Dans cette partie de la thèse, nous présenterons dans un premier temps l'état de l'art des protéines fluorescentes, depuis la découverte du phénomène de fluorescence jusqu'aux protéines fluorescentes photoactivées. Une application futuriste intéressante consiste à utiliser ces protéines pour stocker de manière irréversible des données en utilisant la propriété de photoconversion du vert au rouge ou pour permettre l'effacement et l'écrasement en utilisant la propriété de photocommutation réversible.
History of the fluorescence discovery
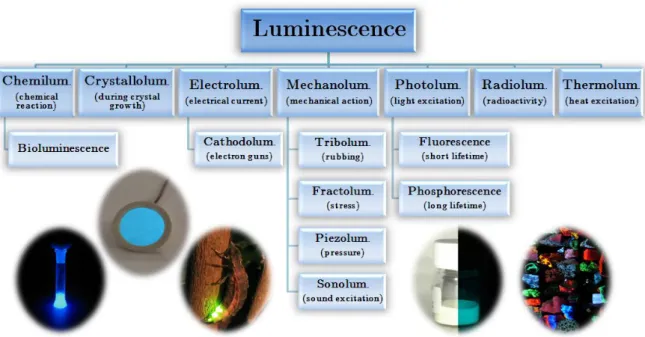
En utilisant une approche historique, ce chapitre d'introduction nous permet de comprendre et de différencier les termes et concepts complexes de phosphorescence, fluorescence et luminescence. De la découverte des premières pierres émettant de la lumière dans l'obscurité jusqu'aux animaux luminescents, les phénomènes physiques d'absorption, de fluorescence, de phosphorescence, de relaxation sans rayonnement et de fluorescence retardée seront expliqués avec des exemples.
Phosphorescence, fluorescence or luminescence?
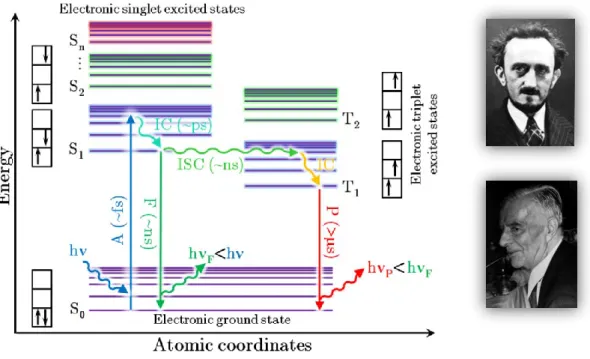
First, by relaxing to the lowest vibrational level of the excited state S1 through internal conversion (IC) in a time range that exists between 10-13 and 10-11 seconds, thanks to the interactions of the molecule with its environment. In the case of fluorescence, the return to the ground state S0 is preceded by the relaxation from the excited vibrational level reached to the lowest vibrational level of the S1.
The explanation of an ancestral mystery
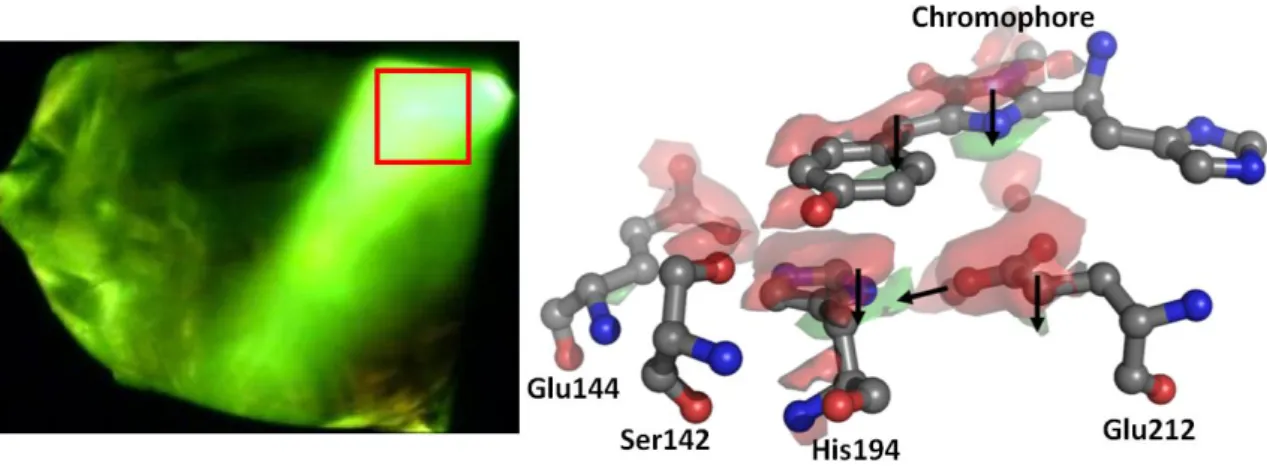
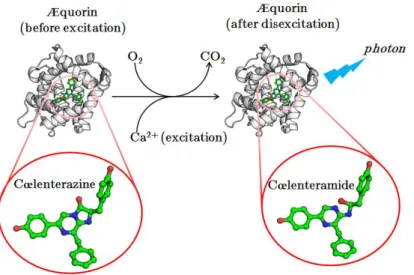
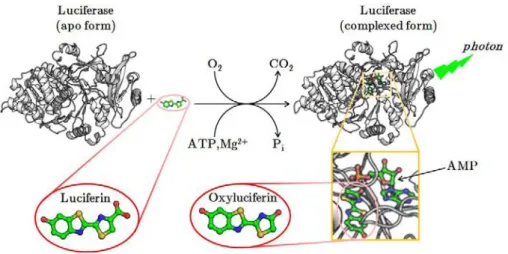
He allowed great improvements in the understanding of bioluminescence (Harvey until the end of his career in the 1950s (he died in 1959), at which date his team became the world leader in bioluminescence research. As in the case of oxyluciferin (excited form of the luciferin), the activated form of the cœlenterazine, the cœlenteramide, will be decarboxylated together with the emission of a blue photon ( max = 470 nm).
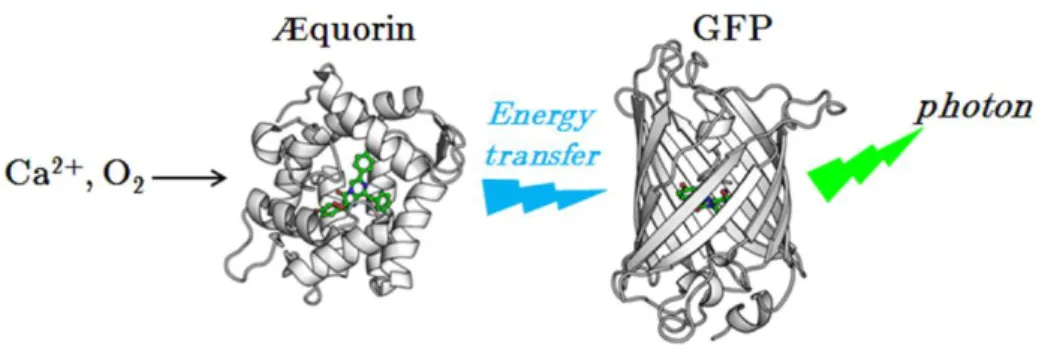
Fluorescent proteins shed a new light on a bioluminescent world
Dans ce chapitre, nous parlons de la protéine fluorescente verte (GFP) et de la révolution qu'elle a apportée au monde de la recherche biomédicale. Le concept de systèmes conjugués et la dépendance au pH de la fluorescence de cette protéine sont présentés.
The Green Fluorescent Protein
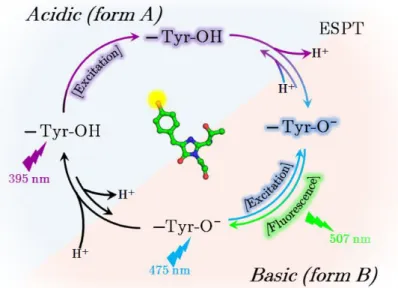
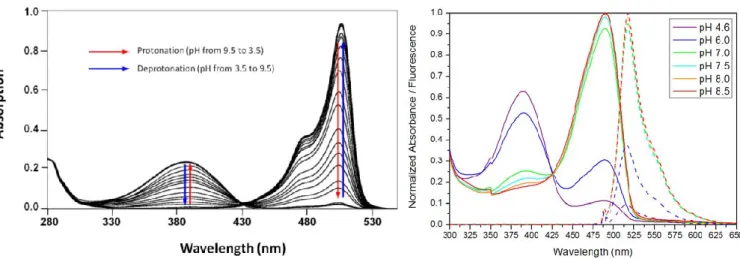
The hydroxybenzylidene part of the GFP chromophore is an important element that can be either protonated (phenol) or anionic (phenolate). Depending on the protonation state and the delocalization, the spectroscopic properties of the chromophore can be drastically modified.

The discovery of anthozoan fluorescent proteins
Structural characterization of mTFP0.7 revealed here again that a cis/trans isomerization of the chromophore was involved in the reversible photobleaching properties of this protein (Henderson et al. 2007). The crystal structure of Kaede confirmed that the green-to-red photoconversion was due to a UV-induced backbone cleavage between the first chromophore residue (His62) and the preceding one (Mizuno et al. 2003).
Applications of PAFPs and aim of the thesis
Ce chapitre aborde l'utilisation de protéines fluorescentes photoactivées et en particulier le suivi des protéines d'intérêt dans les cellules vivantes et leur utilisation en microscopie à fluorescence haute résolution. Enfin, l'objectif de cette thèse sera expliqué : l'étude des phénomènes de phototransformation que subissent les protéines fluorescentes photoactivables : photoconversion, photoswitching et photoblanchiment, grâce à l'utilisation de trois protéines modèles.
Tracking elements within living cells
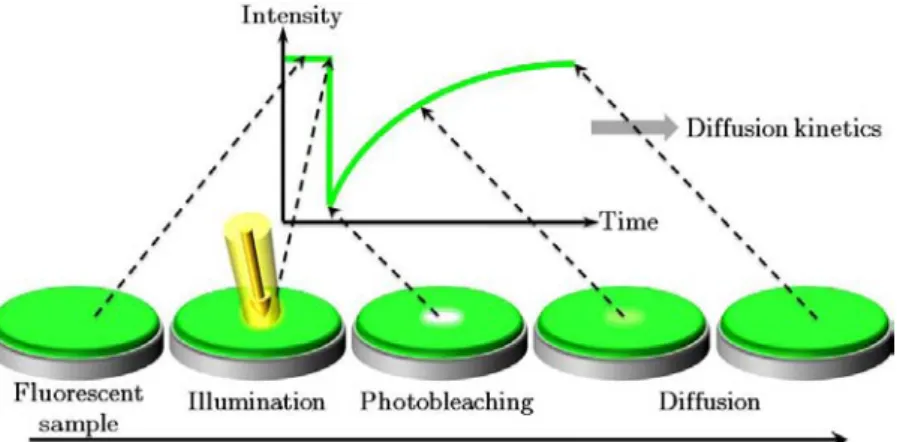
Super-resolution microscopy using PAFPs
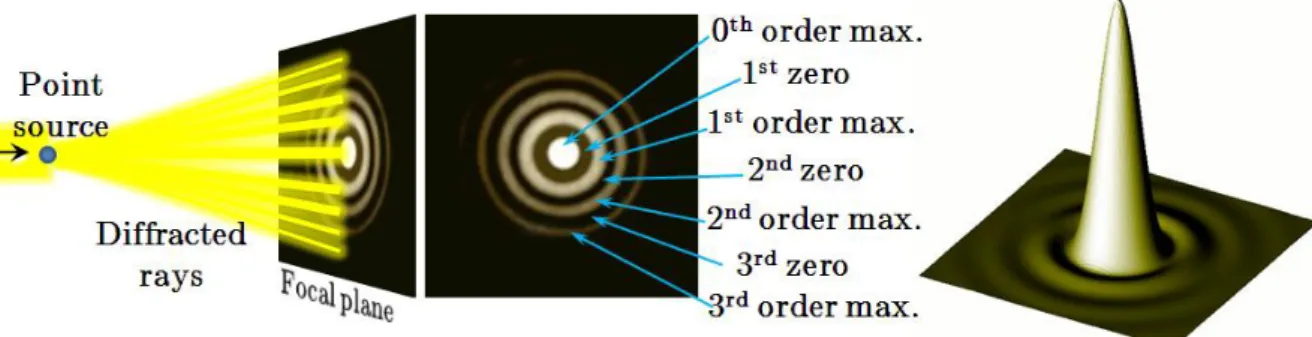
Physicist Ernst Abbe (Figure I.3.3) discovered (Abbe 1873) that in the far-field microscope, using visible light, the smallest distance between two distinguishable objects determines the resolving power (r) of the apparatus. The first high-resolution fluorescence microscopy technique was developed by Stefan Hell (Figure I.3.7) and his colleagues in Göttingen, Germany (where Ernst Abbe received his doctorate in 1861) and is called STED (Stimulated Emission Depletion). Recently, the use of Kaede-like PAFPs in microscopy found an impressive application with the development of photo-activated localization microscopy by Eric Betzig (Figure I.3.7) and colleagues at the Howard Hughes Medical Institute, USA (Betzig et al. 2006).
Aim of this thesis work
Thus, IrisFP represents the first PAFP that brings together the properties of both the Kaede-like family and the Dronpa-like family and allows a better understanding of photoconversion and photochromic behavior in PAFPs. The illustration represents the 3D structures for the four states of the fluorescent protein IrisFP described in this thesis: green cis, green trans, red cis, red trans. The structures are surrounded by absorption and emission spectra of the different phototransformations investigated in this thesis: reversible green-to-dark conversion (left), photobleaching and green-to-red conversion in fluorescence (back) and green-to-red conversion into absorbance (right).
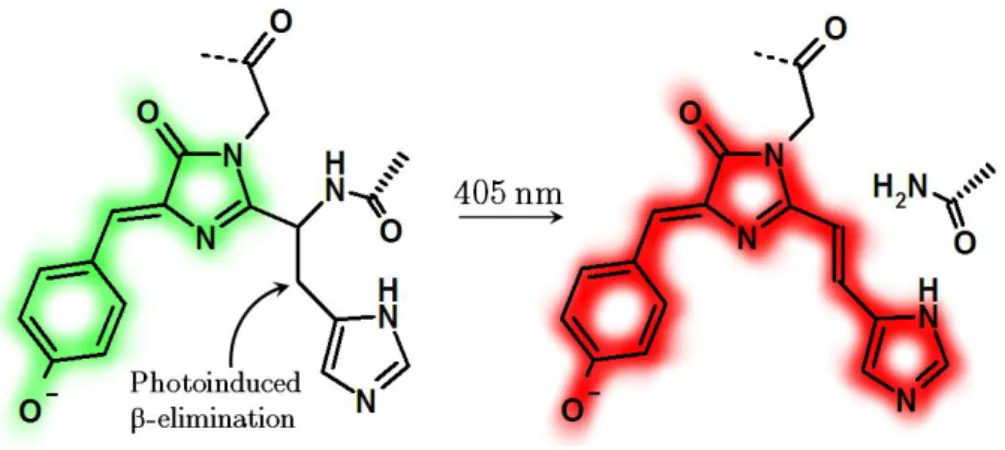
Photoconversion from green to red
Cette propriété permet donc d'étudier quels acides aminés sont impliqués dans les interfaces et de proposer des mutations pour améliorer la stabilité de ces protéines sous leur forme monomère. MONG, très rares protéines fluorescentes photoconvertibles (PCFP) du vert au rouge connues à ce jour (Kaede (Ando et al. 2002), Dendra (Labas et al. 2002), EosFP (Wiedenmann et al. 2004), KikGR (Tsutsui et al. . .. 2005), mcavRFP, cjarRFP et lhemOFP (Oswald et al. 2007)), seules les structures vertes et rouges d'EosFP étaient disponibles lorsque j'ai commencé cette thèse. Comme il n'y avait aucune structure connue pour PCFP Dendra (Gurskaya et al. . , 2006), nous avons également tenté d'obtenir la structure 3D d'une version commerciale monomère brillante de cette protéine appelée Dendra2 (Evrogen, Moscou, Russie).
Study of the photoconversion of EosFP
Looking at the spectral series measured at room temperature during the photoconversion (Figure II.1.4, f), no intermediate state appears to be possible, as there is an isosbestic point between the peaks specific to the green and red states. Our results suggest that we can detect an intermediate state of the photoconversion mechanism. Based on the structures of the green and red forms of EosFP and the experimental results of the photoconversion, Mickael Lelimousin (IBS, Grenoble) during his Ph.D.
Dendra2: an efficient PCFP
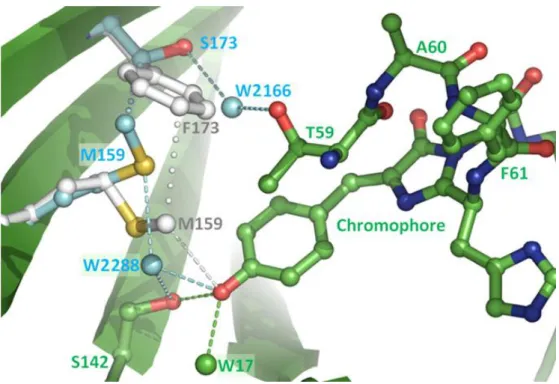
The carbonyl oxygen of the imidazolinone ring hydrogen bonds with the side chain of Arg91 (2.9 Å). These bands are associated with the neutral (phenolic) and anionic (phenolate) forms of the chromophore (Chattoraj et al.). In this variant, the first amino acid of the chromophore triad (Gln66) was mutated into a threonine whose cyclization forms a third chromophore. five-membered heterocycle (2-hydroxy-dihydrooxazole) responsible for the color shift.
Improving the monomerization of PCFPs
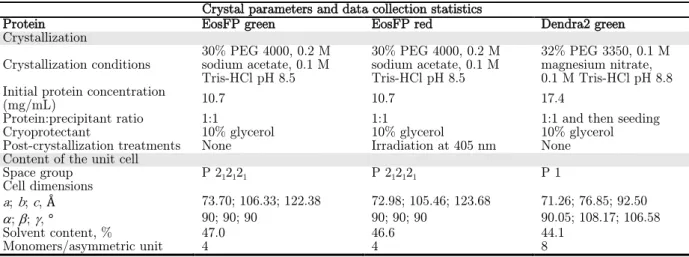
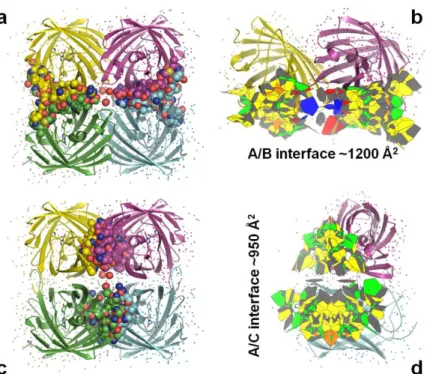
The crystal structure, solved on the MX beamline ID14-3 at the ESRF (Table II.1.6), revealed a tetrameric organization. Regarding the A/C interface, the T158H mutation is very clearly visible in the electron density maps (Figure II.1.24). The interfaces and residues involved in their formation for EosFP, mEosFP and Dendra2 are summarized in Figure II.1.26.
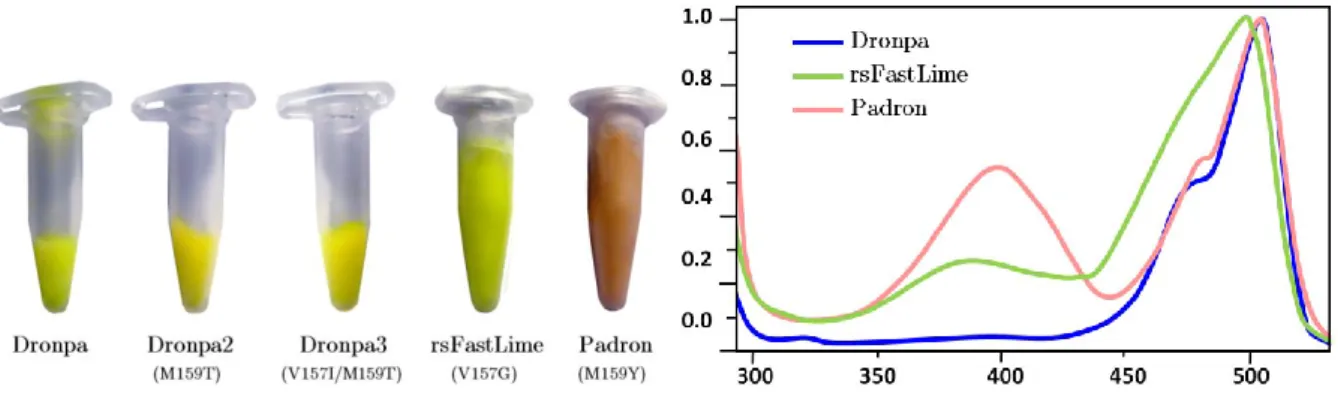
Photoswitching: a reversible bleaching event
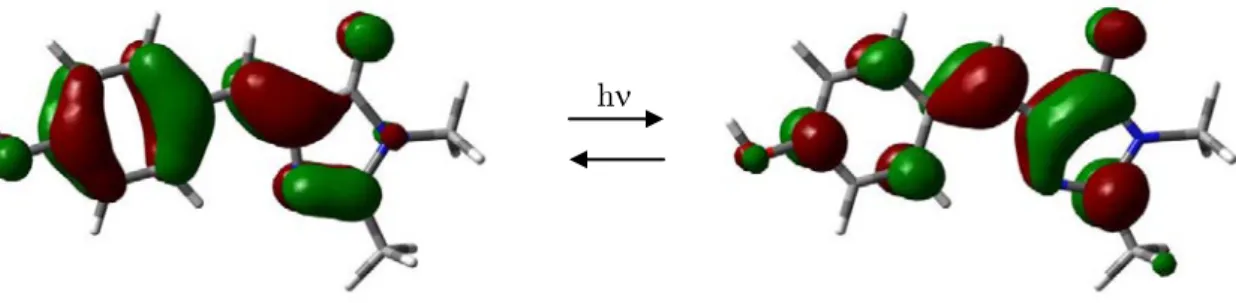
Ce chapitre rassemble les résultats obtenus lors de l'étude du phénomène de photoswitching réversible de certaines protéines fluorescentes photoactivables. En étudiant une variante de l’EosFP, une protéine décrite dans le chapitre précédent, nous avons découvert des propriétés photochromiques, c’est-à-dire que la fluorescence de cette protéine peut être désactivée de manière réversible à volonté. Les raisons de ce phénomène impressionnant s'expliquent grâce à la structure tridimensionnelle de la protéine que nous avons obtenue, qui montre clairement la photoisomérisation du chromophore.
Spectroscopic characterization of a reversible switching
The divergent beam of the 488-nm laser was directed to a crystallization droplet containing crystals of EosFP-F173S (Cf. Emission spectra of the green (red) protein were obtained by exciting at 488 (532) nm and the photoconversion was achieved at 405 nm For studies in the crystalline state, a crystal of the green form of EosFP-F173S was mounted in a modified loop connected by a plastic capillary and.
Structural characterization of the reversible switching
In D, the density map suggests a mixed occupation of the cis and trans states of the chromophore. Free space around the p-hydroxybenzylidene part of the chromophore (Figure II.2.12) can clearly affect the photoswitching kinetics. The superposition of the cis forms of EosFP, Dronpa and mTFP0.7 (Figure II.2.17, A) reveals that these proteins provide quite similar steric.
Further studies about the photoswitching
However, despite this instability, the results (Figure II.2.20, A) are clear and show a decrease in the anionic peak at 488 nm that can be well fitted by a biexponential decay. Both absorption and fluorescence spectra (excited at 502 nm) were measured, and both a thermal recovery and a photoinduced recovery (405 nm) of the partially photo-switched state were followed. The intensity of the 405 nm laser was further increased (76 mW/cm2), resulting in a new monoexponential decay of the green fluorescence (t sec).
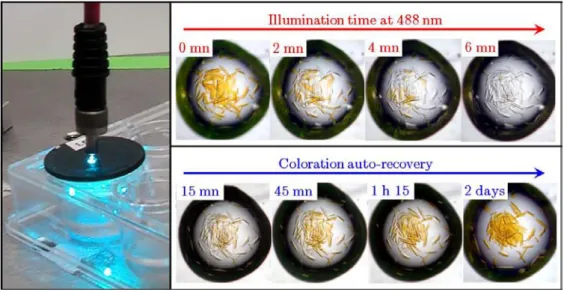
PAFPs as optical data storage devices?
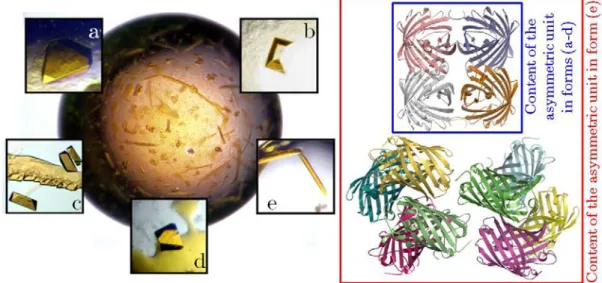
This setup is the same for all experiments shown below, with the exception of the experiment shown in Figure II.2.31. IBS" (stands for Institut de Biologie Structurale) was written on exactly the same crystal scale (Figure II.2.29-d). Green fluorescent crystals of IrisFP were mounted on the goniometer head of the beamline ID14-2 at the ESRF thanks to MicroMesh. supports ( MiTeGen, Ithaca, NY, USA).
About photobleaching
Although photobleaching remains poorly understood, it is widely recognized that the triplet state T1, due to its long lifetime, is the most likely starting point for deleterious reactions leading to chromophore destruction (Donnert et al. 2007). However, with the exception of the engineered protein KillerRed, which has been reported to produce large amounts of 1O2 (Bulina et al. 2006a; Hoogenboom et al. 2005) and we can logically think that such radical formation could also be involved in the photobleaching process of FPs .
Effects of UV-light and X-rays on a fluorescent protein
The protein absorbs very weakly at this wavelength, but at a fairly high power density (760 W/cm²) the fluorescence signal apparently decreases slowly, a victim of photobleaching (Figure II.3.3, B and C). However, as soon as we open the X-ray shutter, the fluorescence signal decreases sharply, indicating that the protein is clearly bleached by X-rays (Figure II.3.3, D). Surprisingly, when the X-ray shutter is closed, there is a partial slow recovery of the fluorescence signal.
Structural basis of photobleaching
The crystallographic statistics for both data sets (non-illuminated and illuminated states of EosFP) are shown in Table II.3.10. The corresponding fluorescence decay is much faster and exhibits a much larger amplitude at comparable doses than in absorbance mode (Figure II.3.5, A). It decreases in two steps and shows a slow monophasic recovery in the absence of X-rays (Figure II.3.6, B).