Article
Printed in Brazil - ©2018 Sociedade Brasileira de Química*e-mail: caile@ynu.edu.cn; ztding@ynu.edu.cn
Improving
Antioxidant
Activity
of
Ophioglossum
thermale
Kom.
by
Fermentation
with
Talaromyces
purpurogenus
M18-11
Jian-weiDong,a,bLeCai,*,aXue-jiaoLi,aRui-fengMei,aPingLuoaand
Zhong-taoDing*,a
aFunctional Molecules Analysis and Biotransformation Key Laboratory of Universities in Yunnan Province, School of Chemical Science and Technology, Yunnan University, 650091 Kunming, P.R. China
bCollege of Chemistry and Environmental Science, Qujing Normal University, 655011 Qujing, P.R. China
Ophioglossum thermale Kom. was fermented with several fungi. The total phenolic and flavonoid contents (TPCs and TFCs) and antioxidant activities of fermented and non-fermented O. thermale (FOT and NFOT) were investigated. The results showed that Talaromyces purpurogenus M18-11 fermented O. thermale possessed significantly improved TPC and TFC and exhibited significantly stronger 1,1-diphenyl-2-picrylhydrazyl (DPPH) (half maximal inhibitory concentration (IC50) = 75.7 ± 2.1 µg mL-1) and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic
acid) diammonium salt (ABTS) (IC50 = 20.52 ± 1.68 µg mL-1) free-radical scavenging activities,
ferric reducing antioxidant power (0.585 ± 0.045 mmol g-1), and reducing power (half maximal
effective concentration (EC50) = 30.52 ± 1.91 µg mL-1) than original material. The determination
of the contents of representative flavonoids and their glucosides revealed that the improvements are attributed to the hydrolysis of homoisoflavonoid and flavonoid glucosides, glycometabolism, as well as fungal metabolites. This paper is the first to report the fermentation of O. thermale with pure strains and T. purpurogenus is an effective strain to process O. thermale for improving the antioxidant activity.
Keywords: Ophioglossum thermale, Talaromyces purpurogenus, fermentation, antioxidant
activity, flavonoid
Introduction
The genus Ophioglossum (Ophioglossaceae) is a
small terrestrial plant that is distributed worldwide.1
Phytochemical researches revealed that the herb of
Ophioglossum contains massive fatty acids and their
esters,2,3 amino acid,4 flavonoids,5-11 and polysaccharoses.12,13 Ophioglossum thermale Kom., “Yizhijian” in traditional
Chinese medicine (TCM), is a plant of the genus
Ophioglossum, which is frequently used as a herbal
medicine for clearing heat and detoxicating. Its main
chemical constituents are flavonoids2,14,15 which possess
significant antioxidant activity.7
Herbal fermentation processing began approximately 4000 years ago in China, which is frequently used to produce secondary metabolites from domestic plants in bulk by utilizing the metabolic mechanisms of microorganisms. Solid-state natural fermentation (SSF)
was very popular in the processing of herbal medicines in
ancient China, such as Semen Sojae Praeparatum, Mass
Galla chinesis et camelliae Fermentata, Massa Medicata Fermentata,and Rhizoma Pinelliae Fermentata, etc. With
the development of microbial technology, pure strain fermentation has become increasingly acceptable and reliable for processing herbal medicines in modern times. In recent years, some fermented traditional medicines (FTMs) have been reported. FTMs exhibit stronger biological activities or higher bioavailability in the human body compared with raw materials. For example, Hsu et al.16 reported that Bacillus subtilis fermented Radix astragali could stimulate the biosynthesis of
type I procollagen in a dose-dependent manner in both aged and young human dermal fibroblast cells.
Aspergillus oryzae-fermented Curcuma longa L. could
effectively prevent CCl4-induced hepatic damage in
rats.17 Wang et al.18 improved bioactivities of polyphenol
extracts from Psidium guajava L. leaves by fermentation
In the present study, the herb of O. thermale was fermented
with several fungi. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical-scavenging activities, reducing power, and ferric reducing antioxidant power (FRAP) of non-fermented and fermented
O. thermale (FOT) were determined for evaluating their
antioxidant activities. Talaromyces purpurogenus
M18-11 was screened to process O. thermale for improving the
antioxidant activity. Further total phenolic and flavonoid contents (TPCs and TFCs) determination and high-performance liquid chromatography (HPLC) analysis were used for explaining the changes of antioxidant.
Experimental
Chemicals
ABTS, DPPH, 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were obtained from J&K Scientific Ltd. (Beijing, China). Rutin and gallic acid were purchased from Aladdin-Reagent (Shanghai, China).
Luteolin (1), quercetin (2), 3-methoxyquercetin (3),
ophioglonol (4), ophioglonol 4’-O-α-D-glucopyranoside
(5), and pedunculosumoside B (6) used in the present study
were isolated from O. thermale.14 The water (resistivity
≥ 18.25 MΩ cm) used was purified with a purity water
system (Chengdu, China). All other chemicals used were of analytical grade.
Plant material
The herbs of O. thermale were collected from Wenshan,
Yunnan, China, in February 2014, and were identified by Assistant Professor Shu-Da Yang from School of Pharmacy, Kunming Medical University, Kunming, China. A voucher specimen (2014-0t-01) has been deposited in School of Chemical Science and Technology, Yunnan University, Kunming, China.
Microorganisms and fermentation
All strains including T. purpurogenus M18-11,
Aspergillus niger YIM3029, Geomyces luteus P18-5, and Penicillium swiecickii F2-7 were obtained from Yunnan
Institute of Microbiology, Yunnan Province, China. Potato dextrose agar (PDA; 1 L water, 200 g potato, 20 g dextrose, and 15 g agar) slant culture mediums were inoculated with fungi above and incubated in a constant temperature incubator at 28 °C for 5 days. Five grams of
O. thermale were added to a 100 mL Erlenmeyer flask to
function as the fermentation culture medium. After being infiltrated with 15 mL water and sterilized at 121 °C for 30 min, the mature slants culture mediums were added and incubated at 28 °C for 30 days.
Extraction
O. thermale (5.0 g) and each of the four individual
FOTs were immersed in 50 mL acetone for 24 h and ultrasonicated three times for 30 min each time. The extracting solution was decanted, filtered under vacuum and concentrated in a rotary evaporator to afford five
extracts: ROT (raw O. thermale, 1.063 g), BFOT (blank
FOT, 1.099 g), FOT1 (A. niger FOT, 0.405 g), FOT2
(T. purpurogenus FOT, 0.549 g), FOT3 (G. luteus FOT,
0.667 g), and FOT4 (P. swiecickii FOT, 0.475 g).
Antioxidant activities
DPPH radical-scavenging activity
The DPPH free radical-scavenging activity was
estimated by the method described previously.19 DPPH
radical-scavenging activity of the sample was calculated as follows:
(1)
where is the absorbance of sample solution mixed
with DPPH and is the absorbance of the blank
solution (ethanol). Rutin was used as a positive control. All tests were performed in triplicate. The half maximal
inhibitory concentration (IC50) value was defined as the
effective concentration at which the DPPH radical was scavenged by 50%. Anti-radical power (ARP) was defined as 1/IC50. ARPi and ARP0 were represented as the ARP of
each sample and ROT, respectively.
ABTS assay
ABTS antioxidant capacity was measured using a
previous ABTS method.19,20 ABTS radical-scavenging
activity was calculated as follows:
(2)
where is the absorbance of sample solution mixed with
ABTS and is the absorbance of the blank solution.
The IC50 value was defined as the effective concentration at
which the ABTS radical was scavenged by 50%. ARP was defined as 1/IC50. ARPi and ARP0 were represented as the
FRAP
The FRAP assay measures the reduction of ferric iron to the ferrous form in the presence of antioxidants. The
assay protocol followed a previously reported procedure.21
The antioxidant capacity of the sample was expressed as
µmol ferrous sulfate per g extract (µmol g-1), which was
calculated from the standard graph obtained with the ferrous sulfate solution.
Reducing power
Reducing power was tested using a reported method.22,23
100 µL of sample at proper concentration was made up to 0.75 mL with phosphate buffer (300 mM, pH 6.6), and
1.5 mL of 1% (m/v) K3Fe(CN)6 was added. The mixture
was shaken vigorously and left to stand for 20 min at 50 °C. After the addition of 1.5 mL of 10% (m/v) trichloroacetic acid, the mixture was centrifuged at 3000 rpm for 10 min. A 1.5 mL of supernatant was mixed with distilled water
(1.5 mL), and 0.3 mL of 0.1% (m/m) FeCl3 was added
before the absorbance was determined at 700 nm. Half
maximal effective concentration (EC50) was defined by
the concentration at absorbance (A) = 0.50; antioxidant
reducing power (ARP) was defined as 1/EC50. ARPi and
ARP0 were represented as the ARP of each sample and
ROT, respectively.
Determination of total phenolic content
The total phenolic content was measured using the Folin-Ciocalteau method.24 Folin-Ciocalteu’s reagent was prepared
according to the method of GB/T 23527-2009 (CN).25 Fifty
microliters of extract solution was added to 2.25 mL of Folin-Ciocalteu’s reagent, which was pre-diluted ten-fold with distilled water. Five minutes later, 1.5 mL of Na2CO3
(7.5%, m/v) solution was added, and the mixture was allowed to stand for 30 min at ambient temperature. Absorbance was measured at 765 nm. The TPC was expressed as mg gallic acid equivalent per g extract (mg GAE g-1) and calculated
according to the calibration curve obtained from the standard solution of gallic acid at various concentrations.
Determination of total flavonoid content
The total flavonoid content was determined by a
colorimetric assay described previously.23,26 The total
flavonoid content was expressed as mg rutin equivalent
per g (mg RE g-1).
HPLC analysis
The determination and quantification of main
flavonoids compounds were measured using an Agilent 1260 Infinity Series HPLC system, equipped with an Agilent G1315D DAD detector, an Agilent G1311C quaternary gradient pump, and Agilent Zorbax SB-C18 (250 × 4.6 mm i.d., 5 µm), and coupled to an Agilent OpenLab data-processing station. A gradient elution system consisting of solvent A (water containing 6 mM acetic acid) and B (acetonitrile) was used for the analysis, and the gradient program was as follows: 0-25 min, 15-65%B; 25-30 min, 65-100%B; 30-35 min, 100%B. The peaks were confirmed by the UV absorptions at 320 and 355 nm and the retention times while the flow rate
was 1.0 mL min-1. The column temperature was set at
35 °C, and the injection volume was 20 µL. Flavonoids were identified by using standard addition method, and quantified according to standard curves.
Results and Discussion
After fermentation of O. thermale with several fungi,
a preliminary experiment by thin layer chromatography (TLC) was measured. Four fermented samples were selected for further experiments.
Antioxidant activities
Oxidation is ubiquitous and has pernicious effects on both food quality and human health. Previous research indicated that oxidative damage can cause browning and off-flavors, change the nutrient value of food, disrupt cellular function and attenuate the formation of compounds used to fight aging and cardio-vascular
disease.27 Zhang et al.7 reported that the extract of
O. thermale possesses antioxidant activity. In the present
study, therefore, the antioxidant activity of ROT, BFOT, and FOT1-4 were tested.
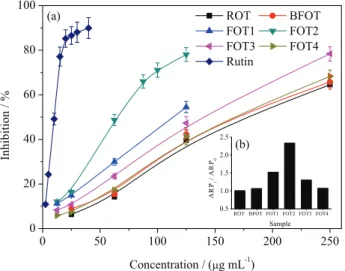
DPPH is a stable free radical that has been used widely to determine the free radical-scavenging activity of natural antioxidants. The DPPH radical scavenging activities shown in Figure 1 revealed that FOT1-3 exhibited more significant activities than ROT, BFOT, and FOT4, in particular, FOT2 possessed 2-fold more antioxidant activity with an IC50 value of 75.7 ± 2.1 µg mL-1 than ROT.
ABTS is another free radical that is frequently used to study free radical-scavenging activity. Notably, the ABTS radical-scavenging activity assay is an important indicator of total antioxidant activity (TAA). As shown in Figure 2, FOT1-3 showed stronger ABTS radical-scavenging activities with the IC50 values of 18.61 ± 1.32,
20.52 ± 1.68, and 19.20 ± 0.92 µg mL-1, than FOT and
Reducing power of the natural plant extracts might be strongly correlated with their antioxidant activity. It is necessary to discuss the reducing power of a natural plant extract to elucidate the relationship between its antioxidant effect and reducing power. The results shown in Figure 3 suggest FOT2 exhibits significantly improved reducing
power with an EC50 value of 30.52 ± 1.91 µg mL-1. The
FRAP is often used as an indicator of phenolic antioxidant activity as important as reducing power. The antioxidant potential of sample was estimated by their abilities, which is to reduce FeIII-TPTZ to FeII-TPTZ. Similar to reducing
power, FOT2 possessed improved antioxidant activity
(0.585 ± 0.045 mmol g-1, Figure 4).
In addition, the DPPH antioxidant activity for
extract of fungal strains A. niger, T. purpurogenus,
G. luteus,and P. swiecickii, individually cultured in PDA
medium was 390.8 ± 25.1, 235.8 ± 21.1, 234.4 ± 10.9,
235.2 ± 31.2 µg mL-1, respectively (for pure PDA medium,
it was 386.5 ± 29.1 µg mL-1). Therefore, the results above
show that FOT2 exhibits significantly improved antioxidant
activity, suggesting that fermentation of O. thermale
with T. purpurogenus might be effective for improving
antioxidant activity.
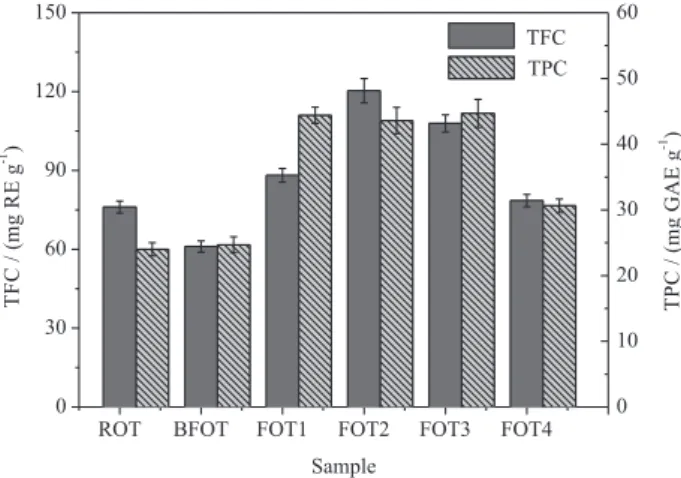
Total phenolic content (TPC)
Phenolic content always shows potent antioxidant activity, in particular, radical-scavenging power. In the present study, the improvement of antioxidant activity might be ascribed to changes of phenolic constituents. Hence, the TPCs of ROT, BFOT, and FOT1-4 were evaluated and the results were shown in Figure 5. The values of TPCs were expressed as
Figure1. DPPH radical scavenging activities of the extracts of ROT, BFOT, and FOT1-4. (a) Inhibition of DPPH radical scavenging activities at different concentrations; (b) relative ARP (ARPi/ARP0) of BFOT and
FOT1-4.
Figure2. ABTS radical scavenging activities of the extracts of ROT, BFOT, and FOT1-4. (a) Inhibition of ABTS radical scavenging activities at different concentrations; (b) relative ARP (ARPi/ARP0) of BFOT and
FOT1-4.
Figure3. Reducing powers of the extracts of ROT, BFOT, and FOT1-4. (a) Reducing powers at different concentrations; (b) relative ARP (ARPi/ARP0) of BFOT and FOT1-4.
mg GAE g-1 and decreased in the following order: FOT1-3
(44.41 ± 1.22, 43.63 ± 2.00, 44.69 ± 2.13 mg g-1) >> FOT4
(30.63 ± 1.03 mg g-1) > ROT (24.01 ± 1.00 mg g-1) ≈ BFOT
(24.69 ± 1.23 mg g-1), suggesting A. niger, T. purpurogenus,
and G. luteus fermentation might improve the TPC of O. thermale.
Total flavonoid content (TFC)
As the main constituents of O. thermale are flavonoids
including quercetin, 3-methoxyquercetin, ophioglonol and their esters, the TFCs of the fermented and non-fermented materials are indicators for evaluating the effects of fermentation. The TFCs of ROT, BFOT, and FOT1-4 are shown in Figure 5. FOT2 and FOT3 possessed obvious improved TFCs with the values of 120.35 ± 0.84 and
107.94 ± 4.22 mg RE g-1, respectively, whereas FOT1,
FOT4, and BFOT exhibited TFC with same grade as ROT. These results suggest that T. purpurogenus might be an
effective strain for processing O. thermale for improving
the TPC, TFC, and antioxidant activity. The improvement
of antioxidant activity might be caused by the changes of flavonoids, as well as fungal metabolites produced by fungi during fermentation.
HPLC analysis
To clarify the flavonoids, it was performed positive antioxidant activities in the fermented and non-fermented
O. thermale. HPLC was employed to identify the changes
of main flavonoids isolated from O. thermale. Six main
flavonoid compounds isolated from O. thermale, luteolin (1),
quercetin (2), 3-methoxyquercetin (3), ophioglonol (4),
ophioglonol 4’-O-α-D-glucopyranoside (5), and
pedunculosumoside B (6) (Figure 6) were used to
identify the peaks in chromatograms of ROT, BFOT, and
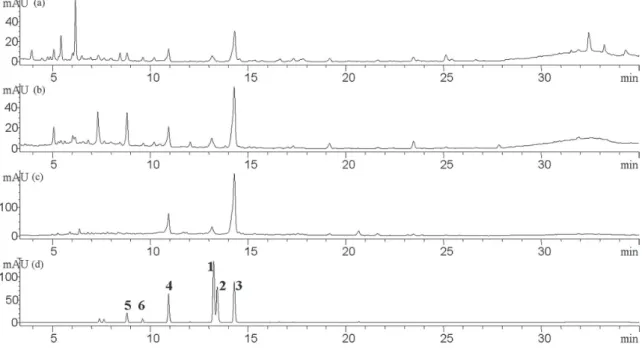
T. purpurogenus FOT. As shown in Figure 7, flavonoid
glucoside 5 could be not detected in T. purpurogenus
FOT, suggesting that flavonoid glucosides are hydrolyzed by T. purpurogenus-fermentation. The peaks at 30-35 min
observed in ROT disappeared after fermentation, suggesting that some low-polarity constituents are metabolized leading to the enrichment of the flavonoids. The contents of three main flavonoids 3-5 were determined by HPLC. The results
in Table 1 show that the contents of flavonoid aglycones 3
and 4 were enriched. This might be caused by the hydrolysis
of flavonoid glucosides and glycometabolism.
Antioxidant activities of flavonoid compounds 1-6
The DPPH radical-scavenging activities of flavonoid
aglycones 1-4 and glucosides 5 and 6 were determined
to evaluate the difference of antioxidant activities between flavonoid aglycones and glucosides. The results
shown in Table 2 reveal that flavonoid aglycones 1-4
exhibited more significant DPPH radical-scavenging
power (IC50 < 10 µg mL-1) than flavonoid glucosides
Figure5. TPCs and TFCs of the extracts of ROT, BFOT, and FOT1-4.
(IC50 > 50 µg mL-1). Therefore, hydrolysis of flavonoid
glucosides in O. thermale could enrich the flavonoid
aglycones, improving the antioxidant activity of O. thermale.
Conclusions
The present study is the first to report that pure strain fermentation processing is effective in improving the TPC,
TFC, and antioxidant activity of O. thermale. Fermentation
with T. purpurogenus could be an innovative approach
to process O. thermale. TFC and antioxidant activity
enhancements with T. purpurogenus might be attributed
to hydrolysis of flavonoid glycosides, decomposition of glycoside and other low-polarity constituents, and fungal metabolites.
Acknowledgments
This work was financially supported by a grant from the Natural Science Foundation of China (No. 81660719), a grant from the Natural Science Foundation of Yunnan Province, a grant of Yunnan Local Colleges Applied Basic Research Project (No. 2017FH001-092), a project of Yunling Scholars of Yunnan Province, the Program for Excellent Young Talents, Yunnan University, a grant from Shanghai Key Laboratory of Rare Earth Functional Materials, and a grant from Functional Molecules Analysis and Biotransformation Key Laboratory of Universities in Yunnan Province.
References
1. Flora of China Editorial Committee; Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1959.
2. Zhang, G. W.; Wu, N. Z.; Fan, Q.; Zhou, X. L.; Huang, S.; Nat. Prod. Res. Dev.2010, 22, 1006.
3. Clericuzio, M.; Burlando, B.; Gandini, G.; Tinello, S.; Ranzato, E.; Martinotti, S.; Cornara, L.; J. Nat. Med.2014, 68, 31.
4. Khandelwal, S.; Biochem. Syst. Ecol.1989, 17, 167. Table1. The contents of compounds 3-5 in non-fermented and fermented
O. thermale
Sample 3 / (mg per 100 g) 4 / (mg per 100 g) 5 / (mg per 100 g) ROT 3.92 ± 0.94 16.88 ± 0.81 15.18 ± 1.18 BFOT 6.76 ± 0.51 17.08 ± 0.43 15.30 ± 2.55
FOT2 17.64 ± 0.42 44.62 ± 0.14 nda
aNot detected. ROT: raw O. thermale; BFOT: blank fermented O. thermale;
FOT2: T. purpurogenus FOT.
Figure7. Chromatograms of (a) ROT; (b) BFOT; (c) T. purpurogenus FOT; (d) compounds 1-6.
Table2. DPPH radical-scavenging activities (IC50) of compounds 1-6
Sample IC50 / (µg mL-1) IC50 / µM
1 7.53 ± 0.10 26.3 ± 0.4
2 2.86 ± 0.09 9.5 ± 0.3
3 9.11 ±0.73 28.8 ± 2.3
4 8.31 ± 0.49 26.3 ± 1.6
5 56.9 ± 2.64 115.2 ± 5.3
6 61.32 ± 5.87 86.6 ± 8.3
Rutina 8.20 ± 0.05 13.4 ± 0.1
aPositive control. DPPH: 1,1-diphenyl-2-picrylhydrazyl; IC 50: half
5. Lin, Y. L.; Shen, C. C.; Huang, Y. J.; Chang, Y. Y.; J. Nat. Prod.
2005, 68, 381.
6. Wan, C. X.; Zhang, P. H.; Luo, J. G.; Kong, L. Y.; J. Nat. Prod.
2011, 74, 683.
7. Zhang, X. Q.; Kim, J. H.; Lee, G. S.; Pyo, H. B.; Shin, E. Y.; Kim, E. G.; Zhang, Y. H.; Am. J. Chin. Med.2012, 40, 279.
8. Wan, C.-X.; Luo, J.-G.; Gu, Y.-C.; Xu, D.-R.; Kong, L.-Y.;
Phytochem. Anal.2013, 24, 541.
9. Clericuzio, M.; Tinello, S.; Burlando, B.; Ranzato, E.; Martinotti, S.; Cornara, L.; La, R. A.; Planta Med.2012, 78,
1639.
10. Wan, C.-X.; Luo, J.-G.; Gu, Y.-C.; Kong, L.-Y.; J. Asian Nat. Prod. Res.2012, 14, 533.
11. Markham, K. R.; Mabry, T. J.; Voirin, B.; Phytochemistry1969,
8, 469.
12. Xue, X.; Fry, S. C.; Ann. Bot.2012, 109, 873.
13. He, X. M.; Ji, N.; Xiang, X. C.; Luo, P.; Bao, J. K.; Appl. Biochem. Biotechnol.2011, 165, 1458.
14. Dong, J. W.; Cai, C.; Li, X. J.; Peng, L.; Xing, Y.; Mei, R. F.; Wang, J. P.; Ding, Z. T.; Fitoterapia2016, 109, 212.
15. Hu, W. C.; Zhou, Z. B.; Wang, C. X.; J. Chin. Med. Mater.2016,
39, 1035.
16. Hsu, M. F.; Chiang, B. H.; Process Biochem.2009, 44, 83. 17. Kim, Y.; You, Y.; Yoon, H. G.; Lee, Y. H.; Kim, K.; Lee, J.; Kim,
M. S.; Kim, J. C.; Jun, W.; Food Chem.2014, 151, 148.
18. Wang, L.; Wei, W.; Tian, X.; Shi, K.; Wu, Z.; Ind. Crops Prod.
2016, 94, 206.
19. Dong, J. W.; Cai, L.; Xiong, J.; Chen, X. H.; Wang, W. Y.; Shen, N.; Liu, B. L.; Ding, Z. T.; Process Biochem.2015, 50, 8.
20. Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.; Free Radical Biol. Med.1999, 26, 1231.
21. Suárez, B.; Álvarez, Á. L.; García, Y. D.; Barrio, G.; Lobo, A. P.; Parra, F.; Food Chem.2010, 120, 339.
22. Dong, J. W.; Cai, L.; Zhu, X. F.; Huang, X.; Yin, T. P.; Fang, H. X.; Ding, Z. T.; J. Braz. Chem. Soc.2014, 25, 1956.
23. Oyaizu, M.; Jpn. J. Nutr.1986, 44, 307.
24. Singleton, V. L.; Rossi, J. A. J.; Am. J. Enol. Vitic.1965, 16,
144.
25. GB/T 23527-2009: Protease Preparations, Administration of
Quality Supervision, Inspection and Quarantine of People’s Republic of China; Standardization Administration of China: China, 2009 (in Chinese).
26. Zhi, S. J.; Meng, C. T.; Jian, M. W.; Food Chem.1999, 64, 555.
27. Cardenia, V.; Rodriguez-Estrada, M. T.; Boselli, E.; Lercker, G.; Biochimie2013, 95, 473.
Submitted: November 12, 2017
Published online: April 2, 2018