Postulated by Ehrlich at the beginning of the last century (Erlich and Herta, 1904), this concept is most often based on the use of the recognition properties of antigens associated with cancerous tumors by means of antibodies [1]. The final part of my work investigated the chemical feasibility and preliminary attempts at the biological feasibility of 44m/44Sc.
Rappels bibliographiques
- Le Scandium : l’élément et sa chimie de coordination
- Radio-isotopes du Scandium et voies de production
- Extraction / purification du Scandium
- Comportement biologique du Scandium
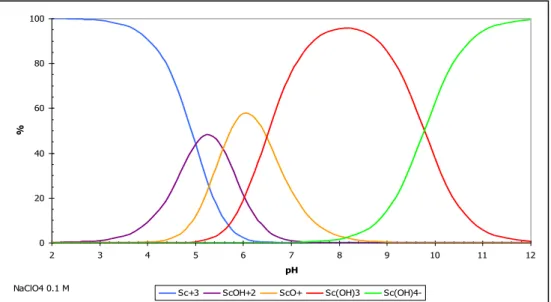
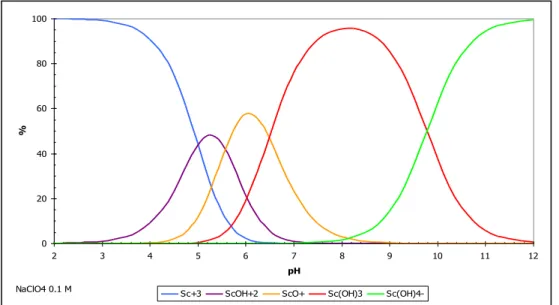
Le scandium est également associé in vivo à des molécules organiques de faible poids moléculaire. L'EDTA et le DTPA présentent une libération de scandium in vivo et plus spécifiquement de DTPA [19].
![Figure 2: schéma de décroissance du Scandium-44 [12]](https://thumb-eu.123doks.com/thumbv2/1bibliocom/464496.69990/35.893.108.715.152.540/figure-2-schéma-décroissance-scandium-44-12.webp)
Compte tenu de la perte d'énergie dans les plaques de Kapton et dans l'air, le deutérium incident atteint la cible avec une énergie de 14,92 MeV et une énergie de sortie de 7,5 MeV. Ils sont déterminés par des techniques batch pour le scandium et autres cations concurrents pouvant provenir de la cible ou être présents dans la station d'irradiation (Ca, Co, Cr, Ni, Zn, Fe, Cu et Al), pour différentes concentrations d'acides, soit chlorhydriques acide ou azote.
Générateur 44m/44 Sc: vers des cibles de plus haute activité
La colonne est ensuite rincée avec 10 mL de HNO3 1 M pour éliminer toutes les impuretés métalliques. Pour l’extraction/purification du scandium, nous avons d’abord utilisé le même protocole que pour le traitement des petites cibles 44CaCO3.
Recyclage des cibles de 44 CaCO 3 et contrôle de pureté
Cependant, il y a eu des pertes significatives dans l’activité produite dans le 44m/44Sc, en particulier lors de l’élution du 44Sc en utilisant 0,1 M de HCl. En effet, des contrôles qualité ont été effectués à chaque étape, ce qui a permis d'assurer et de contrôler la qualité du matériel pour la préparation de nouvelles cibles.
Calculs DFT sur des complexes de Scandium
Les premières études ont été réalisées sur des complexes Sc(H2O)n3+ (n = 1-10). Les résultats obtenus indiquent que le Sc(III) est principalement octa-coordonné avec la première sphère de coordination, formant un complexe de géométrie S8 (Figure 5). Des calculs complémentaires ont ensuite été effectués et ont montré que la Sc est principalement hexacoordonnée avec la première sphère de coordination, qui forme le complexe le plus stable.
Cependant, DOTA forme des complexes beaucoup plus stables avec Sc(III) qu’avec Y(III). Les premières études ont été réalisées sur des complexes Sc(H2O)n3+ (n = 1-10), et les résultats obtenus montrent que Sc(III) est principalement octocoordonné.
Les dérivés de type phosphonique du DOTA nouveaux ligands pour le
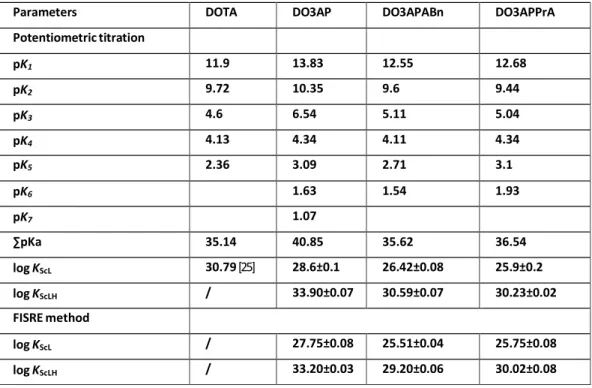
Constantes de stabilité
Dans cette étude, nous avons d’abord déterminé les constantes de stabilité du scandium avec des ligands phosphoniques puis comparé les résultats obtenus avec DOTA. Ces trois ligands présentent des valeurs élevées de constantes de stabilité avec Sc(III).
Optimisation des conditions de radiomarquage
Les paramètres cinétiques, et notamment la température, ont été évalués pour ces ligands lors des études de radiomarquage. La prochaine étape consiste donc à optimiser les conditions physico-chimiques de radiomarquage du 4 4Sc avec les dérivés monophosphoniques du DOTA puis à comparer les résultats obtenus avec le ligand DOTA.
Etudes de stabilité in vitro
DO3AP.2H2O, 44m/44Sc-DO3APABn et 44m/44Sc-DO3APPrA présentent une bonne stabilité dans l'hydroxyapatite, et aucune accumulation dans les os ne semble attendue in vivo en raison de la présence d'un seul groupe phosphonique dans les ligands étudiés. Les dérivés monophosphoriques du DOTA ne constituent donc pas une meilleure alternative pour la complexation du scandium par rapport au DOTA.
Faisabilité chimique du 44m/44 Sc comme potentiel générateur in vivo
Dans une deuxième expérience, une solution de DOTATATE libre a été chargée sur la colonne et éluée avec des solutions de DTPA à des concentrations croissantes. Enfin, une solution de 4 6Sc-DOTATATE, radiomarquée à 97 %, a été chargée sur les strates C-18, augmentant ainsi les concentrations.
Biodistribution et images TEP
De tous ces résultats, il serait possible de conclure que le 44m/44Sc est un possible générateur in vivo. 44mSc, coproduit avec 44Sc, lui permettant d'être considéré comme un générateur 44mSc/44Sc in vivo.

Medicine
- Radiopharmaceuticals
- Selection criteria for radionuclides
- Radionuclides of interest for PET imaging
- Scandium
- Scandium chemistry
- Coordination chemistry: ligands selected for the complexation of Scandium 71
- Biodistribution and toxicity of Scandium
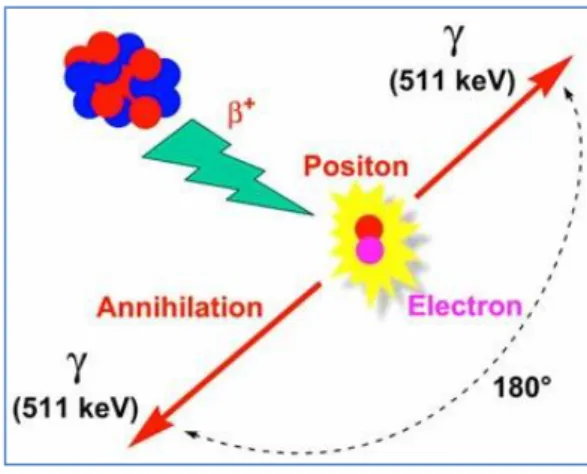
By detecting β+ emitter radionuclide, the in vivo biodistribution of the biological probe can be monitored. The photons (γ) are the most energetic electromagnetic radiation and can travel through the human body with relatively low energy (20 to 600 keV), thus allowing the location of the radionuclide. For example, a large parathyroid absorption of the 46Sc suggested the use of this radionuclide in the treatment of tumors and diagnostic studies [93].
Currently, the high energy intensity of Cyclotron ARRONAX [21] allows the production of 44Sc via the 44Ca (d, 2n) nuclear reaction.
- Using protons as projectile
- Using deuterons as projectile
By choosing the incident proton energy below this value, it is possible to avoid the production of 43Sc. The ratio of the production cross section between the two isomers 44mSc/44Sc was previously measured for the reaction (p, n) [112]. A comparison between the experimental cross sections for production of 44m/44Sc previously measured [110] and the values predicted by the code for the nuclear reaction 44Ca (p, n) was performed [14].
At the ARRONAX cyclotron, 44Sc production is achieved in a production station called Nice3, which is dedicated to the production of small operations (a few mCi at most).

With this device, our target is placed in the air (Figure 3), 6.6 cm downstream of the beamline exit, sealed by a 75 µm thick Kapton foil that allows separation between the vacuum and the air.
Cyclotron production of high purity 44m/44 Sc with deuterons from 4 4 CaCO3
- Introduction
- Materials and Methods
- Chemicals and reagents
- Cyclotron targetry and irradiations
- Partition coefficients determinations
- Radiochemical separations
- Results and discussion
- Cyclotron targetry and irradiations
- Radiochemical separations
- Enriched calcium recycling
- Radiolabelling
- Conclusion
The quality of the final batch with regard to radionuclide purity, specific activity and metal impurities allowed an immediate use for further radiopharmaceutical evaluation. This approach takes advantage of the insolubility of Sc(OH)3 either as a precipitate or coprecipitate, and is similar to a technique developed to purify yttrium from strontium [26]. At the end of the purification process, the scandium solution was evaporated to dryness and the, recycled in a small volume of HCl 0.1 mol.L−1.
Due to the high cost of enriched material, a recycling process was developed for the target.

4. 44m/44 Sc generator: to targets with higher activity
- Preparation of the target
- Conditions of the irradiation
- Extraction and purification
- Recycling of the CaCO 3 targets and purity control
- Monitoring sources of 44m/44 Sc produced: activity and radiochemical purity
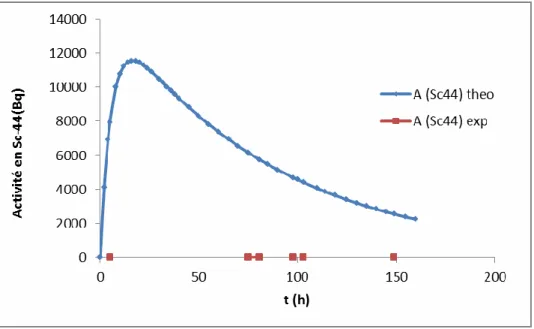
Due to the high cost of the enriched material used to produce 44Sc, a protocol (Figure 6) was developed to recover 44Ca eluted from the DGA resin with HCl 4M after irradiation. Also follow-up on the purity of the different sources of 44m/44Sc produced will be presented. The activity in MBq of the produced radionuclide is not the only one to be taken into account, the specific activity is also a decisive parameter.
Due to the high cost of the raw material used for the production of 44Sc, a protocol was developed to recover 44Ca.

Computational Methods
Density functional theory (DFT) has become a general tool to investigate the structure and properties of inorganic molecules, such as lanthanide(III) coordination compounds, because of the high accuracy that can be achieved at relatively low cost. calculation [122].
DFT studies on Scandium complexes: from water molecule to cyclen moiety
- Computational method
As expected, placement of one water molecule in the second sphere contributes more to the stability of the resulting Sc complexes compared to their counterparts when all water molecules were placed in the first hydration shell. Thus, the presence of six water molecules in the first coordination shell contributes more to the stability of scandium complexes. The present results show that the Sc3+ ion is primarily hexa-coordinated; the presence of six water molecules in the first coordination shell contributes more to the stability of scandium complexes.
The Mulliken charge of the Sc(III) ion is more important in the case of the lowest energy conformer.
![Figure 4: Structures of the [Sc(H 2 O) n ] 3+ cluster](https://thumb-eu.123doks.com/thumbv2/1bibliocom/464496.69990/146.893.74.833.87.879/figure-4-structures-sc-h-2-o-cluster.webp)
Study of a DOTA ligand: estimation of the complexation energy with Scandium
- systems [Sc(CH 3 COO - ) m ] (3-m)
- systems [Sc(H 2 O) (n-m) (CH 3 COO - ) m ] (3-m)
Sc(H2O)(n-m)(CH3COO)m](3-m) cluster and the Mulliken charge of the Sc(III) ion decreases, confirming that the acetate group contributes more effectively to the stability of Sc(III ) complexes formed in relation to the water molecule. When one or two water molecules were replaced by one or two acetate groups, respectively, the complexes formed are located in the first coordination sphere. However, when three water molecules were replaced with three acetate groups, one water molecule in the [Sc-(H2O)5-(CH3COO)3] complex migrates from the first to the second coordination sphere and connected to two water molecules and one acetate group from the first shell.
Even when replacing four water molecules with acetate groups, one acetate group and one water molecule move from the first to the second coordination sphere of the Sc(III) ion and are connected to two water molecules and an acetate group from the first sphere.
![Table 1: Acetate binding energy, Sc-O distance and Mulliken charge for [Sc(CH 3 COO) m ] (3-m) cluster](https://thumb-eu.123doks.com/thumbv2/1bibliocom/464496.69990/155.893.60.836.246.867/table-acetate-binding-energy-distance-mulliken-charge-cluster.webp)
3.3. [Sc(Y)-DOTA] 3+ /[Sc(Y)-DOTA(H 2 O)] 3+ complexes
- Study of the phosphonic derivatives of DOTA-type ligands
- systems [Sc(CH 3 PO 3 ) m ] (3-2m) and [Sc(H 2 O) n’ (CH 3 PO 3 ) m’ ] (3-2m’)
- Scandium and yttrium–complexes with monophosphonic DOTA analogs 164
The primarily obtained results, such as the binding energy and symmetry of the Sc(III) and Y(III) complexes formed with DOTA, average Sc-N, Y-N, Sc-O and Y-O distance and preorganization energy are listed in Table 3. the binding energy of the [Sc(CH3PO3)m] (3-2m) complexes is the lowest compared to that obtained in the case of the [Sc(CH3COO-)m](3-m) clusters, this means. The three Sc-O distances from the acetate arms are relatively higher than the Sc-DOTA complex.
However, for the [Sc-HDO3AP]-1 complex almost all the Sc-O and Sc-N distance remains unchanged compared to the [Sc-DOTA]-1 complex, the binding energy is lower in the presence of the DOTA part relative to the DO3AP once it is protonated.

Experimental
- Chemicals
- Production of 44 Sc
- Equilibrium studies
- NMR measurements
- Free-ion selective radiotracer extraction (FISRE) method
- Radiolabeling with 44 Sc
- Quality control
- Equilibrium studies
- Characterization of the Sc(III) complexes in solution
- Determination of stability constants under trace concentrations
- Labeling of the title ligands with generator- ( 44 Ti/ 44 Sc) and cyclotron-produced
- In vitro stability studies
Job's method was used to determine the stoichiometry of the off-cage species in the Sc(III)-DO3AP system. 48] The overall stoichiometry of Sc(III)-DO3AP species present in acidic solutions can be roughly estimated as 1 : 2 from the Job plot of the NMR signal intensity (Figure S3). Labeling of the title ligands with generator (44Ti/44Sc) and cyclotron (44m/44Sc) produced radioscandium (44m/44Sc).
The specific activity of the labeled chelators was higher for cyclotron-produced 44m/44Sc (~10 MBq/nmol) than for generator-produced 44Sc (~2 MBq/nmol).
Acknowledgments
50] Shubert, The use of ion exchangers for the determination of physicochemical properties of substances, especially radioactive tracers in solutions., 1948; Shubert, Analytical Applications of Ion Exchange Separations., 1950; Shubert, Lind, Westfall, Plefger, & Li Jurkin, Gildehaus, & Wierczinski, Kinetic stability studies of yttrium(III tetraazacyclododecane-1,4,7,10-tetraacetic acid by free ion-selective radiotracer extraction,. 2007; Jurkin & Wierczinski, Analysis of the kinetic stability of yttrium(III) chelates by free ion-selective radiotracer extraction (FISRE)., 2008; Jurkin, Gildehaus, & Wierczinski, Dissociation kinetic determination of yttrium(III) polyaminopolycarboxylates by free ion-selective radiotracer extraction (FISRE) ), 2009.
Behavior of Sc-DOTA complex under irradiation: a theoretical approach
Behavior of Sc-DOTA complex under irradiation: an experimental approach . 210
- Irradiation of Sc-DOTA complex with a Saturn 41: linear electron
Optimization of reaction conditions for the radiolabeling of DOTA and DOTA-
Radiolabeling of scandium with DOTA analogs
- Purification step before injection
- Oasis purification
- HPLC quality control
- In vitro stability of the radiolabelled 44m/44 Sc-DOTA peptides
- In vivo studies (Biodistribution studies and PET imaging)
- Free 44 Sc
4.2. 44 Sc-monophosphonic DOTA analogs complexes
4.3. 46 Sc-DOTATATE complex