Altogether, the study generated new information about the virus-cell interactions that play a role in the development of persistent CVB infections and antiviral drugs that may be effective in their treatment. This study aims to generate new information about the mechanisms of persistent EV infection to facilitate the development of new treatments that may be effective in the prevention of T1D.
Enteroviruses
- Enterovirus classification and structure
- The enterovirus infectious cycle
- Persistent infection
- Enterovirus diseases
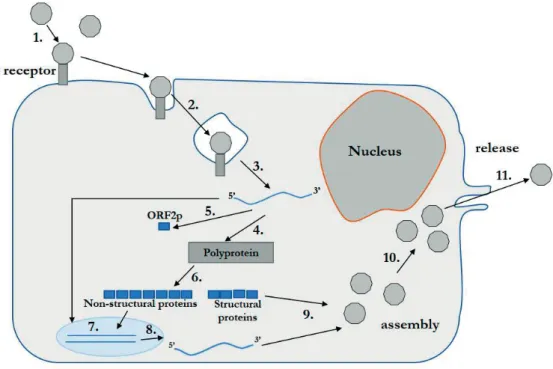
IRES for viral translation, in the 5'-UTR of the EV genome allows the virus bypass shutdown for cap-dependent protein translation (24). The carrier type of persistence appears to be the result of a coevolutionary process of the host cell and the virus (37,38).
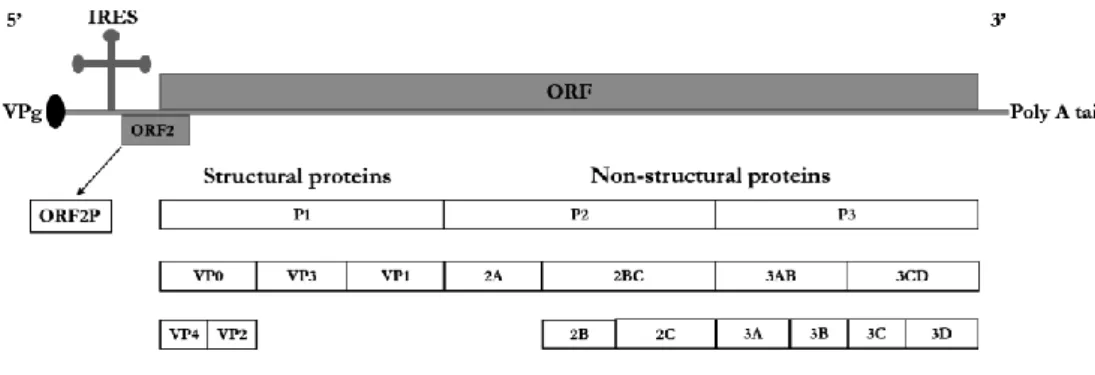
Virus-host interactions
Host cell antiviral response
The adaptive B-cell response produces antibodies against the virus during acute infection, but also protects against re-infection by the same virus type later on. The antibody response appears to be an important factor in eradicating EV infection.
The effect of EVs on the host cell
TFIIIC binds to the promoter, leading to the recruitment of TFIIIB, which is recognized by RNA polymerase III. Some of the host cell proteins required for viral translation or RNA replication are located in the nucleus, or translocate from the nucleus to the cytoplasm, leading to a requirement for their re-localization to the cytoplasm during EV infection.
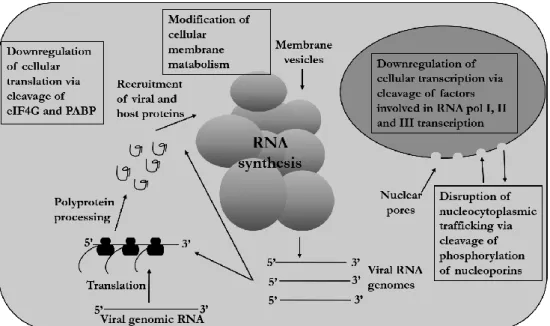
Antiviral drugs against enteroviruses
Pleconaril binds to the capsid pocket of the virus where it inhibits attachment and/or uncoating of the virus, depending on the receptor binding site. Attachment is inhibited if the receptor binding site is located in the canyon of the virus capsid (123).
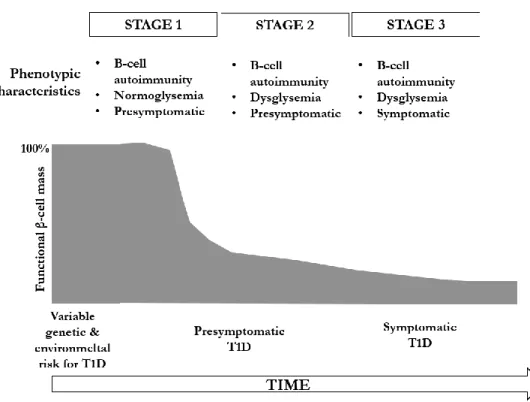
Pathogenesis of type 1 diabetes
Genetic factors
In addition, migration from low-risk areas to high-risk areas increases susceptibility to T1D (168). Therefore, the generally accepted current consensus is that genes determine susceptibility to the development of T1D, but that the disease is caused by some as yet unidentified environmental factors (63).
The environmental factors and T1D
In summary, several environmental factors have been studied as potential risk or predisposing factors for T1D. However, even though there is consensus on the importance of environmental factors in the pathogenesis of T1D, and certain environmental factors have been associated with T1D, causality has not been proven for any of this.
Cells
Viruses
ATCC and 10802 strains induced strong innate immune responses in cultured white blood cells, whereas 10796 and 10797 induced weak responses (213). In addition, two of the strains (strains 10796 and 10802) have also been examined for their ability to damage cultured human pancreatic islets and β-cells.
Persistent infection
- Establishment of viral persistent infection cell models
- Detection of persisting virus
- RT-qPCR
- Plaque assay
- Immunostaining
- Resistance to superinfection
- CAR receptor usage
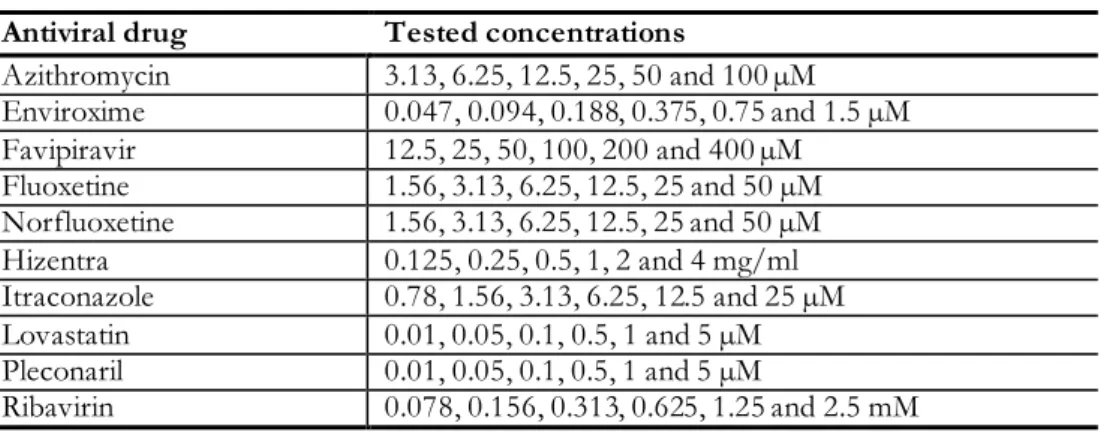
Immunofluorescent staining was performed to verify the presence of the virus from persistent infection models. In addition to immunofluorescent staining, immunohistochemistry was used to detect EV VP1 protein from the persistently infected cells during different stages of the infection.
Host cell evolution in persistent infection
- Proteomics
- Sample collection for proteomics
- Cell pellet lysis and digestion
- Cell culture supernatant digestion
- Mass spectrometric analyses
- Data processing and analysis
- Quantitative real time-PCR
- Western blot analyses
- Live cell immunofluorescent staining
- In situ -hybridization
Samples were then treated with DNase I (Invitrogen, Carlsbad, CA, USA) prior to cDNA synthesis, and SuperScript II reverse transcriptase (Invitrogen) was used for cDNA synthesis. Samples were run on Mini-PROTEAN® TGX™ precast protein gels (BioRad Laboratories, Hercules, CA, USA) and then transferred to polyvinylidene fluoride membranes (Trans-Blot Turbo Transfer Packs) (BioRad Laboratories).
Genomic evolution of viral genome
Next generation sequencing
- Sanger sequencing
The quality of the samples was then analyzed using the Fragment Analyzer following the guidelines of the NGS&RNA Fragment Analysis Analysis Committee (Agilent, Santa Clara, California, USA). Prior to sequencing, samples were diluted based on concentrations estimated by the KAPA Library quantitation kit (Illumina) before and after sample collection.
Analysis of sequence data
The CVB1 virus was modeled in 3D to visualize the locations of the mutations on the virus capsid surface. Then, two CVB3 X-ray diffraction templates, 4GB3 and COV1, (obtained from Protein Data Bank) were used as a template for 3D modeling of CVB1.
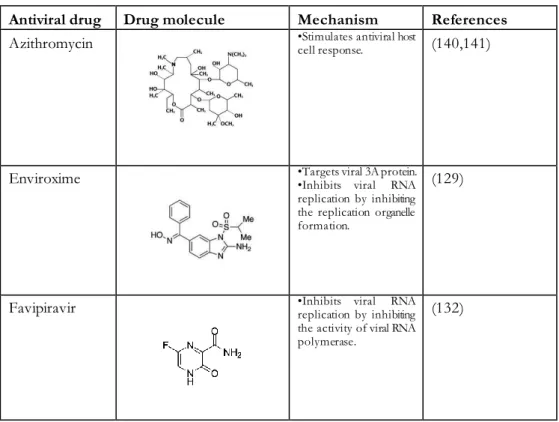
Studies with antiviral drugs
- Virus purification
- Tested antiviral drugs
- Antiviral drug cytotoxicity
- Effect of antiviral drugs in acute infection model
- Effect on Coxsackie B group viruses
- Effect on different Coxsackievirus B1 strains
- Quantification of antiviral effect
- Effect of antiviral drugs in persistent infection models
- Effect on persistent Coxsackievirus B1 infection
- Sanger sequencing
- Re-infection of cured cells
The cells were grown in 96-well plate (Thermo Fisher Scientific Nunc A/S) at a density of 40,000K cells/well grown on HAM's F12 supplemented with 10% FBS and PenSter. Eradication of the virus was verified by RT-qPCR and immunostaining, confirming that the cells were virus-free.
Verification of viral persistency in pancreatic cell lines
When PANC-1 cells persistently infected with CVB1 ATCC or CVB1 10796 were superinfected with CVB1 ATCC or CVB3 ATCC (Nancy strain), no cytopathic effect was observed 2 days after infection. In the upper graphs, the timeline starts after addition of fresh PANC-1 cells to cultures indicated by an arrow, leading to strong cytopathic effect and transient decrease in copy number due to cell lysis.
Host cell modifications during the development of CVB1 persistency
- Expression of cellular proteins during persistent CVB1
- Persistent CVB1 infection usurps the host cell machinery
- Expression of the CVB1 receptor
- The expression of mitochondrial proteins
- The antiviral response in persistently infected cells
- Persistent infection interferes with protein secretion
- The expression of proteins associated with type 1 diabetes
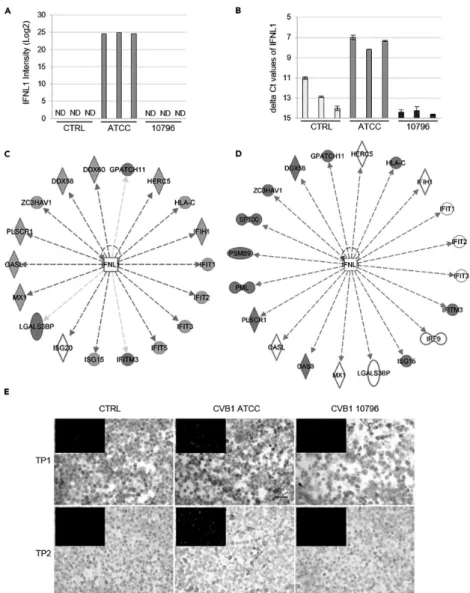
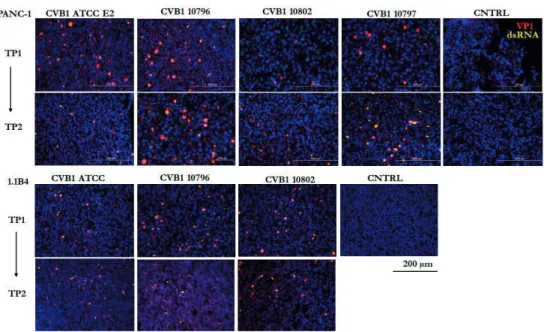
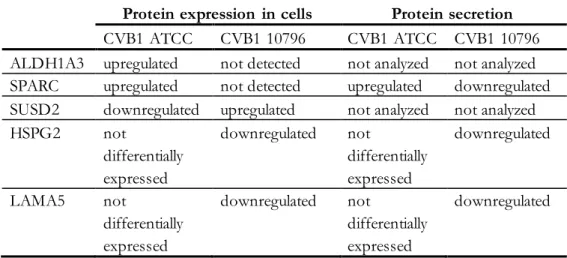
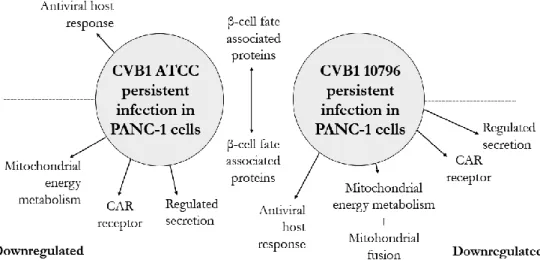
In addition, 14 of the 26 downstream target proteins of IFNL1 were upregulated in cells persistently infected by CVB1 ATCC. CD9 secretion was downregulated in cells persistently infected by CVB1 10796, and CD63 secretion was downregulated in cells persistently infected with CVB1 ATCC.
The adaptation of CVB1 to persistence
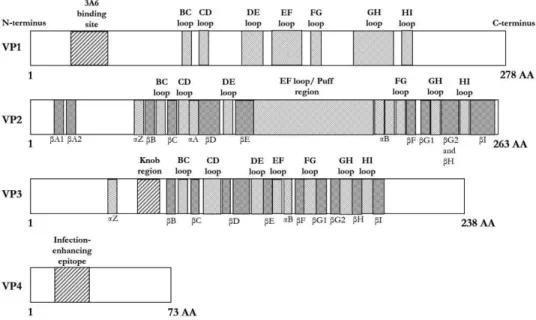
Mutations in persistent infection derived virus strains
- Mutations in viral structural proteins
- Virus mutation generating changes in the surface of the
- Mutations in non-structural CVB1 proteins
In addition, a minimum of three PIDVs had mutations in the CD, GH and HI loop regions (Figures 10 and 11). Mutations were also mapped in the CD and EF loops, and in the Dβ-sheet of the VP3 protein (Figures 10 and 12). This mutation was located near the CAR and DAF binding sites, which are visualized in Figure 9b.
The AA165 is mutated in three binding sites in the CAR and DAF binding site.
Mutations in the second ORF of the CVB1 genome
The effect of antiviral drugs on CVBs
Cytotoxicity of antiviral drugs
The effect of antiviral drugs in acute CVB infection
- Antiviral effect against prototype CVB strains
- The antiviral effect against the CVB1 ATCC and 10796
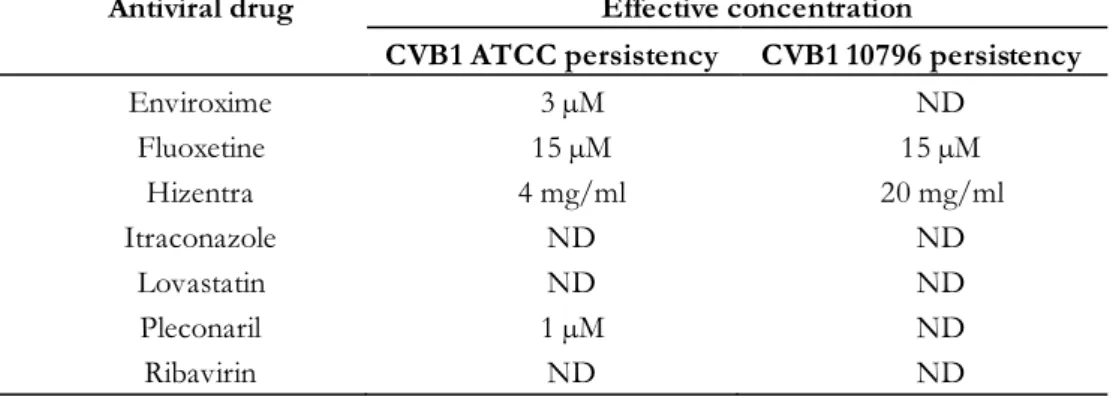
The table shows the half effective dose, ED50, concentrations for each drug and each CVB serotype. The antivirals were further tested against the CVB1 ATCC and 10796 strains, with higher amounts of virus in the assay to mimic the virus concentration achievable in models of persistent CVB1 infection. Fluoxetine and its active metabolite norfluoxetine and Hizentra were effective against both CVB1 strains (Table 10).
The table shows the half effective concentration (EC50) of the effective antiviral drugs for the CVB1 ATCC and CVB1 10796 strains.

The effect of antiviral drugs in persistent CVB1 infection
- The effect on persistent CVB1 infection
- The reinfection of cured cells
- Pleconaril and enviroxime resistance of CVB1 10796. 77
However, none of the Enviroxime concentrations tested affected CVB1 10796, but the highest concentration of Pleconaril (10 µM) caused a transient decrease in CVB1 10796 viral RNA. The concentrations capable of eradicating the persistent CVB1 ATCC and CVB1 10796 infections in PANC-1 cells (effective concentrations). Pleconaril and enviroxime were both able to cure the CVB1 ATCC infection, but not the CVB1 10796 infection.
When adjusting for hydrophobic pocket amino acids that associate with Pleconaril, no differences were observed between CVB1 ATCC and 10796 strains.
EV persistency
The mechanism of the carrier state persistent infection can be partially explained by the downregulation of the CAR receptor in cells persistently infected by the CVB1 ATCC and CVB1 10796 strains, as shown in publication I and in previous publications (37,38). One mutation in a CAR binding site, AA202 in VP1, was shared by three of the eight PIDVs studied. In a DAF binding site, AA162 in VP2 and AA237 in VP3 were mutated in five and three of the PIDVs, respectively.
The AA165 mutation located in both the CAR and DAF binding sites was identified in three of the PIDVs.
Persistent CVB1 infection leads to major changes in protein
- Persistent CVB1 infection affects mitochondrial proteins
- The cell’s antiviral response is either activated or shut
- Persistent CVB1 infection affects the cell’s secretion
- Persistent CVB1 infection affects proteins that are
In addition, the difference in mitochondrial network morphology was noted between cells persistently infected by either CVB1 ATCC or CVB1 10796 strains. In addition, IFNL1 downstream proteins were upregulated in persistent CVB1 ATCC infection, while they were downregulated or undetectable in persistent CVB1 10796 infection. Interestingly, the secretion of the macrophage colony-stimulating factor 1 (CSF1) and its receptor, CSF1R, which plays a role in macrophage proliferation and monocyte development (282), was increased during CVB1 ATCC and CVB1 10796 persistent infections.
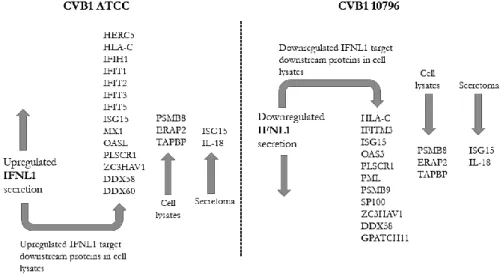
The expression of SUSD2 is the opposite; upregulated on CVB1 10796 and downregulated on CVB1.
The accumulation of mutations in CVB1 genome during viral
The K257R mutation identified from all persistent
One specific mutation of the VP1 structural protein, K257R, was identified from all PIDVs analysed. The location of the mutation is close to the C-terminus of VP1, and it is close to the amino acid K259, the known CAR binding site. The mutation was formed after blind passage of the CVB6 Smith strain, which naturally causes a non-lytic infection in epithelial HPDE cells of the human pancreatic duct.
The occurrence of mutations in all eight studied PIDVs is of great interest for further studies.
Antiviral drug treatment strategies for type 1 diabetes
The virus CVB6 Smith strain changed from non-lytic to lytic form suggesting that the AA257 may play a role in the virus life cycle. It has also been suggested to affect the DAF binding phenotype, but the effect was minor (310). Such studies may include site-directed mutagenesis to alter this AA and study its effect on the course of infection in cell and animal models.
Since DiViDIntervention is the first attempt to modulate the course of T1D with antiviral drugs, a possible beneficial effect of this treatment will open completely new possibilities in the prevention and treatment of T1D.
Antiviral drugs can cure CVB1 persistent infection
Furthermore, the effectiveness of immunoglobulins in the treatment of EV infections can be improved by optimizing their neutralizing antibody spectrum (323). Currently ongoing clinical trial (DiViDIntervention, EudraCT No tests Pleconaril-Ribavirin combination therapy in the treatment of T1D. In the present study, Pleconaril was effective against CVB1 ATCC, but not against persistent CVB1 10796 infection.
In addition, these agents may be useful in the treatment of acute and persistent CVB infections in general.
Limitations of the study
In addition, the combination of Pleconaril and Ribavirin, as used in the ongoing T1D intervention study, may be considered a viable option for testing the efficacy of antiviral drugs in the eradication of persistent EV infection in human patients. It is possible that the different properties of the cell may influence the mechanisms of CVB persistence. For example, the receptors the cell expresses or the level of innate immune response to the virus may dictate the development of sustained infection in a particular direction.
However, this study mainly focused on the possible role of CVB persistence in the pancreas in the pathogenesis of T1D, so the use of exocrine cells and a β-cell line was a natural choice.
Future prospective
In the present study, we were able to examine 10 drugs in a single drug setup. The advantage of the combination therapy is to prevent escape mutants and possibly reduce the necessary concentration of individual drugs in the cocktail. In addition, persistence models also provide an excellent platform to study the development of drug resistance in the persistent viral infection to identify viral mutations that determine drug resistance.
For example, proteins that are markedly downregulated/upregulated during a persistent CVB infection can be identified in pancreatic tissue of T1D patients using immunofluorescent staining.
Graphical summary of the study
B and C) Persistent infection with CVB1 ATCC (B) and 10796 (C) induced changes in the levels of extended granin family proteins, CPE, and peptidylglycine-amidating monooxygenase (PAM) in cell culture supernatants. Persistent CVB1 infection induced changes in the expression levels of (A) ALDH1A3 (cell lysates), (B) SPARC (cell culture supernatants), (C) SUSD2 (cell lysates), and (D) HSPG2 (cell lysates). In particular, proteins of the regulated secretory pathway were greatly downregulated in the secretomes of PANC-1 cells with persistent CVB1 infection.
The presence of virus in persistent infection models was verified by the RT-qPCR method described earlier by Honkanen et al. However, this mutation is not located directly in the DAF-binding region of the viral capsid. All PIDVs had mutations in the DE loop and the N terminus of VP1.
Seven of the PIDVs had mutations in the EF loop and around the BC loop. Laitinen, et al., Human enterovirus 71 strains in the background population and in hospital patients in Finland, J.