Etude parasitologique d'Anguilla anguilla dans deux lagons de Corse et étude ultrastructurale du tégument de trois digènes. Un grand merci également à mes amis de l'association Pro-Doc pour l'aventure que nous avons vécue ensemble.
C HAPITRE( I%
I NTRODUCTION)GENERALE %
1% LE%PARASITISME%
Mésoparasites qui vivent à l'intérieur de l'hôte (par exemple les parasites intestinaux) et se nourrissent du contenu ou de la matière présente dans l'hôte (Marchand, 1994). Les parasites peuvent être classés en fonction de l'écosystème dans lequel ils vivent, de leur emplacement sur l'hôte et de leur cycle de vie.
1.1% Ectoparasites%
Les ectoparasites ont généralement un cycle de vie direct, le parasite nécessitant un seul hôte. Les mésoparasites et les endoparasites ont généralement un cycle de vie indirect, nécessitant deux hôtes ou plus.
1.2% Mésoparasites%
Sa tâche est de maintenir la position du ver dans le tube digestif de l'hôte (Roberts & Janovy, 1996). La femelle produit des œufs avec des embryons partiellement développés (acanthores) et sont libérés dans la lumière intestinale de l'hôte.
2.1% Microscopie%électronique%à%transmission%
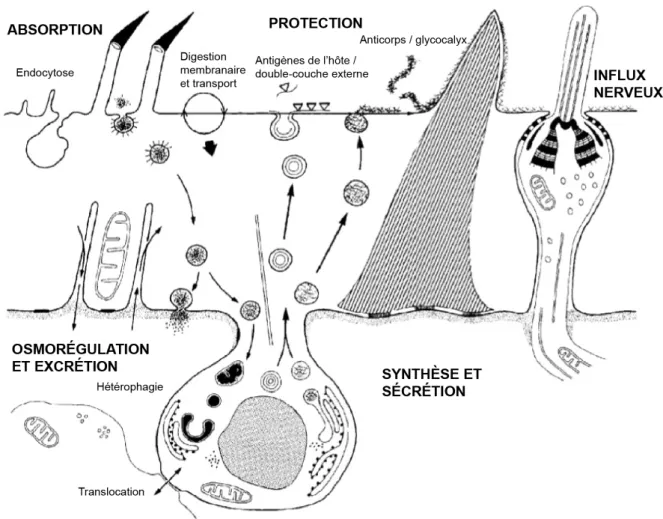
Le contrôle de la différenciation et le bon fonctionnement du tégument sont donc moins vulnérables aux sécrétions de l'hôte. Les observations ultrastructurales du tégument confirment le fait qu'il remplit quatre fonctions principales.
2.2% Microscopie%électronique%à%balayage%
L'objectif principal de cette étude est de fournir une représentation de la parasitofaune de l'anguille européenne dans ces deux lagons. Une autre publication s'intéresse aux différences de paramètres épidémiologiques observés dans les deux lagons en fonction des saisons, de la taille ou du statut argenté de l'hôte.
C HAPITRE( II%
OBSERVEES %
- 2% LES%SITES%ETUDIES%
- 2.1% La%lagune%de%Biguglia%
- 2.2% La%lagune%d’Urbino%
- 3% LES%ESPÈCES%PARASITES%OBSERVÉES%
- 3.2% Monogènes%
- 3.3% Cestodes%
- 3.4% Nématodes
- 3.6% Copépodes%
- 3.7% Myxozoaires%
Goezia anguillae Lèbre & Petter, 1983 est un nématode de la famille des Anisakidae (Figure II.12). Ergasilus gibbus von Nordmann, 1832 est un copépode cyclopoïde de la famille des Ergasilidae (Figure II.14).

C HAPITRE( VI&
TOPOGRAPHY)AND)ULTRASTRUCTURE'OF'THE' TEGUMENT'OF' B UCEPHALUS)ANGUILLAE!
O STEICHTHYEN : ! A NGUILLIDAE )&
1& INTRODUCTION&
The present study deals with the ultrastructural description of the tegument of Bucephalus anguillae Špakulová, Macko, Berrilli & Dezfuli, 2002 (Digenea: Bucephalidae), a. Tegumental secretions are also known to participate in digestion and are suggested to be involved in the defense against immune responses of the hosts (Lumsden, 1975).
2.2& SEM&
In this study, examination using SEM and TEM was performed on the tegument of numerous specimens of B. Fish were brought alive to the laboratory, anesthetized in 10% eugenol solution, killed and dissected on the day of capture. After dissection of the fish intestine, live helminths were collected with a Pasteur pipette and kept active in physiological salt solution (0.7% NaCl).
For each, 20 cilia lengths were measured at the front of the body and 20 at the back. The average cilia length and standard deviation were determined for each sample and for the entire sample.
2.3& TEM&
3& RESULTS&
3.1& SEM&
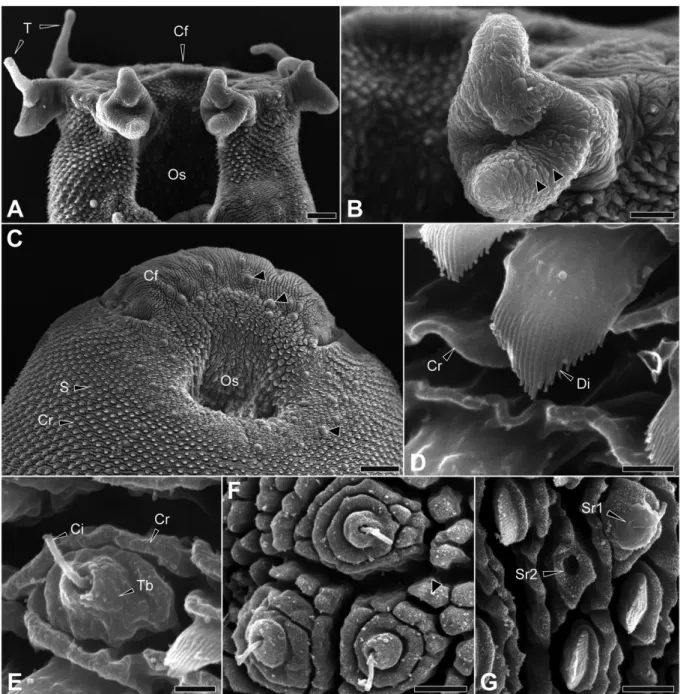
Sensory receptors were clustered around the anterior suction and on the sickle-shaped rhynx formation (Fig. VI.1C). It was observed alone or associated with 1 or 2 receptors of the same type (Figure VI.1F). They were observed alone, associated with one or two receptors of the same type, or associated with type 1 receptors (Figure VI.1G).
They were found alone or associated with 1 or more receptors of the same type (Figures VI.3C,D). Spines appeared to be absent from cobblestone units of the tegument (Figure VI.3C), except near the excretory pore (Figure VI.3D).
3.2& TEM&
An attachment site connected this structure to the top of the bulb (Figure VI.5D, VI.6). They pass through the distal cytoplasm and are released via an eccrine secretion (Figure VI.5G). A second muscle layer is present in the suction tegument below the cytons in the nucleation region (Figure VI.5H).
The inner extremity of the syncytial tegument is located under the cytons or under the second muscle layer (Fig. VI.4A). They had the same variability in shape and size as those in the front of the body (Fig. VI.7B).

4& Discussion&
Cohen et al., 1995), thus explaining the tegumental invagination of spines near excretory pores in this study. The presence of cytoplasmic expansions and cytosine made it difficult to accurately measure the thickness of the distal cytoplasm. However, Hockley and McLaren (1973) argue that the thickness of the digenean tegument probably depends on the environment and the size of the worm.
Cobblestone areas of the tegument are present only on the crescentic formation of the rhynchus and in the posterior part of the body, while they are present on the entire body surface of P. Similar structures have been described for other digeneans such as Cyclocoelum mutabile (Tajrine et al. , 1999), Heterophyopsis continua (Hong et al., 1991), Leucochloridiomorpha constantiae (Font & Wittrock, 1980) and Metagonimus yokogawai (Lee et al., 1984).
4.3& Spines&
Slightly curved with basal plate Shell-shaped with 30 die rings Folded in at the back of the body. Rows of spines on the oral suction cup have been recorded in some other bucephalid species (Moravec & Sey, 1989; Whittington & Cribb, 2001). According to Bakke (1976) and Bennett (1975b), the absence of spines may be less irritating to the host mucosa.
Thus, the decrease in spine density may be related to the specialization of the rhynchus and facilitate attachment to the host tissue (Pandey & Tewari, 1984) without excess. These are thought to be involved in spine motility (Bennett & Threadgold, 1975; . Abbas & Cain, 1987; Ferrer et al., 2001) and associated with the attachment function of the rhynchus.

4.4& Vesicles&
According to Whittington & Cribb (2001), similar secretions are thought to be involved in adhesion to the host mucosa and related to the operation of the rhynchus. The conical electron-dense structure and its attachment site observed in type 1 sensory receptors of B. It is also possible that the SEM preparation may have had an impact on the contraction of the fluke, resulting in the retraction of the cilia at the posterior part of the body.
However, TEM micrographs of these sensory receptors do not show any excessive retraction of the cilia. Thus, Bennett (1975b) suggested that the cilia may be able to register the direction and degree of any pressure acting on the spines since they are elongated to the same height as the surrounding spines.

C HAPITRE( VII&
T OPOGRAPHY(AND( U LTRASTRUCTURE)OF)THE!
T EGUMENT'OF' L ECITHOCHIRIUM*MUSCUL US!
E UROPEAN( E EL# A NGUILLA'ANGUILLA!
O STEICHTHYES : ! A NGUILLIDAE )&
- 1& INTRODUCTION&
- 2.2&SEM&
- 2.3&TEM&
- 3& RESULTS&
- 4& DISCUSSION&
The numerous cobblestone-like units of the tegument enhanced the braided appearance of the tegument on the ventral surface (Figure VII.1C). The dorsal surface of the fluke showed a smoother tegument than on the ventral surface (Figure VII.1D). The ventral suction cup, located on the anterior third of the body, was more developed than the oral one (Figure VII.1A, B).
The deeper layer of the tegument consisted of cytons of different shapes and sizes (Figure VII.2A). The receptors of the upper pair were closer (38 µm) than those of the lower pair (59 µm) (Figure VII.3A).
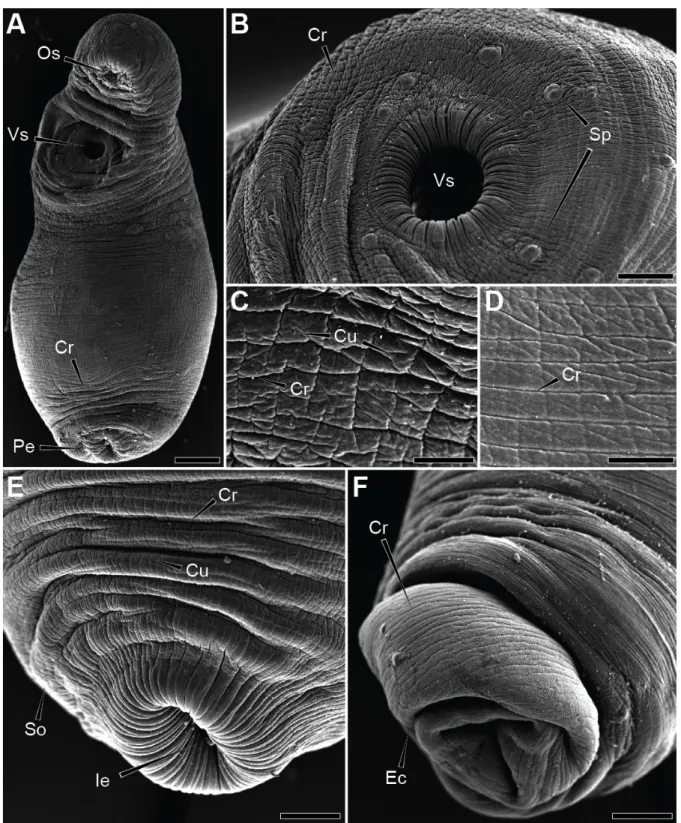
CHAPITRE ! VIII&
T OPOGRAPHY(AND(ULTRASTRUCTURE&OF&THE&
OF#THE# E UROPEAN(EEL( A NGUILLA'ANGUILLA!
1& INTRODUCTION&
The combination of both techniques therefore provides a complete morphological description of the tegumental structures. Among Digenea, the Deropristidae Cable & Hunninen, 1942 is a small group of digenea that are mainly parasitic in the spiral intestine of chondrosteans and, occasionally, the intestine of teleosts in euryhaline conditions in the eastern Nearctic and western Palearctic area (Choudhury & Dick, Dick; Jones et al., 2005). Most recent authors recognized it as belonging to the family Deroprisidae (Choudhury & Dick, 1998; Jones et al., 2005).
However, the authors focused on the collar region of the fluke and its tegumentary spines. To the best of our knowledge, no electron microscopic study using both techniques has been performed on the tegument of a trematode from the family Deropristidae.
2.2&SEM&
2.3&TEM&
3& RESULTS&
A prominent tegumental junction connected the upper cytoplasmic ridge to the tip of the growing spine (Figure VIII.2B). Within the posterior third of the body, the spines were about 3 µm in length, 4 µm in width and showed a crenellated tip (Figure VIII.2E). At the posterior extremity, no ridge was observed rising from the numerous cytoplasmic ridges (Figure VIII.2F).
A layer of interstitial matrix lay just beneath the basal matrix in the nucleated region (Figure VIII.3A, C). This combination was observed in two separate rows of clustered type 1 sensory receptors starting from the ventral side of the oral collar and ending near the mouth (Figure VIII.4A).
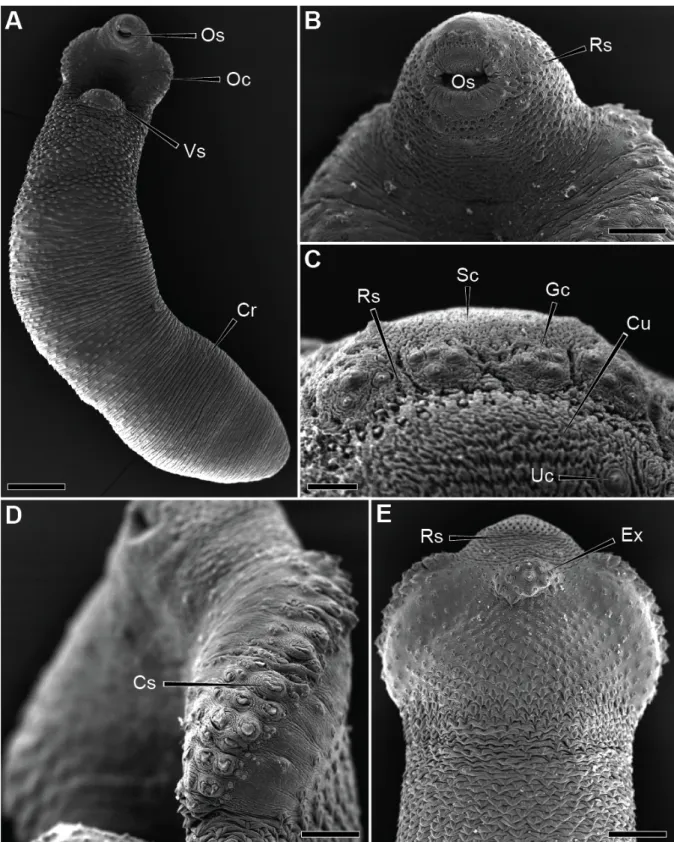
4& DISCUSSION&
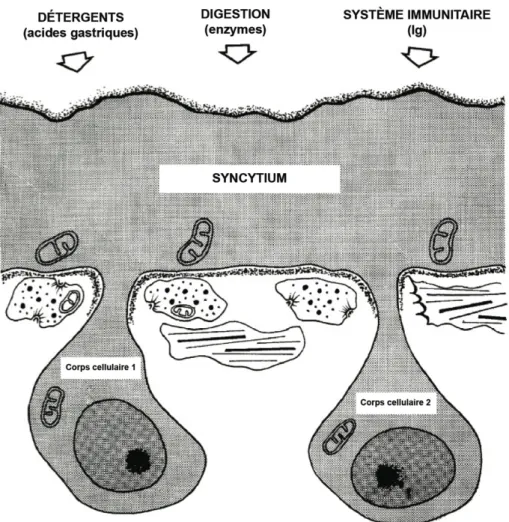
These secretory vesicles are involved in the surface membrane turnover that becomes a protective device for the parasite (Threadgold, 1984). Other authors have suggested that they are involved in the maintenance of the outer membrane or in immuno-protection (Lumsden, 1975; Hanna, 1980a, b). It is assumed that many of the cells of the flatworms are physically connected to the ECM by intercellular junctions (Conn, 1993).
Cytoplasmic bridges that occur through the ECM and connect the cytons to the distal cytoplasm were observed in the integument of D. The cytones can therefore avoid any adverse influence of the host and thus allow regional differentiation and specialization (Halton & Johnston, 1982a).
4.2&Spines&
The long striated root seen in the nerve bulb of the type 1 sensory receptor of D. Sensory receptors with the same external morphology of the type 2 observed here have also been found in other trematodes such as G. These papillae are very similar in morphology and ultrastructure to the type 2 sensory receptor of D.
This central mass, intensely stained with urea-silver nitrate, may be comparable to the electron-dense structure recovered within the type 2 sensory receptor of D. The absence of connection with the external environment observed for the type 2 sensory receptor probably hinders its functioning as a chemoreceptor.

C HAPITRE% IX&
S YNTHESE , !PERSPECTIVES!ET#CONCLUSION
Cependant, les valeurs les plus élevées de diversité spécifique et les valeurs les plus faibles de dominance ont été calculées pour les communautés parasites d'anguilles de la lagune de Biguglia. De nombreux récepteurs sensoriels de ce type ont été observés autour du piston ventral. Des unités tégumentaires en forme de « Klais » ont été observées sur la formation semi-circulaire lisse du piston buccal.
Ces données seront particulièrement importantes dans le cas de l'analyse de l'impact du bassin versant communautaire de Bastia sur la lagune de Biguglia (Pergent-Martini et al., 1997). L'étude de la relation hôte-parasite implique des processus moléculaires complexes (Threadgold, 1963 ; Roberts & Janovy, 1996).
B IBLIOGRAPHIE
Parasites of Anguilla anguilla (L.) from three coastal lagoons of the Ebro River Delta (western Mediterranean).
Résumé&
Abstract&