w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Rapid
assessment
of
chemical
compounds
from
Phyllogorgia
dilatata
using
Raman
spectroscopy
Lenize
F.
Maia,
Rafaella
F.
Fernandes,
Mariana
R.
Almeida,
Luiz
F.C.
de
Oliveira
∗NúcleodeEspectroscopiaeEstruturaMolecular,DepartamentodeQuímica,InstitutodeCiênciasExatas,UniversidadeFederaldeJuizdeFora,JuizdeFora,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received20April2015 Accepted7September2015 Availableonline10November2015
Keywords: Phyllogorgiadilatata Octocoral Ramanspectroscopy Carotenoid Polyenal Fattyacid
a
b
s
t
r
a
c
t
ThegorgonianPhyllogorgiadilatataisendemictotheBraziliancoastwhichislistedasthreatenedwith extinction.Thisspeciesisknowntoproducesterols,mono-totetra-terpenes,conjugatedpolyenalsand peptides.Themainobjectiveofthisstudyistopresentanalternativemethodforidentificationofdifferent classesofcompoundsbaseduponaRamanmappingtechniqueusingFT-Ramanspectroscopy.TheRaman analysisperformeddirectlyonthetissues(insitu)revealedtheoccurrenceofperidinin,diadinoxanthin, conjugatedpolyenalandlinoleicacid,thatwerealsoconfirmedbyRamananalysisofpartitionedcrude extracts.Wehavedemonstratedthatthetechniquehaspotentialforuseinguidingchromatographic separationsandinprovidinginformationwithrespecttotheearlystagesofatissuenecrosisthrough “purpling”.Itmaybecomeavaluablenon-destructivetechniqueformonitoringtheaccumulationor productionofmetabolitesduringabiologicalinteraction.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
ThegorgonianPhyllogorgiadilatataisendemictotheBrazilian coastandislistedasbeingthreatenedwithextinction(MMA,2005). Theleaf-likeformisuniqueamongoctocorals,inwhichbranchesof axesanastomosetoformanetworkfilledbycoenenchyme(Castro etal.,2010).Themorphologyofthecoloniesresemblesanelephant earwheninmovementunderwater.Thebeautyofthesecolonies makesthemattractivetotouristsanddiversexploitthem com-mercially.Fromachemicalpointofview,thisspeciesisknownto producesterols,mono-totetra-terpenes,conjugatedpolyenalsand peptides(Almeidaetal.,2014).
ThefirstchemicalstudyofP.dilatataanditsassociated zoox-anthellaerevealedthepresenceof 23,24-dimethylcholesta-5,22-dien-3-ol(Kelecometal.,1980).Subsequently,theidentification of sesqui-, di- and tetra-terpenes: two nardosinanes (Kelecom etal.,1990;FernandesandKelecom,1995)andthreegermacranes sesquiterpenes(Maia,1991;MartinsandEpifanio,1998),one diter-pene(MartinsandEpifanio,1998)andtwocarotenoids(Martins andEpifanio,1998;Maiaetal.,2012),werereported.Inadditionto terpenes,P.dilatataproducesnon-methylatedconjugatedpolyenes indamagedtissues(Maiaetal.,2012,2013),andbesidessecondary metabolites,a5kDapeptidehasbeenidentified(De Limaetal.,
∗ Correspondingauthor.
E-mail:luiz.oliveira@ufjf.edu.br(L.F.C.deOliveira).
2013).Ecologicalandbiologicalactivitiesofcrudeextractsandpure compoundshavealsobeendescribed.Crudeextractshave been demonstratedtobedeterrentagainstfishinfieldassays(Epifanio etal.,1999),tohaveantifoulantpropertiesagainstbalanids(Pereira et al.,2002)and mussels (Epifanio et al.,2006)and topossess antibacterialactivity(DeLimaetal.,2013).Thefeedingdeterrence andantifouling(musselPernaperna)propertieswereascribedto thediterpene11,12-epoxycembranolide(Epifanioetal.,1999, 2006).Theantimicrobialactivity,activeagainstbacteriaknownto causehospitalinfections,wasattributedtoa5372.66Dapeptide againstbacteriaknowntocausehospitalinfections(DeLimaetal., 2013).Thecarotenoids,peridininanddiadinoxanthinproducedby the symbiotic zooxanthellae, areknown topossess antioxidant activities(Pintoet al.,2000; Lavaudet al.,2002; Dambeck and Sandmann,2014),whichmaybecrucialforcoralsthatliveinthe foticzone.
Inanattempttoperformarapididentificationofchemical con-stituentsfromP.dilatata,wehaveusedRamanspectroscopyas atechniqueforanalyzingtissuesinsitu(priortoextraction)and afterextractionwithorganicsolvents.Ramanspectroscopyisbased ontheinelasticscatteringofphotonsfrommoleculesirradiated withamonochromaticlight(alaser,forinstance),andisrelated topopulationsofthemolecularvibrationalandrotationalenergy levels.Ramanspectroscopyandthecombinationwithmicroscopy (microspectroscopy)provideinformationaboutaparticular chem-icalbond,aswellasthemolecularenvironmentandtheoverall molecularstructures.It is suitable forqualitative and quantita-tiveanalysisoftheindividualcompounds.Ithasbeenextensively
http://dx.doi.org/10.1016/j.bjp.2015.09.002
appliedtoterrestrialplantsandanimalsfortheidentificationof carotenoids(Schulzetal.,2005a;deOliveiraetal.,2010;Baranska etal.,2013),terpenes(Schraderetal.,1999;Talianetal.,2010), essentialoils(Schraderetal.,1999;Schulzetal.,2005b),fattyacids, sterols(Baetenetal.,1998;Yangetal.,2005;SchulzandBaranska, 2007), flavonoids (Schraderet al., 1999; Baranska et al., 2006; SchulzandBaranska,2007),alkaloids(SchulzandBaranska,2007; BaranskaandSchulz,2009;Baranskaetal.,2013),polyacetylenes (Schraderetal.,1999;Schulzetal.,2005b;Baranskaetal.,2013), andwoodresins(Edwardsetal.,2004),amongotheranalyticaluses. Itisalsoreadilyobviousthatthetechniqueisanon-destructive analyticalmethod,whichisveryimportantinthefieldofnatural products.
Theuseof Ramanspectroscopyfortheidentificationof nat-ural products of ecological significance from corals has been previouslyreported(Maiaetal.,2014b).Additionally,Raman anal-ysishasbeen appliedto animals,algae, and dinoflagellates for theidentification of carotenoids, sterols, nonsubstituted conju-gatedpolyenals,chromophoresofgreenfluorescentproteins(GFP), chlorophylls,melanins,mycosporine-likeaminoacids(Maiaetal., 2014b)andpolysaccharides(Bansiletal.,1978;Ellisetal.,2009; Pereiraetal.,2009).Ramanspectroscopymaybecomeavaluable non-destructivetechniqueformonitoringtheaccumulationor pro-ductionofmetabolitesduringabiologicalprocess.Thetechnique canalsobeusedtoobtaininformationwithrespecttothe distribu-tionandconcentrationofmolecularspeciesinaveryshortperiod oftimecomparedwiththeusualtimeandsolventconsuming sep-arationprocedures.
Inthepresentstudywedescribethedistributionofcompounds withinBraziliancoraltissuesbytheuseofmacro,micro-Raman andRamanimagingtechniques.Byusingthesemethodsweseek tounderstandtherelationshipbetweenthepresenceofsuch com-poundsandcolorproperties,whichcouldberelatedtodifferent stages of tissue infectionand/or inducedinflammation in coral species.
Materialsandmethods
Animalmaterial
SamplesofPhyllogorgiadilatataEsper,1806(Alcyonacea, Gor-goniidae)werecollectedinSeptember2009byscubadiversata depthof3matSacodosCardeiros,ArraialdoCabo,RiodeJaneiro coast(23◦44S–42◦02W)andidentifiedbyClovisB.Castro(UFRJ).
Extractionandfractionation
Aftercollection,thecolonieswereimmediatelyfrozenindryice. Ramanspectroscopicanalyseswereperformedinsituwithcream oroff-white,grayandpurpletissuesamplesandusingthecrude extractsmethanol/dichloromethane(MeOH:CH2Cl2)1:1,hexane, ethylacetate(EtOAc)andmethanol(MeOH)extractsaccordingto
Maiaetal.(2012).Fortheextraction,mixturesofMeOH/CH2Cl2 1:1(inthefirstwashingprocedure)andCH2Cl2(forthefollowing washingprocedures)wereused.Theextractswerecombinedand partitionedbetweenhexane/MeOH (nonpolar)andEtOAc/water (mediumpolarity)furnishingthreefurtherextracts:hexane,EtOAc and MeOH. TheEtOAc extractwas submittedtosilica gel (TLC grade)vacuumchromatographyemployingagradientof0–100% of ethylacetate in hexane, followed by methanol, affording 13 fractions(Maiaetal.,2012).Extractiontodetectthepresenceof carotenoidsinanimaltissueswasmadeaccordingtoMcGrawetal. (2005),whereasmallpieceofthetissuewasextractedand par-titionedbetweenaqueouspyridineandhexane-tert-methylbutyl ether(TBME).Sclerites,whicharethemineralcontentofskeleton
andcomposedofcalcite(CaCO3),wereisolatedaccordingtothe methodologydescribedbyLeewisandJanse(2008).
SpectroscopicanalysisandRamandataprocessing
FouriertransformRamanmeasurementswerecarriedoutusing a Bruker RFS100 instrument and a Nd:YAG laser operating at 1064nm.Theinstrumentwith4cm−1ofspectralresolutionwas
equipped with a Ge detector cooled with liquid nitrogen and coupledtoaRamanScopeIIImicroscopesystem.Good signal-to-noiseratiosformacro-Ramanspectroscopywereobtainedusing 300 and 1000 scans and a range of laser powers at the sam-ple(100–300mW).Thescatteredradiationwascollectedat180◦
geometry. The collectedspectra were processed by the Bruker Opussoftwarepackageversion6.0.Insamplessubmittedto micro-spectroscopy,thelaser is focusedonthetissue byan Olympus BX40×microscopeobjective.Micro-spectroscopyRamanimaging wasperformedby consecutiveRamanmeasurementsthatwere spatiallyresolvedpointbypointondifferentplacesinasample selectedaftervisualinspectionthroughthemicroscope.A collec-tionof Ramanspectraacrossa defined areaof thecoral tissue generatedamaprecordedonaRamanScopeIIIBrukermicroscope withanOlympusBX40×objectiveandamotorizedstagecoupled toaMultiRAMFT-Ramanspectrometerequippedwitha1064nm laserlineandagermaniumdetector.Allspectrawerecollectedin a50–4000cm−1rangewith4cm−1resolution,300scansperpoint
andthelaserpowersetat300mW.Anareaofapproximately2cm2 wasanalyzed.TheRamanimageswereconstructedinMatlab
soft-ware7.10.0(R2010a);theSimplisma(Windigetal.,1990)algorithm
wasemployedtoseparatethepurevariablesinthedataset.
Resultsanddiscussion
Predation, competition (Coll, 1992; Chiappone et al., 2003; Lesser,2004;PaulandRitson-Williams,2008;Pauletal.,2011), dis-eases(Rosenbergetal.,2007;Francini-Filhoetal.,2008;Mydlarz etal.,2008),thermalstress(Hughesetal.,2003),andcurrentflux (Lin and Dai, 1996)are biotic andabiotic agents thatinfluence growth,form,healthinessandpopulationdensityofcoralcolonies (Thompsonetal.,2015).TheoctocoralP.dilatatahasbeenfoundto bechemicallydefendedagainstdiversemarineorganisms(Almeida etal.,2014).However,theagentthatcausesthepurplingdisease inducingtissuenecrosishasnotbeenidentified(Alvesetal.,2010).
Alvesetal.(2010)havedemonstratedthatapparentlyhealthyand diseasedcoral(P.dilatata)possessavibriomicrobiotaassociatedin themucus;thisoctocoralcouldbeareservoirofpotentiallyvirulent strainsofvibrio.Ontheotherhand,itmightbeanormalcomponent oftheholobiont.Inourstudy,purplinghasbeenobservedinareas withnecrosisandinareaswithnoapparenttissuedamage. Sam-pleswereanalyzedbymeansofmacro,micro-RamanandRaman imagingspectroscopy.
Insituanalysisusingmacro-Ramanspectroscopy
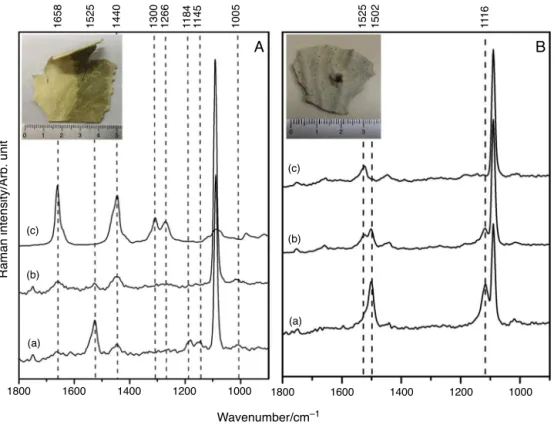
Samplesfromdifferentpartsofthecoloniesaswellasfrom differentcolonies submittedtomacro and micro-Raman analy-sis have revealed variations of chemical constituents. In Fig. 1
theinsituanalysisofapparentlyhealthycoloniesshowedmajor bandsat1525,1184,1145,1128,1020and1005cm−1addressed
tocarotenoidsand bandsattributed toCaCO3 (calcite)at1752, 1089,715cm−1(Maiaetal.,2012).Deconvolutionofthesharpband
centeredat1525cm−1 andbroadbandsat1159and1005cm−1
2000 1800 1600
1560
A
B
C
1530 15001200 1180 1160
1020
2173 1929 1537 1525 1145 1159 1021
1010 1000 1140
1400 1200 1000
Wavenumber/cm–1
Raman intensity/Arb. unit
2200 2000 1800 1600 1400 1200 1000 800 600
600 800
Fig.1. MacroRamananalysis:PanelA–insituFT-RamanspectrumfromP.dilatataanddeconvolutionforvibrationalmodesrangingfrom1560to1500cm−1,from1200to 1140cm−1andfrom1040to980cm−1;PanelB–FT-Ramanspectrumfromdiadinoxanthin;PanelC–FT-Ramanspectrumfromperidinin.
etal.,2012,2013).Theidentificationofperidinin1wasperformed byanalysisofthefingerprintbandsat1929cm−1assignedtothe
symmetricalvibrationof theallene functional group(C C C), togetherwithbandsat1750(C O),1616(C C),1525(C C), 1184ı(C H),1145ı(C H),1021ı(C CH3)(Dietzeketal.,2010;
Maiaetal.,2012).Thediadinoxanthin2spectrumshoweda char-acteristickeyacetylenebandat2173cm−1assignedtothe(C C) anddistinctbandsat15371(C C),11592(C C)and1018cm−1
ı(C CH3)(Maiaetal.,2013).
O O
O
OH AcO
OH
1
HO
O
OH
2
In situanalysis of purpletissues and purplesclerites (Fig.2
and Table 1) showed bands at 1502 (C C), 1116 (C C), 1018cm−1ı(C CH3)attributedtoconjugatedpolyenals3and addi-tionalbandsrelatedtocalcite(Maiaetal.,2012,2014a;Fernandes etal.,2015).Althoughcarotenoidsandpolyenalsareconjugated polyenes,theypresentdistinctfeatures(Fernandesetal.,2015).
The observation of the band at 1658cm−1 together with
bandsat1301and1266cm−1suggestedfattyacidsinthetissues
(Figs. 2 and 3), which were confirmed by analysis of the hex-aneextractshowingacollectionofbandsat3014( CH),2925
(CH2),2898(CH3),1743(C O),1658(C C)cis,1440ı(CH2),
1301ı(CH2),1266ı(CH)cis,1073(C C)thatcouldbeassignedto linoleicacid4bycomparisonwithliteraturedata(Meurensetal., 2005;Afsethetal.,2006;SchulzandBaranska,2007).
O
3
HO2C
4
Table1
MainobservedRamanbands(incm−1)andtentativevibrationalassignmentsfrominsituanalysisoftissues,crudeextractsandfractionsfromPhyllogorgiadilatatawithlaser excitationat1064nminmacroscopicmode.
Off-whitetissue Purpletissue Hexaneextract Hex/TBMEextract EtOAcextract Peridinin Diadinoxanthin Tentativeassignment
2173m (C C)
1930w 1929w (C C C)
1750w 1750 1743w 1754w 1752w (C O)
1662m 1658s 1658s 1656w 1654w (C C)/(C O)
1612w 1615w 1616w (C C)
1525s 1502s 1530m 1525s 1525s 1537s (C C)
1445m 1440s 1440m 1448m 1450w 1446m ı(C H)
1301m 1301m ı(CH2)
1266w 1266m 1266m ı(C H)
1184m 1184m 1184m ı(C H)
1159m 1118s (C C)
1147m 1145m 1145m 1159 ı(C H)
1089s 1089s 1(CO3)a
1073w 1068w (C C)
1020w 1014w 1020w 1021w 1018w ı(C CH3)
1005w 1004w ı(C CH3)
716m 715m 4(CO3)a
1800 (d)
1525 1502 1184 1145 111
6
1089 1018 715
(c)
(b)
(a)
1600 1400 1200
Wavenumber/cm–1
Raman intensity/Arb. unit
1000 800 600
Fig.2. MacroRamananalysis:(a)FT-Ramanspectrumfrompurplesclerites;(b) purpletissue;(c)colorlesssclerites;(d)P.dilatatatissueinsitu.
Thisspectral pattern wasalsoobserved inthehexane/TBME extract (Fig. 3 and Table 1) and the fraction was eluted with hexane/EtOAc7:3and6:4fromtheEtOAccrudeextract(Fig.4). Thehexane/TBMEextractwaspreparedinordertodeterminethe presenceofcarotenoidsinthetissuesbyasimplechemicaltestas proposedbyMcGrawetal.(2005);however,thisproceduredid notyieldcarotenoidsbutresultedintheisolationoffattyacids.The fractionelutedwithhexane/EtOAc2:8showedmajorRamanbands at1650–1611(C C),1445ı(CH2),1380ı(CH),whichis charac-teristicof terpenes(Daferera et al.,2002; Schulzand Baranska, 2007).Increasingthegradientofthehexane/ethylacetateto1:9, andto100%ethylacetate,resultedintheobservationofRaman bandsat2171(C C),1930(C C C),1747(C O),1544(C C), 1451 ı(CH), 1211, 1187 ı(CH), 1157 ı(CH3), 1020 ı(CH), 941 (C O)(Dietzek etal.,2010;Maiaetal.,2012,2013)thatcould beassignedtoamixtureofperidininanddiadinoxanthin(Fig.4).
1800 (i)
(h)
(g) (f)
1660 1611 1537 1525 1300 1266 1186 1159 1144 1020
(b) (c) (d) (e)
(a)
1600 1400 1200
Wavenumber/cm–1
Raman intensity/Arb. unit
1000
Fig.4.MacroRamananalysis:FT-Ramanspectraofthe(a)ethylacetatefraction; (b)hex/ethylacetate1:9fraction;(c)hexane/ethylacetate2:8fraction;(d) hex-ane/ethylacetate3:7;(e)hexane/ethylacetate6:4fraction;(f)hexane/ethylacetate 7:3fraction;(g)ethylacetateextract;(h)hexaneextractand(i)MeOH/CH2Cl21:1 extract.
However,thepresenceofthesebandscouldalsobeattributedtoa mixtureofdifferentxanthophyllswithsimilarchemicalstructures oracompoundthatbearsalleneandacetylenefunctionalgroups commontodinoflagellates(Johansenet al.,1974; Maoka etal., 2002;DembitskyandMaoka,2007).
Insituanalysisusingmicro-Ramanspectroscopy
Micro-Ramanspectroscopywasusedtoobtaintheinsitu spec-traofspecificregionsofthetissues.Coloniespresentingdark(gray) andclear(off-white)colorationshoweddistinctRamanspectra: thedarkregionsrevealedmajorbandsthatwereassignedtothe carotenoidperidinin1(1525, 1616,1446,1180and1145cm−1)
3100 (d)
(c)
(b)
(a)
3000 2900
A
B
1320130012801260
3014 2925 2898 2852 1658 1440 1089 1073
2800 2700
Wavenumber/cm–1
Raman intensity/Arb. unit
1800 1600 1400 1200 1000 800
1800 (c)
0 1 2 3 4 5 0 1 2 3
(b)
(a)
(c)
(b)
(a)
1600 1400
A
B
1658 1525 1440 1300 1266 1184 1145 1005 1525 1502 111
6
1200 1000
Wavenumber/cm–1
Raman intensity/Arb. unit
1800 1600 1400 1200 1000
Fig.5.MicroRamananalysis:PanelA:insituRamanspectrafromPhyllogorgiadilatatatissues.(a)Darkregion;(b)whiteregionand(c)Ramanspectrumoflinoleicacid. PanelB:insituRamanspectrafromP.dilatatatissues.(a)Purpletissue;(b)purpleandoff-whitetissueand(c)off-whitetissue.
andbandsattributedtoCaCO3(calcite)(1752,1089and715cm−1). Theoff-whiteportionrevealedcalcitevibrationalmodestogether withmajorRaman bandsattributed tofattyacids (1658,1450, 1300–1200cm−1)andminorbandscharacteristicofcarotenoids
(1527,1186,1159,1016,1020cm−1).Tissuescontaininginjuries
and purplepigmentationwerealsoinvestigated (Fig. 5).Insitu irradiationonsitepresentingoriginalcolor(offwhiteorcream) showedtypicalbandsofperidinin1at1525,1446,1180,1145cm−1.
Injuredpurpletissueshowedbandsat1500,1118and1017cm−1
thatwereidentifiedastheconjugatedpolyenal3.Theregionsof tis-suewherethepurplepigmentshowsupasapurpleshadowshowed bandsattributedtobothclassofcompounds(carotenoidsand con-jugatedpolyenals) (Fig.5).Polyenals and carotenoidsarelinear conjugatedpolyenesthathavebeencharacterizedbythe analy-sisofvibrationalbandsattributedto(C C),(C C)andı(C CH3) modes.Therearethreemaindifferencesbetweenpolyenalsand carotenoids observed by Ramanspectroscopic analysis: (1) the red-shiftedwavenumberpositionofthe(C C)stretchingmode ofpolyenalsbyca.30cm−1whencomparedwithcarotenoids,(2)
theabsenceinpolyenalsatresonanceconditionsofthe deforma-tionmoderelated totheı(C CH3)groupatca.1000cm−1,and
(3)thepresenceofı(C CH3)atca.1020cm−1(Maiaetal.,2014a;
Fernandeset al.,2015).Thewavenumberpositionof the(C C) stretchingvibrationsmaybeinfluencedbythelengthofthe conju-gatedcarbon–carbonchain,bythesubstitutionpatterninthechain, andbytheinteractionwithotherchemicalconstituentspresentin thebiologicalmatrix(Schulzetal.,2005a;deOliveiraetal.,2010, 2011).
DiscriminatingcompoundsbyRamanmicro-imaging
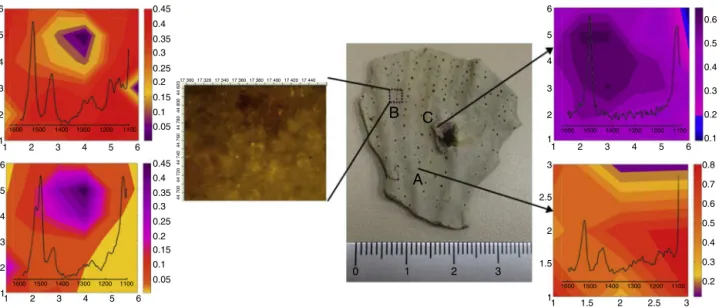
The Raman mapping was applied for the assessment of carotenoidsandpolyenalsdistributionacrossthesurfaceofcoral tissuespresentingornotvariationsincolorationpattern(Fig.6).For
thispurpose,measurementsweredonepointbypointinanareaof 160m×120mandtheRamanimagesweregeneratedbymeans ofRamanintensitiesfromcarotenoidsandpolyenals.Ascanbe seeninFig.6,gradientofcolorationinchemicalmapsiscorrelated withtheintensityofRamanbandsindicatingtherelativecontent ofeachsubstancepresentinthetissue.TheRamanimageofthe areaAobtainedfromoff-whitecoloniesapparentlyhealthyshowed Ramanmajorbandsat1526,1446,1184,1145cm−1characteristic
ofperidinin1coloredaccordingtobandintensities(Fig.6A);the darkorangecolorrepresentstheregionwithhighestamountofthe carotenoidandtheyellowcolorrepresentstheregionwithlowest contentofcarotenoid.RamanimagesfromtheareaB,where pur-plepigmentationco-occurswithundamagedoff-whitetissues,and showedthepresenceofbothcarotenoidsandpolyenals(Fig.6B) due to bands at 1526, 1184, 1145cm−1 and 1502, 1118cm−1,
respectively.Themapshowedareaswithgradientsoforangeto yellowcolorsaddressedtocarotenoidsandareaswithpinkto pur-plecolorsaddressedtopolyenals.TheRamanimageofthearea Cbelongstothedamagedtissue,whichshowedRamanbandsat 1502and1118cm−1 typicalofpolyenals,representedbypurple
color(Fig.6C).
6 0.45 0.35 0.25 0.15 0.05 0.4 0.3 0.2 0.1 0.45 0.35 0.25 0.15 0.05 0.4 0.3 0.2 0.1 5 4 3 2 1 6 5 4 3 2 1 1 2
1600 1500 1400 1300 1200 1100
1600 1500 1400 1300 1200 1100
3 4 5 6
1 2 3 4 5 6
1600 1500 1400 1300 1200 1100 1600 1500 1400 1300 1200 1100
1 1.5
0 1 2
A
B
C
3
2 2.5 3 1 1.5 2.5 2 3 1
1 2 3 4 5 6 2 3 4 5 6 0.6 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.5 0.4 0.3 0.2 0.1
Fig.6. InsituRamanimaginganalysisfromPhyllogorgiadilatatatissues:(A)off-whitetissue;(B)purpleandoff-whitetissueand(C)purpletissue.Thechemicalmapsare coloredaccordingtotheintensityofRamanbandscharacteristicforcarotenoid(1and2),polyenal(3)andcarotenoidandpolyenalcompounds(1,2and3).
Onthe otherhand,carotenoidsare produced bythe photosyn-thetic endosymbionts which provide nutrients tothe host and enablethemtosurviveinoligotrophicenvironment(Fujiseetal., 2014).The reduction orabsenceof carotenoid Ramanbands in
P.dilatatatissues mayindicateexpulsionof thesymbiontfrom
the host or/and a decrease in production of these compounds in the symbiont; both hypotheses maycompromise the main-tenanceandsurvival ofthecolony. Studiesperformedwiththe phytoplanktonic coccolithophoridEmiliania huxleyi have shown theactivityofthediadinoxanthin2cycle(conversionof diatox-anthinindiadinoxanthin)occurringinresponsetoviralinfection (Llewellynetal.,2007).Variationintheconcentrationof individ-ualcarotenoidswasobserved,suchasanincreaseofdiatoxanthin relativetochlorophyll-aversusadecreaseindiadinoxanthinand
-carotenecontent(Llewellynetal.,2007).Differencesinpattern
ofcolorationandchemicalcompositionobservedintissuesamples presentingdark(gray)andnonpigmented(white)areas(Fig.5) may suggest that the apparent bleached colony contained the endosymbiontduetothepresenceofminorbandsofcarotenoids; the predominance of fatty acid bands in the non-pigmented portionmaybe attributedto the animal metabolism or dietary input.
It has been straightforwardly to noticed that Raman maps obtainedfromdamagedtissuehaveshownbandsattributed exclu-sivelytopolyenals,accordingtoFig.6C;thiscanberationalized asfollows:coralproducespigmentsaspolyenalsintothesclerites asaninflammatoryresponsetoaninvadingagent;purplesclerites maybepositionedonorunderneathcolorlessscleriteswhichin
P.dilatatahavenopigmentsincecarotenoidsarenotembeddedin
calcitematrix(Fig.2).Mappingperformedinoff-whitetissueswith purpleshadowsclearlyshowedthepurplescleritesemergingfrom thesurfaceofcoralpriortothenecrosis.
Intheexperimentperformedwithdoublecoloredtissue,dark regionshowedthepresenceofcarotenoidbands,whereasthewhite regionshowedthesamecompoundswithdifferentspectral pat-tern.Theconclusionthatcanbeachievedfromthisfactisveryclear, healthy tissues present carotenoid in its composition, whereas damagedornon-pigmentedtissuesdonotshowsuchchemicals analyzedbyRamanspectroscopydespitetheagent:bioticor abi-otic.
ThesedatahaveindicatedthatRamanspectroscopyissuitable to detect variations in the chemical composition of P.dilatata
tissues and thetechnique is ableto provideinformation about earlystagesofatissueinfectionand/oranyotheragentthatinduce inflammation.The analysisof thefractionedcrude extractmay have a potential use in guiding chromatographic and spectro-scopicstudies.ItisexpectedthatdifferentRamanspectroscopic approaches(individualRamanspectra,Raman-mapping,the com-binationofchromatographicseparationandRaman)canbeapplied tosimultaneousandrapidstudyinsituofchemicalcompounds frommarineorganism.Summingup,Ramandata,mainlybased onmicro-spectroscopyRamanimaging,haveclearlyshownthat healthytissuesofP.dilatatapresentverystrongbandsassignedto carotenoidsindicatingtheassociationwiththedinoflagellate sym-biont.DamagedtissuesdonotpresentRamanbandsaddressedto carotenoids,meaningthatthehosthaslostthesourceofnutritional inputfromthesymbiontleavingcoralcoloniesmoresusceptible todiseaseandmortality.Itcouldbeunderstoodasatypical finger-printofthecoralhealthcondition:healthytissuespresentRaman spectrum containing carotenoids bands, in bleached or dam-agedtissues,andRamanspectrumisdevoidoftypicalsymbiont compounds.
Authors’contributions
LFM(Postdoc)contributedinrunningthelaboratorywork, anal-ysisofthedataanddraftingthepaper.RFF(PhDstudent)performed thespectroscopy analysisand elaboration of figures.MRA con-tributedtospectroscopyanalysisandRamanimagesprocessing. LFCOsupervisedthelaboratoryworkandcontributedtocritical reading of themanuscript. Allthe authors have read the final manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
research.Thisworkisacollaborativeresearchprojectwith mem-bersofRedeMineiradeQuímica(RQ-MG)supportedbyFAPEMIG (Project:CEX-RED-0010-14).
References
Afseth,N.K.,Wold,J.P.,Segtnan,V.H.,2006.ThepotentialofRamanspectroscopyfor characterisationofthefattyacidunsaturationofsalmon.Anal.Chim.Acta572, 85–92.
Almeida,M.T.R.,Moritz,M.I.G.,Capel,K.C.C.,Pérez,C.D.,Schenkel,E.P.,2014. Chem-icalandbiologicalaspectsofoctocoralsfromtheBraziliancoast.Rev.Bras. Farmacogn.24,446–467.
AlvesJr.,N.,Neto,O.S.M.,Silva,B.S.O.,DeMoura,R.L.,Francini-Filho,R.B.,Barreirae Castro,C.,Paranhos,R.,Bitner-Mathé,B.C.,Kruger,R.H.,Vicente,A.C.P., Thomp-son,C.C.,Thompson,F.L.,2010.Diversityandpathogenicpotentialofvibrios isolatedfromAbrolhosBankcorals.Environ.Microbiol.Rep.2,90–95. Baeten,V.,Hourant,P.,Morales,M.T.,Aparicio,R.,1998.Oilandfatclassificationby
FT-Ramanspectroscopy.J.Agric.FoodChem.46,2638–2646.
Bansil,R.,Yannas,I.V.,Stanley,H.E.,1978.Ramanspectroscopy:astructuralprobe ofglycosaminoglycans.Biochim.Biophys.Acta541,535–542.
Baranska,M.,Roman,M.,Dobrowolski,J.C.,Schulz,H.,Baranski,R.,2013.Recent advances in Raman analysis of plants: alkaloids, carotenoids, and poly-acetylenes.Curr.Anal.Chem.9,108–127.
Baranska,M.,Schulz,H., 2009.Chapter 4.Determinationofalkaloidsthrough infraredandRamanspectroscopy.In:Geoffrey,A.C.(Ed.),TheAlkaloids: Chem-istryandBiology.AcademicPress,pp.217–255.
Baranska,M.,Schulz,H.,Joubert,E.,Manley,M.,2006.Insituflavonoidanalysis byFT-Ramanspectroscopy:identification,distribution,andquantificationof aspalathiningreenrooibos(Aspalathuslinearis).Anal.Chem.78,7716–7721. Castro,C.B.,Medeiros,M.S.,Loiola,L.L.,2010.Octocorallia(Cnidaria:Anthozoa)from
Brazilianreefs.J.Nat.Hist.44,763–827.
Chiappone,M.,Dienes,H.,Swanson,D.W.,Miller,S.L.,2003.Densityandgorgonian host-occupationpatternsbyFlamingoTonguesnails(Cyphomagibbosum)inthe Floridakeys.Caribb.J.Sci.39,116–127.
Coll,J.C.,1992.Thechemistryandchemicalecologyofoctocorals(Coelenterata, Anthozoa,Octocorallia).Chem.Rev.92,613–631.
Daferera,D.J.,Tarantilis,P.A.,Polissiou,M.G.,2002.Characterizationofessentialoils fromLamiaceaespeciesbyFouriertransformRamanspectroscopy.J.Agric.Food Chem.50,5503–5507.
Dambeck,M.,Sandmann,G.,2014.Antioxidativeactivitiesofalgalketocarotenoids actingasantioxidativeprotectantsinthechloroplast.Photochem.Photobiol.90, 814–819.
DeLima,L.A.,Migliolo,L.,Castro,C.B.,DeOliveiraPires,D.,López-Abarrategui,C., Gonc¸alves,E.F.,Vasconcelos,I.M.,DeOliveira,J.T.A.,DeJesúsOtero-Gonzalez,A., Franco,O.L.,Dias,S.C.,2013.Identificationofanovelantimicrobialpeptidefrom BraziliancoastcoralPhyllogorgiadilatata.ProteinPeptideLett.20,1153–1158. deOliveira,V.E.,Almeida,E.W.C.,Castro,H.V.,Edwards,H.G.M.,DosSantosH.F.,
deOliveira,L.F.C.,2011.Carotenoidsand-cyclodextrininclusioncomplexes: Raman spectroscopy and theoreticalinvestigation. J. Phys. Chem. A 115, 8511–8519.
deOliveira,V.E.,Castro,H.V.,Edwards,H.G.M.,deOliveira,L.F.C.,2010.Carotenes andcarotenoidsinnaturalbiologicalsamples:aRamanspectroscopicanalysis. J.RamanSpectrosc.41,642–650.
Dembitsky,V.M.,Maoka,T.,2007.Allenicandcumuleniclipids.Prog.LipidRes.46, 328–375.
Dietzek,B.,Chábera,P.,Hanf,R.,Tschierlei,S.,Popp,J.,Pascher,T.,Yartsev,A.,Polívka, T.,2010.Optimalcontrolofperidininexcited-statedynamics.Chem.Phys.373, 129–136.
Edwards,H.G.M.,DeOliveira,L.F.C.,Prendergast,H.D.V.,2004.Ramanspectroscopic analysisofdragon’sbloodresins–basisfordistinguishingbetweenDracaena (Convallariaceae),Daemonorops(Palmae)andCroton(Euphorbiaceae).Analyst 129,134–138.
Ellis,R.,Green,E.,Winlove,C.P.,2009.Structuralanalysisofglycosaminoglycansand proteoglycansbymeansofRamanmicrospectrometry.Connect.TissueRes.50, 29–36.
Epifanio,R.,Martins,D.,Villac¸a,R.,Gabriel,R.,1999.Chemicaldefensesagainstfish predationinthreeBrazilianoctocorals:11,12-epoxypukalideasafeeding deterrentinPhyllogorgiadilatata.J.Chem.Ecol.25,2255–2265.
Epifanio,R.d.A.,daGama,B.A.P.,Pereira,R.C.,2006.11,12-Epoxypukalideasthe antifoulingagentfromtheBrazilianendemicseafanPhyllogorgiadilatataEsper (Octocorallia,Gorgoniidae).Biochem.Syst.Ecol.34,446–448.
Fernandes,L.,Kelecom,A.,1995.Afurthernardosinanesesquiterpenefromthe gor-gonianPhyllogorgiadilatata(Octocorallia,Gorgonacea).An.Acad.Bras.Cienc.67, 171–173.
Fernandes,R.F.,Maia,L.F.,Couri,M.R.C.,Costa,L.A.S.,deOliveira,L.F.C.,2015.Raman spectroscopyasatoolindifferentiatingconjugatedpolyenesfromsyntheticand naturalsources.Spectrochim.ActaA:Mol.Biomol.Spectrosc.134,434–441. Francini-Filho,R.B.,Moura,R.L.,Thompson,F.L.,Reis,R.M.,Kaufman,L.,Kikuchi,
R.K.P.,Leão,Z.M.A.N.,2008.Diseasesleadingtoaccelerateddeclineofreefcorals inthelargestSouthAtlanticreefcomplex(AbrolhosBank,easternBrazil).Mar. Pollut.Bull.56,1008–1014.
Fujise,L.,Yamashita,H.,Suzuki,G.,Sasaki,K.,Liao,L.M.,Koike,K.,2014. Moder-atethermalstresscausesactiveandimmediateexpulsionofphotosynthetically damagedzooxanthellae(Symbiodinium)fromcorals.PLOSONE9.
Hughes,T.P.,Baird,A.H.,Bellwood,D.R.,Card,M.,Connolly,S.R.,Folke,C., Gros-berg,R.,Hoegh-Guldberg,O.,Jackson,J.B.C.,Kleypas,J.,Lough,J.M.,Marshall, P.,Nyström,M.,Palumbi,S.R.,Pandolfi,J.M.,Rosen,B.,Roughgarden,J.,2003. Climatechange,humanimpacts,andtheresilienceofcoralreefs.Science301, 929–933.
Johansen,J.E.,Svec,W.A.,Liaaen-Jensen,S.,Haxo,F.T.,1974.Carotenoidsofthe dinophyceae.Phytochemistry13,2261–2271.
Kelecom,A.,Brick-Peres,M.,Fernandes,L.,1990.Anewnardosinane sesquiter-penefromtheBrazilianendemicgorgonianPhllogorgiadilatata.J.Nat.Prod.53, 750–752.
Kelecom,A.,SoleCava, A.M.,Kannengiesser, G.J.,1980.Occurrenceof 23,24-dimethylcholesta-5, 22-dien-3-ol in the Brazilian gorgonian Phyllogorgia dilatata(octocorallia,gorgonacea)andinitsassociatedzooxanthella.Bull.Soc. Chim.Belg.89,1013–1014.
Lavaud,J.,Rousseau,B.,VanGorkom,H.J.,Etienne,A.L.,2002.Influenceofthe diadinoxanthinpoolsizeonphotoprotectioninthemarineplanktonicdiatom Phaeodactylumtricornutum.PlantPhysiol.129,1398–1406.
Leewis,R.J.,Janse,M.,2008.AdvancesinCoralHusbandryinPublicAquariums. Burgers’Zoo.
Lesser,M.P.,2004.Experimentalbiologyofcoralreefecosystems.J.Exp.Mar.Biol. Ecol.300,217–252.
Lin,M.-C.,Dai,C.-F.,1996.Drag,morphologyandmechanicalpropertiesofthree speciesofoctocorals.J.Exp.Mar.Biol.Ecol.201,13–22.
Llewellyn,C.A.,Evans,C.,Airs,R.L.,Cook,I.,Bale,N.,Wilson,W.H.,2007.Theresponse ofcarotenoidsandchlorophyllsduringvirusinfectionofEmilianiahuxleyi (Prym-nesiophyceae).J.Exp.Mar.Biol.Ecol.344,101–112.
Maia,L.F.,1991.TerpenosdecoraisdaordemGorgonacea.UFRRJ,Itaguaí,pp.200. Maia,L.F.,deOliveira, V.E.,deOliveira, M.E.R.,Fleury,B.G.,de Oliveira,L.F.C.,
2012. Polyenicpigmentsfrom the Brazilianoctocoral Phyllogorgiadilatata Esper,1806characterizedby Ramanspectroscopy. J.RamanSpectrosc.43, 161–164.
Maia, L.F., de Oliveira, V.E., Oliveira, M.E.R., Reis, F.D., Fleury, B.G.,Edwards, H.G.M.,deOliveira,L.F.C.,2013.Colourdiversificationinoctocoralsbasedon conjugatedpolyenes:aRamanspectroscopic view.J.RamanSpectrosc.44, 560–566.
Maia,L.F.,Fernandes,R.F.,Lobo-Hajdu,G.,DeOliveira,L.F.C.,2014a.Conjugated polyenesaschemicalprobesoflifesignature:useofRamanspectroscopyto differentiatepolyenicpigments.Philos.Trans.R.Soc.A372.
Maia,L.F.,Fleury,B.G.,Lages,B.G.,Creed,J.C.,deOliveira,L.F.C.,2014b.Chapter10. NewStrategiesforidentifyingnaturalproductsofecologicalsignificancefrom corals:nondestructiveRamanspectroscopyanalysis.In:Attaur,R.(Ed.),Studies inNaturalProductsChemistry.Elsevier,pp.313–349.
Maoka, T., Tsushima, M., Nishino, H.,2002. Isolationand characterization of dinochromeAandB,anti-carcinogenicactivecarotenoidsfromthefreshwater redtidePeridiniumbipes.Chem.Pharm.Bull.50,1630–1633.
Martins,D.L.,Epifanio,R.D.A.,1998.Anewgermacranesesquiterpenefromthe BrazilianendemicgorgonianPhyllogorgiadilatataEsper.J.Braz.Chem.Soc.9, 586–590.
McGraw,K.,Hudon,J.,Hill,G.,Parker,R.,2005.Asimpleandinexpensivechemical testforbehavioralecologiststodeterminethepresenceofcarotenoidpigments inanimaltissues.Behav.Ecol.Sociobiol.57,391–397.
McGraw, K.J.,Nogare, M.C.,2004. Carotenoid pigments andthe selectivityof psittacofulvin-basedcolorationsystemsinparrots.Comp.Biochem.Phys.B138, 229–233.
Meurens,M.,Baeten,V.,Yan,S.H.,Mignolet,E.,Larondelle,Y.,2005.Determination oftheconjugatedlinoleicacidsincow’smilkfatbyFouriertransformRaman spectroscopy.J.Agric.FoodChem.53,5831–5835.
MMA,2005.Normativan◦005,21demaiode2004.anexo1.Brazil.,pp.1.
Mydlarz,L.D.,Holthouse,S.F.,Peters,E.C.,Harvell,C.D.,2008.Cellularresponsesin seafancorals:granularamoebocytesreacttopathogenandclimatestressors. PLoSONE3,e1811.
Paul,V.J.,Ritson-Williams,R.,2008.Marinechemicalecology.Nat.Prod.Rep.25, 662–695.
Paul,V.J.,Ritson-Williams,R.,Sharp,K.,2011.Marinechemicalecologyinbenthic environments.Nat.Prod.Rep.28,345–387.
Pereira,L.,Amado,A.M., Critchley,A.T.,vande Velde,F.,Ribeiro-Claro, P.J.A., 2009.Identificationofselectedseaweedpolysaccharides(phycocolloids)by vibrationalspectroscopy (FTIR-ATRand FT-Raman). Food Hydrocolloid 23, 1903–1909.
Pereira,R.C.,Carvalho,A.G.V.,Gama,B.A.P.,Coutinho,R.,2002.Fieldexperimental evaluationofsecondarymetabolitesfrommarineinvertebratesasantifoulants. Braz.J.Biol.62,311–320.
Pinto,E.,Catalani,L.H.,Lopes,N.P.,DiMascio,P.,Colepicolo,P.,2000.Peridininas themajorbiologicalcarotenoidquencherofsingletoxygeninmarinealgae Gonyaulaxpolyedra.Biochem.Biophys.Res.Commun.268,496–500. Rosenberg,E.,Kellogg,C.A.,Rohwer,F.,2007.Coralmicrobiology.Oceanography20,
146–154.
Schrader,B.,Klump,H.H.,Schenzel,K.,Schulz,H.,1999.Non-destructiveNIRFT Ramananalysisofplants.J.Mol.Struct.509,201–212.
Schulz,H.,Baranska,M.,2007.Identificationandquantificationofvaluableplant substancesbyIRandRamanspectroscopy.Vib.Spectrosc.43,13–25. Schulz,H.,Baranska,M.,Baranski,R.,2005a.PotentialofNIR-FT-Ramanspectroscopy
innaturalcarotenoidanalysis.Biopolymers77,212–221.
Sorauf,J.E.,1980.Biomineralization,structureanddiagenesisofthecoelenterate skeleton.ActaPalaeontol.Pol.25,327–343.
Talian,I.,Ori ˇnák,A.,Efremov,E.V.,Ariese,F.,Kaniansky,D.,Ori ˇnáková,R.,Hübner,J., 2010.DetectionofbiologicallyactivediterpenoicacidsbyRamanspectroscopy. J.RamanSpectrosc.41,964–968.
Thompson,J.R.,Rivera,H.E.,Closek,C.J.,Medina,M.,2015.Microbesinthecoral holo-biont:partnersthroughevolution,development,andecologicalinteractions.
Front. Cellular Infect. Microbiol. 4, http://dx.doi.org/10.3389/fcimb.2014. 00176.
Windig,W.,Lippert,J.L.,Robbins,M.J.,Kresinske,K.R.,Twist,J.P.,Snyder,A.P., 1990.Interactiveself-modelingmultivariateanalysis.Chemometr.Intell.Lab.9, 7–30.