pela grande amizade, companherismo e apoio que sempre me deu em minhas decisões;
Ao professor Albenísio, pela excelente orientação;
Ao Yugo, por todas as vezes que me ajudou, me ouviu nos meus aperreios e me aconselhou com seu jeito tranquilo de ver a vida;
Ao Vicente, por toda a ajuda concedida na excecução dos experimentos; Aos colegas do LABPLANT, pelos bons momentos vividos e tantas ajudas a mim concedidas.
“Há pessoas que desejam saber só por saber, e isso é curiosidade; outras, para alcançarem fama, e isso é vaidade; outras, para enriquecerem com a sua ciência, e isso é um negócio torpe; outras, para serem edificadas, e isso é prudência; outras, para edificarem os outros, e isso é caridade.”
A produção de espécies reativas de oxigênio (ERO) é um processo natural em celulas vegetais. A acumulação excessiva de ERO resulta em danos em processos celulares essenciais, como fotosíntese e síntese proteica. No entanto, várias evidências têm apontado para o envolvimento de ERO em processos de sinalização. Entre elas, o peróxido de hidrogênio (H2O2) é considerado a principal molécula sinalizadora, visto que ele possui longa meia vida e pode migrar para diferentes compartimentos celulares. Diversos trabalhos destacam o papel de sinalização do H2O2 na tolerância ao estresse oxidativo. Os peroxisomos são o principal local de produção de H2O2. Por isso, há muitos antioxidantes enzimáicos e não enzimáicos presentes nessa organela e, dentre eles, a catalase (CAT) é a enzima mais importante na remoção de H2O2. A ausência de CAT resulta em diversos danos para o metabolismo vegetal, principalmente redução no crescimento e na eficiêncica da fotossíntese. O ciclo ascorbato/glutationa (ASC/GSH) é outro sistema eficaz na remoção de H2O2, por meio da enzima ascorbato peroxidase (APX). No entanto, pouco se conhece acerca do papel da isoforma peroxisomal de APX (pAPX). Já é conhecido que o silenciamento da APX4, uma isoforma de pAPX, em plantas de arroz induz menor sensibilidade a inibição da CAT, mas não é completamente compreendido o papel de APX nos peroxissomos. Portanto, abordagens proteômicas e fisiológicas foram utilizadas neste trabalho para avaliar quais sistemas antioxidantes são acionados por plantas de arroz silenciadas em APX4 com atividade de CAT inibida e quais as consequências na eficiência fotossintética. Nesssas condições plantas APX4 apresentaram elevada resiliência da fotossíntese e acumulação de proteínas do metabolismo antioxidante, principalmente as do ciclo ASC/GSH. A maior integridade da maquinaria fotosintética em plantas APX4 sob alta fotorrespiração foi associada a um maior fluxo fotorrespiratório e acumulação de proteínas antioxidantes nos cloroplastos. Em conclusão, o silenciamento de APX4 aciona um proccesso de sinalização, induzindo uma eficiente resposta antioxidante à acumulação de H2O2 peroxissomal, através de diferentes mecanismos de proteção que protegem o aparato fotossintético dos efeitos tóxicos causados por altos níveis de H2O2. Os resultados obtidos neste trabalho auxiliam no entendimento do papel da isoforma peroxisomal de APX no processo de sinalização por H2O2 peroxisomal.
Synthesis of reactive oxygen species (ROS) is a natural process in plant cells. The excessive ROS accumulation implies in damage for important cellular processes, such as photosynthesis and protein synthesis. However, several evidences have shown the role of ROS as signaling molecules. Among them, hydrogen peroxide (H2O2) is considered the main signaling molecule, since it has a relative high half-life and can migrate into different cellular compartments. Several works have reported H2O2 signaling inducing tolerance to oxidative stress. Peroxisome is the main site of H2O2 production. Many antioxidants act in the H2O2 detoxification in peroxisomes and catalase (CAT) is the most important of them. The absence of CAT results in several damages for plant metabolism by excessive H2O2 accumulation, such as strong impairment in growth and photosynthesis. The H2O2 detoxification by ascorbate/glutathione cycle, through the enzyme ascorbate peroxidase (APX), is also an important H2O2 scavenger. However, the role of peroxisomal ascorbate peroxidases (pAPX) is still unclear. It has shown that APX4 knockdown, a pAPX isoform, triggers less sensibility to CAT inhibition in rice plants. Nevertheless, it is not completely understood how pAPX knockout induces this favorable outcome. In the present study, proteomic and physiological approaches were utilized to evaluate which antioxidant systems are triggered by pAPX-knocked-down plants upon CAT inhibition and how this impacts the photosynthetic performance. High photosynthesis resilience and accumulation of protein from antioxidant metabolism, mainly the ascorbate/glutathione cycle, were observed in APX4-knocked-down plants (APX4) under inhibition of CAT. Additionally, the participation of photorespiration in photosynthesis resilience of APX4 plants was evaluated. The better photosynthesis performance of APX4 plants upon induced photorespiration was associated to enhanced photorespiratory flux and accumulation of chloroplastic antioxidant proteins. Therefore, the present thesis allows the conclusion that the deficiency of APX4 induces a previous signaling that triggers an efficient antioxidant response to peroxisomal H2O2 accumulation by different protective mechanisms. This response protects the photosynthetic apparatus against oxidative damage caused by high H2O2. The results obtained in this work expand the understanding of the role of pAPX and H2O2 signaling in peroxisomes.
1O2: Singlet oxygen 2PG: 2-phosphoglycolate 3-AT: 3-amino-1,2,4-triazol 3PG: 3-phosphoglicerate ABA: Abiscisic acid
Apot: Potential photosynthesis APX: Ascorbate peroxidase APX4: APX4-silenced rice plants ASC: Ascorbate
Asn: Asparagine Asp: Aspartate CAT: Catalase
CBC: Calvin–Benson cycle
DHAR: Dehydroascorbate reductase ETR: Electron transport rate of PSII
Fd-GOGAT: Ferredoxin-dependent glutamate synthase FNR: ferredoxin-NADP reductase
Fv/Fm: Potential quantum yield of PSII G6PDH: glucose-6-phosphate dehydrogenase GDC: Glycine decarboxylase complex
GGAT: Glutamate:glyoxylate aminotransferase Gln: Glutamine
Gln: Glutamine Glu: Glutamate
GLYK: Glycerate-3-kinase GPX: Glutathione peroxidase GR: Glutathione reductase
GS2: Chloroplastic gluthamine synthetase GSH: Glutathione
HPR: Hydroxypyruvate reductase HSP: Heat shock protein
LHCB: Light-harvesting complex B MAPK: Mitogen-activated protein kinases MDHAR: Monodehydroascorbate reductase NO: Nitric oxide
O2.-: Superoxide radical
pAPX: Peroxisomal ascorbate peroxidase PCD: Plant cell death
PGLP: 2PG phosphatase PN: Net photosynthesis Pr: Photorespiration Prx: Peroxiredoxins
PSBO: PSII subunit O protein PSBP: PSII subunit O protein PSBQ: PSII subunit Q protein PSII: Photosystem II
rbcL: Rubisco large subunit ROS: Reactive oxygen species
Rubisco: D-ribulose-1,5-bisphosphate carboxylase/oxygenase SA: Salicylic acid
sAPX: Stromal ascorbate peroxidase SGAT: Serine:glyoxylate aminotransferase SHMT: Serine-hydroxymethyl transferase SOD: Superoxide dismutase
1 CHAPTER I: LITERATURE REVIEW 13
1.1 Introduction 13
1.2 Redox metabolism in peroxisomes 14
1.3 Physiologial consequences of CAT deficiency 18
1.4 Role of asscorbate peroxidase isoforms 20
1.5 H2O2 as a signaling molecule 23
1.6 Photorespiration and its impact in photosynthesis 29
2 CHAPTER II: PEROXISOMAL APX KNOCKDOWN AND CAT
INHIBITION ENHANCE SYNTHESIS OF ANTIOXIDANT PROTEINS MITIGATING PHOTOSYNTHESIS IMPAIRMENT IN RICE
45
2.1 Introduction 47
2.2 Material and methods 50
2.3 Results 58
2.4 Discussion 64
2.5 Acknowledgements 69
3 CHAPTER III: IMPROVEMENT OF PHOTOSYNTHETIC
PERFORMANCE UNDER HIGH LIGHT CONDITION ACHIEVED BY PEROXISOMAL APX4 KNOCKDOWN RICE PLANTS AS CONSEQUENCE OF COMBINED PROTECTIVE MECHANISMS
95
3.1 Introduction 97
3.2 Material and methods 100
3.3 Results 106
3.4 Discussion 113
4 CONCLUSION 135
REFERENCES 136
OBJECTIVES
1. Understand the role of peroxisomal ascorbate peroxidase in the antioxidant metabolism.
2. Better understand the relation between photosynthesis regulation and photorespiration.
3. Indicate protection mechanisms triggered by H2O2 signaling.
1 CHAPTER I: LITERATURE REVIEW
1.1Introduction
Hydrogen peroxide is a reactive oxygen species (ROS) which in high concentration can damage biomolecules, such as DNA, lipids and proteins, inactivating key cellular functions (Gill and Tuteja, 2010; Del Río, 2015). However, H2O2 in subtle cellular levels can act as a signaling molecule for biological processes, such as development and stress perception, triggering plant tolerance to stress (Hossain et al., 2015; Corpas, 2015; Del Río, 2015). Taking into account the double role of H2O2, an efficient antioxidant system is crucial to control the H2O2 levels (Gill and Tuteja, 2010).
In C3 plants, peroxisome is the main site of H2O2 production, by the photorespiratory glycolate oxidase enzyme (GO) (Foyer and Noctor, 2003). Plants have a robust antioxidant system in peroxisomes composed by ascorbate peroxidase (APX), catalase (CAT), glutathione peroxidase (GPX) and glutathione S-transferase (GST) (Del Río et al., 2016). Among these enzymes, CAT is highlighted as the main H2O2 scavenger in the plant cell (Mhamdi et al., 2012). The function of the peroxisomal APX (pAPX) is controversial but this enzyme has been reported to act in the H2O2 signaling in pAPX knocked down rice, triggering less oxidative damage under CAT inhibition (Sousa et al., 2015).
enzymes. However, despite the eventual damages in photosynthetic apparatus originated from photorespiratory H2O2 over-accumulation, the photorespiratory flux is essential for the photosynthesis performance. Several works have shown that disruption of photorespiratory flux by suppression of photorespiratory enzymes results in decreased photosynthetic activity (Timm et al., 2012a; Dellero et al., 2015; Lu et al., 2014). In this context, this review will discuss some important issues about the antioxidant metabolism in peroxisome, the importance of CAT and pAPX in redox regulation and, finally the synergism between photorespiration and photosynthesis.
1.2Redox metabolism in peroxisomes
Peroxisomes are small organelles with diameter 0,1-1,7 μm, surrounded by a single membrane (Kaur et al., 2009). This organelle acts in important cellular processes, such as lipid metabolism (β-oxidation), hormone synthesis, glyoxylate cycle, ureides metabolism and photorespiration (Kaur et al., 2009; Hu et al., 2012). The most of these processes result in production of ROS, such as H2O2, superoxide radical (O2.-), and singlet oxygen (1O2). The peroxisome homeostasis is highly controlled by the cellular redox status (Wang et al., 2015). For example, under oxidative stress, a high H2O2 accumulation affects peroxisome proliferation and induces peroxisome aggregation (Shibata et al., 2013).
disproportionation of O2.- into H2O2 and O2. The presence of Mn-SOD, CuZn-SOD, and Fe-SOD has been demonstrated in peroxisomes of different plant species (Corpas et al., 2017). It has already been reported the presence of 1O2 in peroxisomes and, different than 1O2 generated in chloroplasts, this ROS is produced in peroxisomes by a light-independent reaction, the Haber-Weiss mechanism (Mor, et al., 2014).
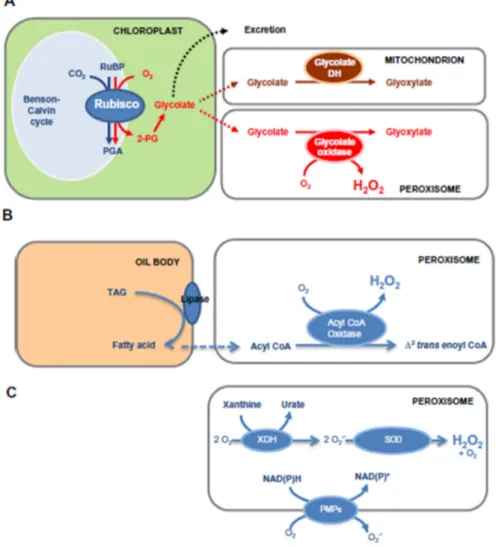
The peroxisomes are the main site of H2O2 production in plant cells. In C3 plants, the main process generating H2O2 in peroxisomes is the photorespiration, followed by the acyl-CoA oxidase in the fatty acid β-oxidation, the flavin oxidases and the spontaneous or enzymatic O2.- dismutation (Baker and Graham, 2013; DEL Río et al. 2002, Foyer et al. 2009) (Figure 1). The glycolate oxidase (GO) is a photorespiratory enzyme that catalyzes the oxidation from glycolate to glyoxylate with H2O2 production. This reaction is the main source of H2O2 in peroxisomes with about 2- and 50-fold higher rates of H2O2 production than that reported for chloroplasts and mitochondria, respectively (Foyer et al., 2009). Another enzyme involved in H2O2 production in peroxisomes is the acyl-CoA oxidase, the first enzyme of the β-oxidation which catalyzes the formation of 2-trans-enoyl-CoA using oxygen as the electron acceptor and generating H2O2 (Arent et al., 2008). Peroxisomes also contain many H2O2-producing flavin oxidases depending on the organism and tissue origins, such as enzymes of polyamine catabolism, xanthine oxidase, sulfite oxidase and sarcosine oxidase (Corpas et al., 2009; Sandalio and Romero-Puertas, 2015).
Figure 1. Some of the principal pathways of H2O2 production in plant peroxisomes. For discussion of other reactions, see text. (A) The photorespiratory pathway through peroxisomal glycolate oxidase. Other fates of glycolate, mainly described in unicellular algae, are shown on upper right. (B) b-Oxidation of fatty acids; (C) generation of H2O2 from superoxide. The xanthine dehydrogenase (XDH) reaction is shown as an example of a superoxide-generating enzyme. 2-PG, 2-phosphoglycolate. PGA, 3-phosphoglycerate. PMP, peroxisomal membrane polypeptide. RuBP, ribulose-1,5-bisphosphate. TAG, triacylglyceride. Reproduced from Mhamdi et al., (2012) - Archives of Biochemistry and Biophysics.
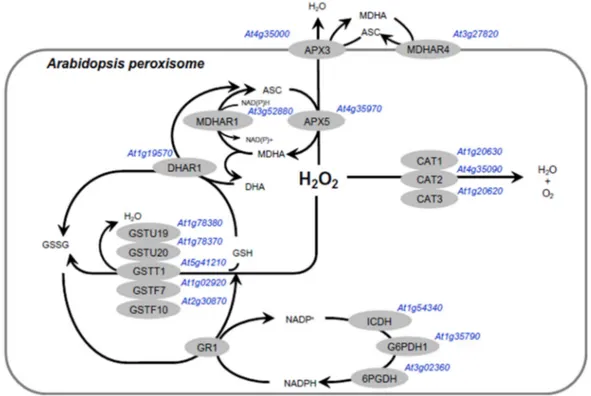
H2O2 in addition to participating in the detoxification of xenobiotic compounds and lipid peroxide products (Dixon et al., 2009). It has been reported another peroxidase, glutathione peroxidase, in leaf peroxisome of tomato plants (Kużniak and Sklodowska 2005). The presence of a protein immunorelated to peroxiredoxins (Prx) in the matrix of pea leaf peroxisomes differently regulated by oxidative stress suggests that peroxiredoxin could also be involved in the mechanism of regulation of H2O2 in peroxisomes (Corpas et al., 2017). Although peroxisomes have several H2O2-scavengers, the catalase is highlighted as the main antioxidant involved in removing H2O2 (Mhamdi et al., 2012). The figure 2 shows the major H2O2 scavenger enzymes in peroxisomes from Arabidopsis.
MDHAR, monodehydroascorbate reductase; 6PGDH, 6-phosphogluconate dehydrogenase. Reproduced from Mhamdi et al., (2012) - Archives of Biochemistry and Biophysics.
1.3Physiological consequences of CAT deficiency
The presence of CAT in peroxisomes is known since the first characterization of these organelles by De Duve and Baudhuin, (1966). Different from peroxidases which require a small reducing molecule, CAT evolves directly O2 from H2O2 (Loew, 1900). It is necessary two H2O2 molecules for the CAT reaction, where the first molecule is transformed to water and the second converted to O2 (Zamocky et al., 2008). Apart from its function in the control of H2O2, CAT also has a key role in the maintenance of cellular redox homeostasis (Mhamdi et al., 2012, Sandalio and Romero-Puertas, 2015). CAT has low affinity for H2O2, with apparent Km value in the range 40–600 mM, and is susceptible to the inhibitor 3-amino-1,2,4-triazole (Chelikani and Fita, 2004; Regelsberger et al., 2002). The molecular mass of plant catalase monomers is approximately 55 kDa (Corpas et al., 1999).
The CAT activity can be reduced for some stresses as cold or salt under light condition, drought and high light (Streb and Feierabend, 1996; Nahar et al., 2015; Hertwig and Feierabend, 1992; Schmidt et al., 2002; Hasanuzzaman and Fujita, 2011). For example, in high light stress, CAT activity can be decreased through insufficient rates of resynthesis to replace photoinactivated enzyme (Streb and Feierabend, 1996). In general, decreases in CAT activity by stress are associated with similar effects to those described in deficient mutants in CAT2, such as reduction of the glutathione redox state (Streb and Feierabend, 1996; Volk and Feierabend, 1989).
Arabidopsis cat2 mutants developed lesions day length and light intensity dependent, associated with reduced glutathione redox status (Mhamdi et al., 2010). However, under high CO2 growth, these responses are abolished, once photorespiration and hence H2O2 production are suppressed (Mhamdi et al., 2010). Deficient plants in CAT have always high levels of H2O2 (Bueso et al., 2007; Hu et al., 2010). CAT-lacking tobacco plants show bleaching and more susceptibility to diseases, while young leaves are less susceptible than the older leaves and show reduction in glutathione redox status, upon high light exposure (Willekens et al., 1997; Chamnongpol et al., 1998).
similar to those triggered by CAT knockdown. Rice plants CAT-inhibited by 3-AT showed reduced glutathione redox status, membrane damage and reduction in net photosynthesis (Sousa et al., 2015).
1.4Role of ascorbate peroxidase isoforms
Ascorbate peroxidase (APX) is a member from the plant-type heme peroxidase superfamily that reduces hydrogen peroxidase to water, using ASC (Shigeoka et al., 2002). This enzyme contains a heme prosthetic group in which iron plays an important role for the enzymatic reaction and it has been shown that iron deficiency results in reduced APX activity even with high ASC concentration (Zaharieva and Abadia, 2003). This enzyme is involved in the fine regulation of H2O2 since APX has a high affinity for H2O2, with Km values below 100 μM (Mittler and Zilinskas, 1991). The APX activity is inhibited for Cyanide and azide, once APX is a heme peroxidase (Mittler and Zilinskas, 1991).
In higher plants, APX is encoded by multigenic families. Arabidopsis contains nine APX genes, three were found to be encoded in cytosol whereas the other six were distributed in chloroplast and peroxisome (Chew et al., 2003; Mittler et al., 2004). The presence of eight isoenzymes has been confirmed in Arabidopsis, with the following localization: two in chloroplast (sAPX and tAPX), three in cytosol (APX1, APX2, APX6) and three in microsome (APX3, APX4, APX5) (Jespersen et al., 1997; Panchuk et al., 2002). Rice has eight APX genes: two cytosolic (OsAPx1 and OsAPx2), two peroxisomal (OsAPx3 and OsAPx4), three chloroplastic (OsAPx5, OsAPX7 and OsAPx8) and one mitochondrial (OsApx6) (Teixeira et al., 2004; Teixeira et al., 2006). The existence of multiple isoforms of APX within different cellular compartments indicates the important and specific role played by APXs in antioxidant defense (Ishikawa et al., 1998; Shigeoka et al., 2002).
In chloroplast, APX integrates the water-water cycle, involved in ROS production and scavenging (Asada, 1999; Shigeoka et al., 2002, Foyer and Shigeoka, 2011).In the water–water cycle, electrons excised from water at PSII are transferred to oxygen by PSI, resulting in the formation of O2.– which is subsequently converted into H2O2 by SOD and, APX, in turn, reduces H2O2 back into water (Asada, 1999). Chloroplast APXs are indispensable for photosynthetic activity and photoprotection under photooxidative stress (Kangasjärvi et al., 2008). In Arabidopsis, chloroplastic APXs have contributed for photoprotection regulating H2O2-responsive genes during photooxidative stress (Maruta et al., 2009).
The importance of APX in peroxisomes is not fully clear, whereas this organelle has CAT, an efficienty H2O2-scavenger (Mhamdi et al., 2010). However, several works have reported protective role of pAPX against stress. Transgenic plants overexpressing pAPX were significantly more tolerant to heat stress (Shi et al., 2001; Wei-Feng et al., 2008). Enhanced protection against oxidative stress was reported in tobacco plants overexpressing peroxisomal APX (Wang et al., 1999). Tabacco plants overexpressing pAPX from Halophyte salicornia have shown salt and drought stress tolerance (Singh et al., 2014).
Overexpression of a Populus peroxisomal ascorbate peroxidase (PpAPX) gene in transgenic tobacco has been reported for enhanced cellular protection against drought and salinity (Li et al., 2009). However, Narendra et al., (2006) working with
inhibition (Sousa et al., 2015). Therefore, there is no a consensus in concern the importance of peroxisomal APX isoform.
1.5H2O2 as a signaling molecule
The Hydrogen peroxide (H2O2) molecule is the result of two reductions via O.-2, and can be produced in different cellular sites by several processes. The major players in H2O2 generation in plants are the photorespiratory GO, the photosynthetic electron transport chain, the respiratory electron transport chain, and NADPH oxidases located at the plasmalemma (Noctor et al., 2017). Initially, H2O2 had been considered a toxic molecule without any function in the cellular metabolism. However, in the last decades H2O2 has been receiving highlight as an important signaling molecule (Neil et al., 2002; Maruta et al., 2012; Noctor et al., 2014).
The highest half-life (1 ms) of H2O2 among the other ROS and its small size, associated to selective reactive, ability to cross membrane through aquaporins and stability make H2O2 an ideal signaling molecule (García-Mata and Lamattina, 2013; Noctor et al., 2014). Thus, as consequence of these early mentioned properties, H2O2 can diffuse to different cellular compartments and affect protein redox status, alter its function and structure, activate or inactivate target proteins (Suzuki et al., 2012; Maruta et al., 2012; Jacques et al., 2013).
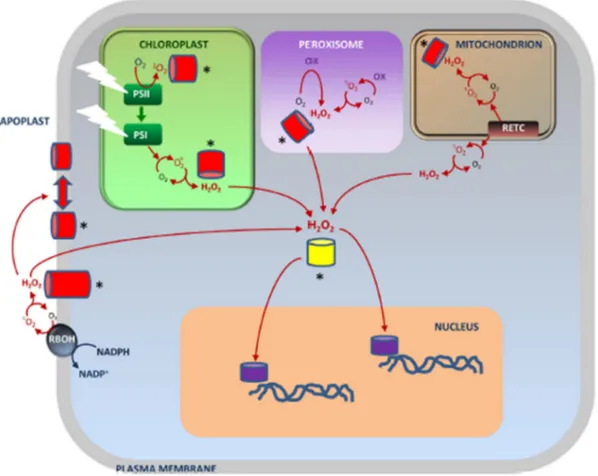
chloroplast-derived H2O2 can be detected in the nucleus, suggesting that a nuclear gene expression respond to changes in photosynthetic activity, via H2O2 signaling. The figure 3 shows schematically the different types of H2O2 signaling in the plant cell, highlighting that the signaling can be in the site of H2O2 production or the H2O2 molecule can migrate to trigger a signaling response in another compartment.
Figure 3. Integration of multiple pathways of ROS signaling in plant cells. The most stable ROS, H2O2, can move from the compartments in which it is mainly produced to alter cytosolic and nuclear redox states, which can be perceived by receptor proteins (yellow barrel). In addition, site-specific receptor systems (red barrels) may perceive singlet oxygen- or H2O2-driven redox changes more locally, leading to (in)activation of signaling networks (*). This might involve redox modifications of protein–protein interactions or second messengers. Ultimately, gene expression will be modified by altered activity of transcription factors (purple) that may not themselves be redox-modified. This extensive vocabulary of generic and site-specific signaling confers both specificity and flexibility in the redox regulation of gene expression. Reproduced from Noctor et al., (2017) - Seminars in Cell & Developmental Biology.
residues more reactive than the protonated cysteine thiol group (SH) and provide selectivity and specificity (Finkel, 2012). Thus, H2O2 oxidization of the thiolate anion to the sulfenic form (SO _) by H2O2 results on cellular signaling by changing protein conformation and activity. Recently, König et al. (2018) working with glutathione and ascorbate deficient mutants pad2 and vtc1, concluded that the light‐triggered rapid gene regulation is a glutathione‐dependent processes, independent on cellular H2O2.
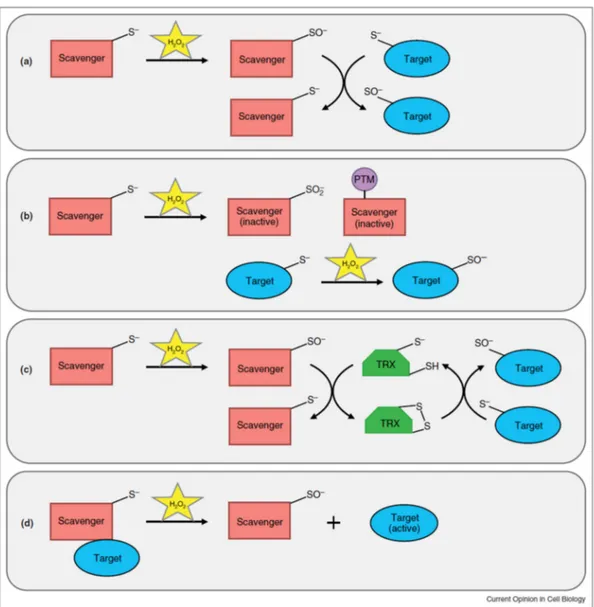
Figure 4. Possible mechanisms for H2O2-dependent signal transduction. (a) The redox relay mechanism uses a scavenging enzyme such as glutathione peroxidase (GPX) or peroxiredoxin (PRX) to transduce the H2O2 signal and oxidize the target protein. (b) With the floodgate model, H2O2 inactivates the scavenger, perhaps through hyperoxidation to sulfinic (SO2 _) acid or through a posttranslational modification (PTM), to allow for H2O2-mediated oxidation of the target protein. (c) The scavenging enzyme accepts H2O2 oxidation and transfers the oxidation to an intermediate redox protein such as thioredoxin (TRX), which subsequently oxidizes the target protein. (d) Dissociation of the target protein from the oxidized scavenging enzyme results in target protein activation. Reproduced from Reczek & Chandel, (2015) – Current Opinion in Cell Biology.
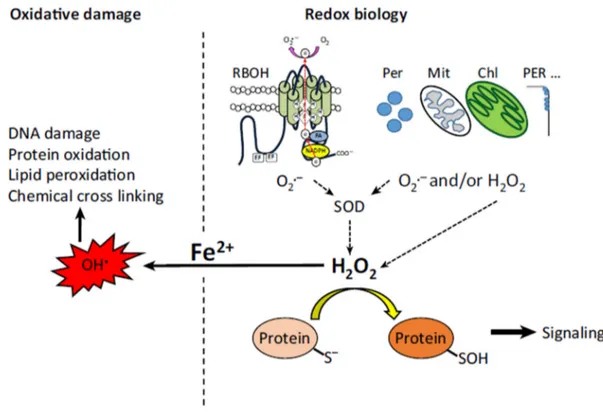
to explain (STÖKER et al., 2017). The signaling process displayed for ROS to regulate and maintain normal physiological functions mainly via interacting with cysteine (Cys) residues of proteins is called redox biology (SCHIEBER and CHANDEL, 2014; RECZEK and CHANDEL, 2015; TRUONG and CARROLL, 2013). The figure 5 shows an example of H2O2 altering the structure and function of a target protein through oxidation of Cys thiolate anions (Cys–S _) to sulfenic form (Cys–SOH).
Figure 5. ROS and Redox Biology. ROS produced by respiratory burst oxidase homologs (RBOHs), peroxisomes (Per), mitochondria (Mit), chloroplasts (Chl), and cell wall bound peroxidases (PER) result in the accumulation of H2O2 that mediates the oxidation of cysteine residues on proteins, affecting their structure and function and triggering/regulating cellular signaling pathways (Signaling). However, the presence of labile iron in the form of Fe2+ can tip the cellular balance of ROS/redox reactions and cause oxidative stress via the formation of hydroxyl radicals. Maintaining the cellular pool of labile iron as low as possible is therefore crucial for redox biology and for the regulation of metabolism and other cellular functions by ROS. Copied from Mittler, (2017) – Trends in Plant Science.
1.6Photorespiration and its impact in photosynthesis
each O2 metabolized (OGREN and BOWES, 1971). The 2PG accumulation can result in photosynthesis collapse, once this molecule is a potent inhibitor of, at least, two CB cycle enzymes: triosephosphate isomerase and phosphofructokinase (ANDERSON, 1971; KELLY and LATZKO, 1976). To salvage the 3PG from 2PG, takes place a complex process that involves more than 20 enzymes in three organelles, the photorespiration (HAGEMANN and BAUWE, 2016).
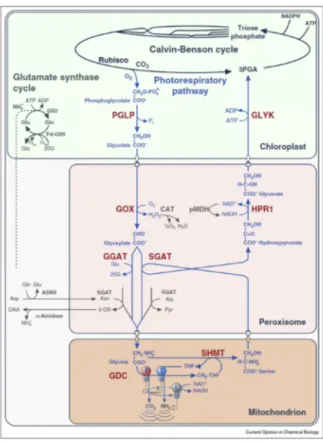
Figure 6. The photorespiratory pathway and its interconnection with photosynthetic Calvin–Benson cycle and NH3 assimilation in higher plants. (2OG, 2- oxoglutarate; 2-OS, 2-oxosuccinamate; 3PGA, 3-phosphoglycerate; Ala, alanine; Asn, asparagine; ASNS, asparagine synthetase; Asp, aspartate; CAT, catalase; FD-GltS, ferredoxin-dependent glutamate synthase; GDC, glycine decarboxylase complex; GGAT, glutamate:glyoxylate aminotransferase; Gln, glutamine; Glu, glutamate; GLYK, glycerate 3-kinase; GOX, glycolate oxidase; GS2, glutamine synthetase; HPR1, hydroxypyruvate reductase; OAA, oxaloacetate; PGLP, phosphoglycolate phosphatase; Pyr, pyruvate; Rubisco, ribulose 1,5-bisphosphate carboxylase/oxygenase; SGAT, serine:glyoxylate aminotransferase; SHMT, serine hydroxymethyltransferase.). Reproduced from Hagemann and Bauwe, (2016) – Current Opinion in Chemical Biology.
higher CO2 concentration than normal, where RuBP oxygenation becomes inhibited (Xu et al., 2009; Timm et al., 2012a; Schjoerring et al., 2006).
For example, a study with Arabidopsis knocked down in the major photorespiratory isoform of glutamate:glyoxylate aminotransferase 1 (GGT1) has shown reduced photosynthesis in consequence of glyoxylate accumulation (Dellero et al., 2015). Arabidopsis knocked out in PGLP1 displayed a strong reduction of net photosynthesis and growth even under 1% CO2, suggesting that a significant amount of 2PG is produced even in high CO2 concentration and this compound could be affecting harmfully CB cycle enzymes (Timm et al., 2012a). Glycerate is other photorespiratory metabolite with potential impact on the CB cycle since this compound inhibits sedoheptulose 1,7-bisphosphatase and fructose 1,6-bisphosphatase (Schimkat et al., 1990). Glycine chelates magnesium ions and, thus could potentially affect Calvin– Benson cycle activity (Eisenhut et al., 2007).
Timm et al., (2016) propose two hypotheses for the fact that blocked photorespiration cycle implies in photosynthesis impairment. Firstly, some photorespiratory metabolites could inhibit Calvin–Benson cycle enzymes that could inhibit RuBP regeneration and CO2 fixation and, in a feedback loop, cause a reduction of photorespiration by lower RuBP supply (Timm et al., 2012a). Secondly, with a slower response time, some photorespiratory metabolites could be involved in transcriptional and translational regulation, as was experimentally shown at least for serine and glycine (Timm et al., 2013).
Additionally, some works have been shown that overexpression of photorespiratory enzymes implies in improvement photosynthesis. Arabidopsis
GDC and rice overexpressing SHMT1 (Zhang et al., 2015) displayed improved grown higher, net photosynthesis and lowered CO2 compensation point. These results highlight the metabolic importance and the complexity of the photorespiration pathway.
References
Agrawal, G. K., Jwa, N. S., Iwahashi, H., & Rakwal, R. (2003). Importance of ascorbate peroxidases OsAPX1 and OsAPX2 in the rice pathogen response pathways and growth and reproduction revealed by their transcriptional profiling. Gene, 322, 93-103.
Agrawal, G. K., Rakwal, R., & Jwa, N. S. (2001). Stress signaling molecules involved in defense and protein phosphatase 2A inhibitors modulate OsCATC expression in rice (Oryza sativa) seedlings. Journal of plant physiology, 158(10), 1349-1355.
Anderson, L. E. (1971). Chloroplast and cytoplasmic enzymes II. Pea leaf triose phosphate isomerases. Biochimica et Biophysica Acta (BBA)-Enzymology, 235(1), 237-244.
Andersson, I., & Backlund, A. (2008). Structure and function of Rubisco. Plant Physiology and Biochemistry, 46(3), 275-291.
Arent, S., Pye, V. E., & Henriksen, A. (2008). Structure and function of plant acyl-CoA oxidases. Plant Physiology and Biochemistry, 46(3), 292-301.
Asada, K. (1992). Ascorbate peroxidase–a hydrogen peroxide‐scavenging enzyme in plants. Physiologia Plantarum, 85(2), 235-241.
Asada, K. (1999). The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual review of plant biology, 50(1), 601-639. Baker, A., & Graham, I. A. (Eds.). (2013). Plant peroxisomes: biochemistry, cell biology and biotechnological applications. Springer Science & Business Media. Barba‐Espín, G., Diaz-Vivancos, P., Job, D., Belghazi, M., Job, C., & Hernández, J. A. (2011). Understanding the role of H2O2 during pea seed germination: a combined proteomic and hormone profiling approach. Plant, cell & environment, 34(11), 1907-1919.
Chamnongpol, S., Willekens, H., Moeder, W., Langebartels, C., Sandermann, H., Van Montagu, M., ... & Van Camp, W. (1998). Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proceedings of the National
Academy of Sciences, 95(10), 5818-5823.
Chao, Y. Y., Hsu, Y. T., & Kao, C. H. (2009). Involvement of glutathione in heat shock–and hydrogen peroxide–induced cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant and soil, 318(1-2), 37.
Chelikani, P., Fita, I., & Loewen, P. C. (2004). Diversity of structures and properties among catalases. Cellular and Molecular Life Sciences CMLS, 61(2), 192-208. Chew, O., Whelan, J., & Millar, A. H. (2003). Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. Journal of Biological Chemistry, 278(47), 46869-46877.
Corpas, F. J., Barroso, J. B., Palma, J. M., & Rodriguez-Ruiz, M. (2017). Plant peroxisomes: a nitro-oxidative cocktail. Redox biology, 11, 535-542.
Corpas, F. J., Chaki, M., Leterrier, M., & Barroso, J. B. (2009). Protein tyrosine nitration: a new challenge in plants. Plant signaling & behavior, 4(10), 920-923.
Corpas, F. J., Palma, J. M., Sandalio, L. M., López-Huertas, E., Romero-Puertas, M. C., Barroso, J. B., & Del Río, L. A. (1999). Purification of catalase from pea leaf
peroxisomes: identification of five different isoforms. Free Radical Research, 31(sup1), 235-241.
De Duve, C. A. B. P., & Baudhuin, P. (1966). Peroxisomes (microbodies and related particles). Physiological reviews, 46(2), 323-357.
del Río, L. A., Corpas, F. J., Sandalio, L. M., Palma, J. M., Gómez, M., & Barroso, J. B. (2002). Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes.
Journal of Experimental Botany, 53(372), 1255-1272.
Dellero, Y., Lamothe‐Sibold, M., Jossier, M., & Hodges, M. (2015). Arabidopsis
thaliana ggt1 photorespiratory mutants maintain leaf carbon/nitrogen balance by reducing RuBisCO content and plant growth. The Plant Journal, 83(6), 1005-1018. Dixon, D. P., Hawkins, T., Hussey, P. J., & Edwards, R. (2009). Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase
superfamily. Journal of Experimental Botany, 60(4), 1207-1218.
Eisenhut, M., Bauwe, H., & Hagemann, M. (2007). Glycine accumulation is toxic for the cyanobacterium Synechocystis sp. strain PCC 6803, but can be compensated by supplementation with magnesium ions. FEMS microbiology letters, 277(2), 232-237. Exposito-Rodriguez, M., Laissue, P. P., Yvon-Durocher, G., Smirnoff, N., &
Finkel, T. (2012). From sulfenylation to sulfhydration: what a thiolate needs to tolerate.
Sci. Signal., 5(215), pe10-pe10.
Foyer, C. H., & Noctor, G. (2003). Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia plantarum, 119(3), 355-364.
Foyer, C. H., & Shigeoka, S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant physiology, 155(1), 93-100.
Foyer, C. H., Bloom, A. J., Queval, G., & Noctor, G. (2009). Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annual review of plant biology, 60, 455-484.
García-Mata, C., & Lamattina, L. (2013). Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Science, 201, 66-73. Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant physiology and biochemistry, 48(12), 909-930.
González, A., de los Ángeles Cabrera, M., Henríquez, M. J., Contreras, R. A., Morales, B., & Moenne, A. (2012). Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant physiology, 158(3), 1451-1462.
Hagemann, M., & Bauwe, H. (2016). Photorespiration and the potential to improve photosynthesis. Current opinion in chemical biology, 35, 109-116.
Hasanuzzaman, M., & Fujita, M. (2011). Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biological Trace Element Research,
143(3), 1758-1776.
Hertwig, B., Streb, P., & Feierabend, J. (1992). Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiology, 100(3), 1547-1553.
Jacques, S., Ghesquière, B., Van Breusegem, F., & Gevaert, K. (2013). Plant proteins under oxidative attack. Proteomics, 13(6), 932-940.
Jespersen, H. M., KJÆRSGÅRD, I. V., Østergaard, L., & Welinder, K. G. (1997). From sequence analysis of three novel ascorbate peroxidases from Arabidopsis thaliana to structure, function and evolution of seven types of ascorbate peroxidase. Biochemical Journal, 326(2), 305-310.
Jimenez, A., Hernandez, J. A., del Río, L. A., & Sevilla, F. (1997). Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant physiology, 114(1), 275-284.
Joo, J., Lee, Y. H., & Song, S. I. (2014). Rice CatA, CatB, and CatC are involved in environmental stress response, root growth, and photorespiration, respectively. Journal of Plant Biology, 57(6), 375-382.
Kangasjärvi, S., Lepistö, A., Hännikäinen, K., Piippo, M., Luomala, E. M., Aro, E. M., & Rintamäki, E. (2008). Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochemical Journal, 412(2), 275-285. Kaur, N., Reumann, S., & Hu, J. (2009). Peroxisome biogenesis and function. The Arabidopsis book, e0123.
Kelly, G. J., & Latzko, E. (1976). Inhibition of spinach‐leaf phosphofructokinase by 2‐ phosphoglycollate. FEBS letters, 68(1), 55-58.
König, K., Vaseghi, M. J., Dreyer, A., & Dietz, K. J. (2018). The significance of glutathione and ascorbate in modulating the retrograde high light response in Arabidopsis thaliana leaves. Physiologia plantarum, 162(3), 262-273.
Koussevitzky, S., Suzuki, N., Huntington, S., Armijo, L., Sha, W., Cortes, D., ... & Mittler, R. (2008). Ascorbate peroxidase 1 plays a key role in the response of
Arabidopsis thaliana to stress combination. Journal of Biological Chemistry, 283(49), 34197-34203.
Kużniak, E., & Skłodowska, M. (2005). Fungal pathogen-induced changes in the antioxidant systems of leaf peroxisomes from infected tomato plants. Planta, 222(1), 192-200.
Leegood, R. C., Lea, P. J., Adcock, M. D., & Häusler, R. E. (1995). The regulation and control of photorespiration. Journal of Experimental Botany, 1397-1414.
Li, Y. J., Hai, R. L., Du, X. H., Jiang, X. N., & Lu, H. (2009). Over‐expression of a Populus peroxisomal ascorbate peroxidase (PpAPX) gene in tobacco plants enhances stress tolerance. Plant Breeding, 128(4), 404-410.
Lu, Z., Liu, D., & Liu, S. (2007). Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant cell reports, 26(10), 1909-1917. Maruta, T., Noshi, M., Tanouchi, A., Tamoi, M., Yabuta, Y., Yoshimura, K., ... & Shigeoka, S. (2012). H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. Journal of Biological Chemistry, 287(15), 11717-11729.
Maruta, T., Tanouchi, A., Tamoi, M., Yabuta, Y., Yoshimura, K., Ishikawa, T., & Shigeoka, S. (2009). Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant and Cell Physiology, 51(2), 190-200.
Mhamdi, A., Hager, J., Chaouch, S., Queval, G., Han, Y., Taconnat, L., ... & Noctor, G. (2010). Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant physiology, 153(3), 1144-1160.
Mhamdi, A., Noctor, G., & Baker, A. (2012). Plant catalases: peroxisomal redox guardians. Archives of Biochemistry and Biophysics, 525(2), 181-194.
Mittler, R., & Zilinskas, B. A. (1991). Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiology, 97(3), 962-968.
Mittler, R., Vanderauwera, S., Gollery, M., & Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends in plant science, 9(10), 490-498.
Mor, A., Koh, E., Weiner, L., Rosenwasser, S., Sibony-Benyamini, H., & Fluhr, R. (2014). Singlet oxygen signatures are detected independent of light or chloroplasts in response to multiple stresses. Plant physiology, 165(1), 249-261.
Morita, S., Tasaka, M., Fujisawa, H., Ushimaru, T., & Tsuji, H. (1994). A cDNA clone encoding a rice catalase isozyme. Plant physiology, 105(3), 1015.
Mullineaux, P. M., Karpinski, S., & Baker, N. R. (2006). Spatial dependence for
hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiology, 141(2), 346-350.
Nahar, K., Hasanuzzaman, M., Alam, M. M., & Fujita, M. (2015). Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biologia plantarum, 59(4), 745-756.
Narendra, S., Venkataramani, S., Shen, G., Wang, J., Pasapula, V., Lin, Y., ... & Zhang, H. (2006). The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development.
Journal of experimental botany, 57(12), 3033-3042.
Noctor, G., & Foyer, C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annual review of plant biology, 49(1), 249-279.
Noctor, G., Mhamdi, A., & Foyer, C. H. (2014). The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiology, 164(4), 1636-1648. Noctor, G., Reichheld, J. P., & Foyer, C. H. (2017, July). ROS-related redox regulation and signaling in plants. In Seminars in cell & developmental biology. Academic Press. Ogren, W. L., & Bowes, G. (1971). Ribulose diphosphate carboxylase regulates soybean photorespiration. Nature New Biology, 230(13), 159.
Panchuk, I. I., Volkov, R. A., & Schöffl, F. (2002). Heat stress-and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in
Arabidopsis. Plant physiology, 129(2), 838-853.
Panchuk, I. I., Zentgraf, U., & Volkov, R. A. (2005). Expression of the Apx gene family during leaf senescence of Arabidopsis thaliana. Planta, 222(5), 926-932.
Petrov, V. D., & Van Breusegem, F. (2012). Hydrogen peroxide—a central hub for information flow in plant cells. AoB plants, 2012.
Pick, T. R., Bräutigam, A., Schulz, M. A., Obata, T., Fernie, A. R., & Weber, A. P. (2013). PLGG1, a plastidic glycolate glycerate transporter, is required for
photorespiration and defines a unique class of metabolite transporters. Proceedings of the National Academy of Sciences, 110(8), 3185-3190.
Pnueli, L., Liang, H., Rozenberg, M., & Mittler, R. (2003). Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic
ascorbate peroxidase (Apx1)‐deficient Arabidopsis plants. The Plant Journal, 34(2), 187-203.
Queval, G., Jaillard, D., Zechmann, B., & Noctor, G. (2011). Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant, cell & environment, 34(1), 21-32.
Reczek, C. R., & Chandel, N. S. (2015). ROS-dependent signal transduction. Current opinion in cell biology, 33, 8-13.
Regelsberger, G., Jakopitsch, C., Plasser, L., Schwaiger, H., Furtmüller, P. G., Peschek, G. A., ... & Obinger, C. (2002). Occurrence and biochemistry of hydroperoxidases in oxygenic phototrophic prokaryotes (cyanobacteria). Plant Physiology and Biochemistry,
40(6-8), 479-490.
Rodríguez‐Peña, J. M., García, R., Nombela, C., & Arroyo, J. (2010). The high‐
osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: a yeast dialogue between MAPK routes. Yeast, 27(8), 495-502.
leaves: response to abiotic stress and characterization of the peroxisomal isozyme. New phytologist, 170(1), 43-52.
Rosa, S. B., Caverzan, A., Teixeira, F. K., Lazzarotto, F., Silveira, J. A., Ferreira-Silva, S. L., ... & Margis-Pinheiro, M. (2010). Cytosolic APx knockdown indicates an
ambiguous redox responses in rice. Phytochemistry, 71(5-6), 548-558.
Sage, R. F., Sage, T. L., & Kocacinar, F. (2012). Photorespiration and the evolution of C4 photosynthesis. Annual review of plant biology, 63, 19-47.
Sandalio, L. M., & Romero-Puertas, M. C. (2015). Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Annals of botany,
116(4), 475-485.
Sandalio, L. M., Rodríguez-Serrano, M., Romero-Puertas, M. C., & Luis, A. (2013). Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. In Peroxisomes and their key role in cellular signaling and metabolism (pp. 231-255). Springer, Dordrecht.
Saxena, I., Srikanth, S., & Chen, Z. (2016). Cross talk between H2O2 and interacting signal molecules under plant stress response. Frontiers in plant science, 7.
Schieber, M., & Chandel, N. S. (2014). ROS function in redox signaling and oxidative stress. Current biology, 24(10), R453-R462.
Schimkat, D., Heineke, D., & Heldt, H. W. (1990). Regulation of sedoheptulose-1, 7-bisphosphatase by sedoheptulose-7-phosphate and glycerate, and of fructose-1, 6-bisphosphatase by glycerate in spinach chloroplasts. Planta, 181(1), 97-103.
Schjoerring, J. K., Mäck, G., Nielsen, K. H., Husted, S., Suzuki, A., Driscoll, S., ... & Bauwe, H. (2006). Antisense reduction of serine hydroxymethyltransferase results in diurnal displacement of NH4+ assimilation in leaves of Solanum tuberosum. The Plant Journal, 45(1), 71-82.
Schmidt, M., Dehne, S., & Feierabend, J. (2002). Post‐transcriptional mechanisms control catalase synthesis during its light‐induced turnover in rye leaves through the availability of the hemin cofactor and reversible changes of the translation efficiency of mRNA. The Plant Journal, 31(5), 601-613.
Sewelam, N., Jaspert, N., Van Der Kelen, K., Tognetti, V. B., Schmitz, J., Frerigmann, H., ... & Maurino, V. G. (2014). Spatial H 2 O 2 signaling specificity: H 2 O 2 from chloroplasts and peroxisomes modulates the plant transcriptome differentially.
Molecular Plant, 7(7), 1191-1210.
Shi, W. M., Muramoto, Y., Ueda, A., & Takabe, T. (2001). Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by
overexpressing in Arabidopsis thaliana. Gene, 273(1), 23-27.
Shibata, M., Oikawa, K., Yoshimoto, K., Kondo, M., Mano, S., Yamada, K., ... & Nishimura, M. (2013). Highly oxidized peroxisomes are selectively degraded via autophagy in Arabidopsis. The Plant Cell, 25(12), 4967-4983.
Shigeoka, S., Ishikawa, T., Tamoi, M., Miyagawa, Y., Takeda, T., Yabuta, Y., & Yoshimura, K. (2002). Regulation and function of ascorbate peroxidase isoenzymes.
Journal of experimental Botany, 53(372), 1305-1319.
Singh, N., Mishra, A., & Jha, B. (2014). Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Marine biotechnology, 16(3), 321-332. Sousa, R. H., Carvalho, F. E., Ribeiro, C. W., Passaia, G., Cunha, J. R., LIMA‐MELO, Y., ... & Silveira, J. A. (2015). Peroxisomal APX knockdown triggers antioxidant mechanisms favourable for coping with high photorespiratory H2O2 induced by CAT deficiency in rice. Plant, cell & environment, 38(3), 499-513.
Stöcker, S., Van Laer, K., Mijuskovic, A., & Dick, T. P. (2017). The conundrum of H2O2 signaling and the emerging role of peroxiredoxins as redox relay hubs.
Antioxidants and Redox Signaling, (ja).
Streb, P., & Feierabend, J. (1996). Oxidative Stress Responses Accompanying
Photoinactivation of Catalase in NaCI‐treated Rye Leaves. Plant Biology, 109(2), 125-132.
Suzuki, N., Koussevitzky, S., Mittler, R. O. N., & Miller, G. A. D. (2012). ROS and redox signalling in the response of plants to abiotic stress. Plant, Cell & Environment,
35(2), 259-270.
Teixeira, F. K., Menezes-Benavente, L., Galvão, V. C., Margis, R., & Margis-Pinheiro, M. (2006). Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta, 224(2), 300.
Teixeira, F. K., Menezes-Benavente, L., Margis, R., & Margis-Pinheiro, M. (2004). Analysis of the molecular evolutionary history of the ascorbate peroxidase gene family: inferences from the rice genome. Journal of Molecular Evolution, 59(6), 761-770. Timm, S., Florian, A., Arrivault, S., Stitt, M., Fernie, A. R., & Bauwe, H. (2012b). Glycine decarboxylase controls photosynthesis and plant growth. FEBS letters, 586(20), 3692-3697.
Timm, S., Florian, A., Wittmiß, M., Jahnke, K., Hagemann, M., Fernie, A. R., & Bauwe, H. (2013). Serine acts as a metabolic signal for the transcriptional control of photorespiration-related genes in Arabidopsis. Plant physiology, 162(1), 379-389. Timm, S., Mielewczik, M., Florian, A., Frankenbach, S., Dreissen, A., Hocken, N., ... & Bauwe, H. (2012a). High-to-low CO2 acclimation reveals plasticity of the
photorespiratory pathway and indicates regulatory links to cellular metabolism of
Arabidopsis. PLoS One, 7(8), e42809.
Timm, S., Wittmiß, M., Gamlien, S., Ewald, R., Florian, A., Frank, M., ... & Bauwe, H. (2015). Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of Arabidopsis thaliana. The Plant Cell, 27(7), 1968-1984.
Truong, T. H., & Carroll, K. S. (2013). Redox regulation of protein kinases. Critical reviews in biochemistry and molecular biology, 48(4), 332-356.
Volk, S., & Feierabend, J. (1989). Photoinactivation of catalase at low temperature and its relevance to photosynthetic and peroxide metabolism in leaves. Plant, Cell & Environment, 12(7), 701-712.
Wang, J., Zhang, H., & Allen, R. D. (1999). Overexpression of an Arabidopsis
peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant and Cell Physiology, 40(7), 725-732.
Wang, Y., Zhang, J., Li, J. L., & Ma, X. R. (2014). Exogenous hydrogen peroxide enhanced the thermotolerance of Festuca arundinacea and Lolium perenne by increasing the antioxidative capacity. Acta physiologiae plantarum, 36(11), 2915-2924.
Wei-Feng, X. U., Wei-Ming, S. H. I., Ueda, A., & Takabe, T. (2008). Mechanisms of Salt Tolerance in Transgenic Arabidopsis thaliana Carrying a Peroxisomal Ascorbate Peroxidase Gene from Barley1. Pedosphere, 18(4), 486-495.
Willekens, H., Chamnongpol, S., Davey, M., Schraudner, M., Langebartels, C., Van Montagu, M., ... & Van Camp, W. (1997). Catalase is a sink for H2O2 and is
indispensable for stress defence in C3 plants. The EMBO journal, 16(16), 4806-4816. Wu, G., Wang, G., Ji, J., Gao, H., Guan, W., Wu, J., ... & Wang, Y. (2014). Cloning of a cytosolic ascorbate peroxidase gene from Lycium chinense Mill. and enhanced salt tolerance by overexpressing in tobacco. Gene, 543(1), 85-92.
Xu, F. J., Jin, C. W., Liu, W. J., Zhang, Y. S., & Lin, X. Y. (2011). Pretreatment with H2O2 Alleviates Aluminum‐induced Oxidative Stress in Wheat Seedlings. Journal of integrative plant biology, 53(1), 44-53.
2 CHAPTER II: PEROXISOMAL APX KNOCKDOWN AND CAT INHIBITION ENHANCE SYNTHESIS OF ANTIOXIDANT PROTEINS MITIGATING PHOTOSYNTHESIS IMPAIRMENT IN RICE
Rachel H.V. Sousa1, Fabricio E. L. Carvalho1, Yugo Lima-Melo1,2, Vicente T.C.B Alencar1, Marcia Margis-Pinheiro3,Setsuko Komatsu4 & Joaquim A. G. Silveira1*
1Department of Biochemistry and Molecular Biology, Federal University of Ceará, Fortaleza, Ceará, Brazil.
2Molecular Plant Biology, Department of Biochemistry, University of Turku, Turku, Finland.
3Department of Genetics, Federal University of Rio Grande do Sul, Porto Alegre, Rio Grande
do Sul, Brazil.
4Graduate School of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Japan.
*Corresponding author:
Prof. J.A.G. Silveira; Departamento de Bioquímica e Biologia Molecular, Laboratório de Metabolismo de Plantas, Universidade Federal do Ceará. Av. Humberto Monte 2825, Campus do Pici, Bloco 907. Fortaleza, CP 6020, CEP 60451-970, Ceará, Brasil. Phone: +55 85 3366 9821. E-mail: silveira@ufc.br
Running title: Antioxidant protection and photosynthesis in rice.
Abstract – Signaling pathways triggered by excess of peroxisomal H2O2 and downstream oxidative changes are still not completely understood. Here we employed an integrated approach involving peroxisomal ascorbate peroxidase knockdown (RNAiOsAPX4) and CAT inhibition by 3-AT in rice plants, and performed proteomics and physiological analyses. The aim was to elucidate why RNAiOsAPX4 plants under CAT inhibition display protective mechanisms more effective to mitigate photosynthesis impairment. APX4 deficiency differently regulated several proteins, inducing a metabolic rearrangement. Higher photosynthetic efficiency displayed by RNAiOsAPX4 plants under CAT inhibition was related to higher amount andactivityof Rubisco and photochemical efficiency of PSII, which was associated with alterations in the protein profile. The remarkable increase in proteins belonging to the ascorbate-glutathione cycle and other antioxidant pathways were probably crucial for photosynthesis protection against excess ROS. Besides, these plants also accumulated other important proteins related to sugar metabolism, photorespiration, protein translation and heat shock. We propose that RNAiOsAPX4 plants exhibit a metabolic reprogramming in response to CAT inhibition, possibly induced by signaling involving H2O2 or other oxidative signals. This biochemical rearrangement triggers a more effective antioxidant system to mitigate photosynthesis impairment under CAT deficiency.
2.1 Introduction
Plant peroxisomes are the most important cellular site for H2O2 production in C3 plant species exposed to light. Several studies have revealed that redox changes in this organelle are able to affect the metabolic regulation in other cellular compartments by cross-talking mechanisms (Nyathi & Baker, 2006; Sewelam et al. 2014; Corpas, 2015). The majority of these finds have been achieved by using different CAT-deficient plants, evidencing that such responses are associated with photorespiratory H2O2 accumulation and downstream oxidative signaling events (Willekens et al. 1997; Rizhsky et al. 2002; Vandenabeele et al. 2004; Vanderauwera et al. 2011; Han et al. 2013; Rahantaniaina et al. 2017). Transcriptomic and proteomics analyses have revealed that these alterations in peroxisome metabolism are able to trigger several transcriptional and translational modifications, affecting some metabolic pathways including changes in various ribosomal and heat shock proteins (Vandenabeele et al. 2004; Vanderauwera, 2005; Vanderauwera et al. 2011).
The comprehension of how the deficiency in CAT activity and the H2O2 signaling can affect such processes is still plenty of gaps (Sousa et al. 2015). This problem has a practical importance because the majority of the common abiotic stresses can enhance the photorespiratory H2O2 production and some of them are capable of inducing CAT degradation and consequently decreasing its activity (Hertwig et al. 1992; Polidoros et al.
Arabidopsis and rice plants deficient in peroxisomal APXs have suggested minor importance for these proteins as H2O2 scavengers (Narendra et al. 2006; Sousa et al. 2015).
CAT and pAPX present very contrasting KM values for H2O2 and this fact has suggested that these peroxidases could display complementary or different metabolic roles in the peroxisomes (Mhamdi et al. 2012). In this context, pAPX could be important to keep fine-tuning and low concentrations of H2O2, allowing cellular signaling (Corpas, 2015). It has been proposed that these enzymes are externally bound to peroxisome membranes, with the catalytic site facing the cytosol (Yamaguchi et al. 1995; Kavitha et al. 2008; Ribeiro et al.
2017). This fact could be favorable for establishment of a signaling interface between peroxisomes and cytoplasm since pAPX activity could interfere in the H2O2 flux intensity from peroxisome to cytosol. Indeed, experimental evidences have demonstrated that peroxisomal H2O2 might migrate to cytosol and other neighbor organelles throughout membranes and triggers specific signals for gene expression (Mubarakshina et al. 2010; Corpas, 2015; Exposito-Rodriguez et al. 2017).
The complexity of H2O2 signaling might explain some unexpected results reported in literature. For instance, tobacco and Arabidopsis double-mutant lines deficient in CAT2 and APX1 were more resistant to paraquat-induced oxidative stress than single mutants (Rizhsky
et al. 2002; Vanderauwera et al. 2011). The authors have proposed that specific ROS signaling due to CAT and APX deficiency contributed for a more effective DNA repair system associated with cellular cycle control and suppression of the plant cell death (PCD) pathway, which resulted in higher resistance to tobacco double mutants (Vanderauwera et al.
2011). Our group has reported similar results in rice plants deficient in peroxisomal or cytosolic APX followed by pharmacological CAT inhibition (Sousa et al. 2015; Bonifacio et al. 2016). These results reinforce that physiological responses generated by signaling involving H2O2 and other ROS are very intricate since they are dependent on multifaceted metabolic and gene networks.
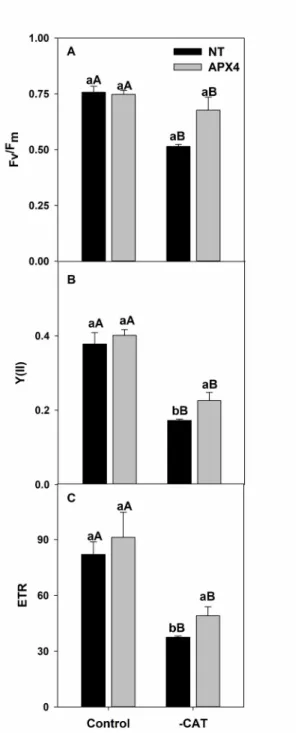
Cytosolic or peroxisomal APX-silenced rice plants exposed to CAT inhibition displayed lower ROS accumulation and higher photosynthesis resilience in comparison to CAT-inhibited NT plants (Sousa et al. 2015; Bonifacio et al. 2016). The mechanisms underlying such responses, especially a more effective antioxidant protection associated to a relative better photosynthetic performance, are still unknown to date. We have postulated that the increased constitutive-peroxisomal H2O2 due to APX deficiency is able to trigger over-expression of some antioxidant genes favoring the oxidative defense for a further oxidative stress. We have hypothesized that this mechanism is able to mitigate oxidative dangerous effects generated by CAT inhibition on the photosynthetic apparatus.
To test this hypothesis, we have performed an integrated approach employing reverse genetics by silencing of peroxisomal APX4 (RNAi-knockdown) combined with CAT pharmacological inhibition by 3-AT associated with high-throughput proteomic analysis and
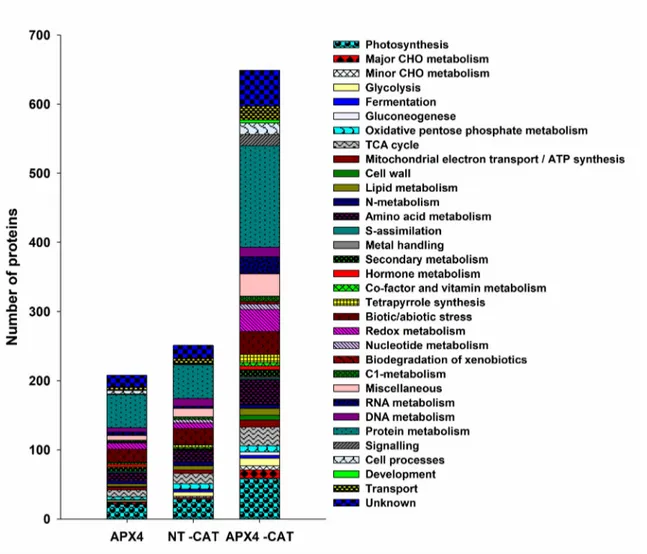
peroxisomal APX deficiency followed by CAT pharmacological inhibition triggered a strong increase in several important proteins involved with crucial metabolic processes. Especially, accumulation of proteins belonging to the ASC-GSH cycle addressed to cytosol and chloroplasts, additionally to other antioxidants and protective proteins, were important to improve photosynthetic capacity. The physiological significance of these results for photosynthesis resilience under oxidative stress is discussed.
2.2 Material and methods
Growth and treatment conditions
Knockdown rice (Oryza sativa japonica CV. Niponbare) plants deficient in peroxisomal ascorbate peroxidase 4 (RNAiOsAPX4) were obtained as previously described (Ribeiro et al. 2017). Non-transformed (NT) and RNAiOsAPX4 seedlings were transferred to 2-L pots containing Hoagland-Arnon’s nutritive solution (Hoagland & Arnon 1950) in a controlled growth chamber (day/night mean temperature of 29/24 °C, mean relative humidity of 68%, photoperiod of 12 h and 400 µmol photons m-2 s-1 PPFD) for 40 days.
control plants were sprayed with a similar 3-AT-free solution. For the short-term CAT inhibition and H2O2 accumulation experiment, 40-day-old NT and RNAiOsAPX4 leaves were sprayed once with 50 mL AT and control solutions similarly to described above and leaf sampling was performed at 0, 0.5, 1.5 and 2.5 hours after 3-AT exposure. During this time course experiment, plants were kept under moderate light regime (400 µmol photons m-2 s-1). Aiming to investigate the consequences of CAT and APX4 deficiencies on PSII activity, 40-day-old NT and RNAiOsAPX4 plants were sprayed with 10 mM 3-AT solution and kept in moderate light regime (400 µmol photons m-2 s-1) during 1 hour. Subsequently, plants were exposed to high light (1000 µmol photons m-2 s-1) for one hour and in vivo chlorophyll a fluorescence measures were performed. Controls were sprayed with a similar 3-AT-free solution.
Gas exchange measurements
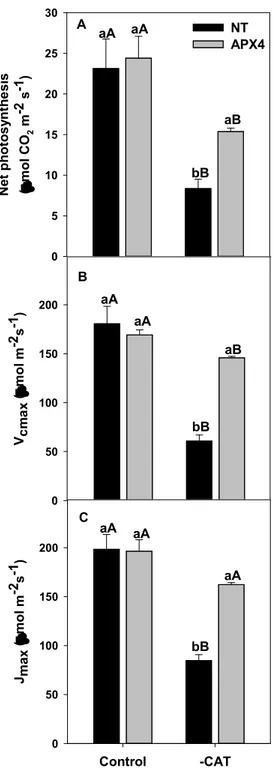
The intercellular CO2 partial pressure (CI)-dependent photosynthesis (A) curve was measured using a portable infrared gas analyzer system, equipped with an LED source and a leaf chamber (IRGA LI-6400XT, LI-COR, Lincoln, NE, USA). For the A/CI curve, the CI was varied between 50 and 1,800 ppm CO2 and the photosyntheticaly active radiation (PAR) was set as 1,000 μmol photons m-2 s-1. The maximum Rubisco carboxylation rate (Vcmax) and maximum photosynthetic electron transport rate (Jmax) were calculated from the A/CI curve (Sharkey et al. 2007).
The saturation pulse method (Schreiber et al. 1995) was used in order to evaluate the
in vivo photosystem II activity. To perform the measurements, a Dual-PAM-100 fluorometer (Walz, Germany) was employed. Initially, detached rice leaves were dark-acclimated during 30 minutes in order to assess the maximum quantum efficiency of PSII estimated as [Fv/Fm = (Fm-Fo)/Fm], where Fo and Fm represent the minimal and the maximum fluorescence after a saturation pulse in the dark, respectively. The saturation pulse intensity and duration were 8,000 μmol photons m-2 s-1 and 0.6 s, respectively. Next, the actinic light (500 μmol photons m-2.s-1) was turned on and a second saturation pulse was triggered after 3 min under this light condition, in order to assess the actual quantum efficiency estimated as [YII = (Fm’-Fs)/Fm’], where Fm’ and Fs consisted in the maximum and steady state fluorescence in the light, respectively. The electron transport ratio from PSII was estimated as [ETRII = YII×500×0.5×0.8], assuming that 80 percent of incident light reached the PSII antennas and an equal energy distribution between PSII and PSI had occurred.
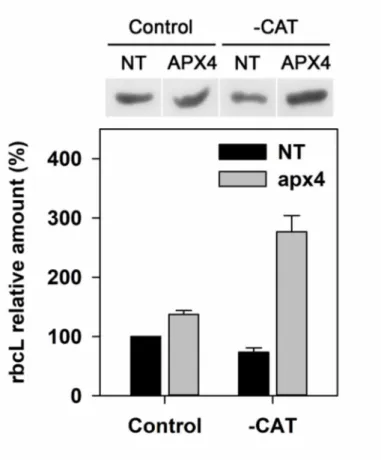
Western blot
through the reaction between the second antibody bound to the target protein and the ECL reagent (GE ref. RPN2106).
Hydrogen peroxide concentration
The H2O2 concentration was assayed by the resorufin production method (Zhou et al.
1997), using the Amplex-redTM kit. Leaf extracts were incubated with a reaction buffer containing Amplex Red (Invitrogen) and horseradish peroxidase (Sigma). After 30 min of reaction, the resorufin was quantified in spectrophotometer set 560 nm and the H2O2 concentration was estimated by a standard curve.
Catalase activity
Protein extraction, digestion and desalt for proteomic analysis
Approximately 700 mg of leaves fresh matter from each plant were ground to a powder, frozen and dried by lyophilization. The extracted proteins were precipitated in 10% trichloroacetic acid and 0.07% 2-mercaptoethanol in acetone, and resuspended in a lysis buffer containing 8 M urea, 2 M thiourea, 5% CHAPS and 2 mM tributylphosphine. The protein concentrations were determined using the Bradford method (Bradford, 1976) with bovine serum albumin as standard. The samples were purified with methanol and chloroform to remove detergent from samples and centrifuged at 20,000 × g for 10 min to achieve phase separation. The upper phase was discarded and methanol was added to the lower phase. The solutions were again centrifuged at 20,000 × g for 10 min and the resulting pellets were dried. Dried samples were reduced with 25 mM dithiothreitol and alkylated with 30 mM iodoacetamide. Alkylated proteins were digested with trypsin and lysyl endopeptidase (Wako, Osaka, Japan) at 1:100 enzyme:protein ratio at 37 C for 16 h in the dark. Peptides were acidified with 20% formic acid (pH < 3) and desalted with a C18-pipette tip (Nikkyo Technos, Tokyo, Japan). The samples were analyzed by nano-liquid chromatography with in tandem mass spectrometry (LC-MS/MS).
Protein identification using nano-liquid chromatography mass spectrometry
acquisition mode with Xcalibur software (version 2.1, Thermo Fisher Scientific). The peptide samples were loaded onto a C18 PepMap trap column (300 µm I.D. × 5 mm, Thermo Fisher Scientific) equilibrated with 0.1% formic acid and eluted from the trap column with a linear acetonitrile gradient in 0.1% formic acid at a flow rate of 200 nL min-1. The eluted peptides were loaded and separated on a C18 capillary tip column (75 µm I.D. × 120 mm, NikkyoTechnos) with a spray voltage of 1.5 kV. Full-scan mass spectra were acquired in the Orbitrap MS over 400-1,500 m/z with a resolution of 30,000. The top ten most intense precursor ions were selected for collision-induced fragmentation in the linear ion trap at normalized collision energy of 35%. Dynamic exclusion was employed within 90 sec to prevent the repetitive selection of peptides (Zhang et al. 2009).
Data acquisition by mass spectrometry analysis
automatic decoy database search was performed as part of the search. Mascot results were filtered with the Percolator function to improve the accuracy and sensitivity of peptide identification. The acquired Mascot results were imported to SIEVE software (version 2.1, Thermo Fisher Scientific).
Differential analysis of proteins using mass spectrometry data
The commercial label-free quantification package SIEVE was used for the differential analysis of relative abundances of peptides and proteins between samples. The chromatographic peaks detected by MS were aligned, and the peptide peaks were detected as a frame on all parent ions scanned by MS/MS using 5 min of frame time width and 10 ppm of frame m/z width. Chromatographic peak areas within a frame were compared for each sample, and the ratios between samples in a frame were determined. The frames detected in the MS/MS scan were matched to the imported Mascot results. The peptide ratio between samples was determined from the variance-weighted average of the ratios in frames that matched the peptides in the MS/MS spectrum. The ratios of peptides were further integrated to determine the ratio of the corresponding proteins. In the differential analysis of protein abundance, total ion current was used for normalization. The minimum requirement for identification of a protein was two matched peptides. Significant changes in the abundance of proteins between samples were analyzed (p< 0.05).
Functional analysis of identified proteins was performed using MapMan bin codes (http://mapman.gabipd.org/) (Usadel et al. 2005).
Assay of pAPX activity