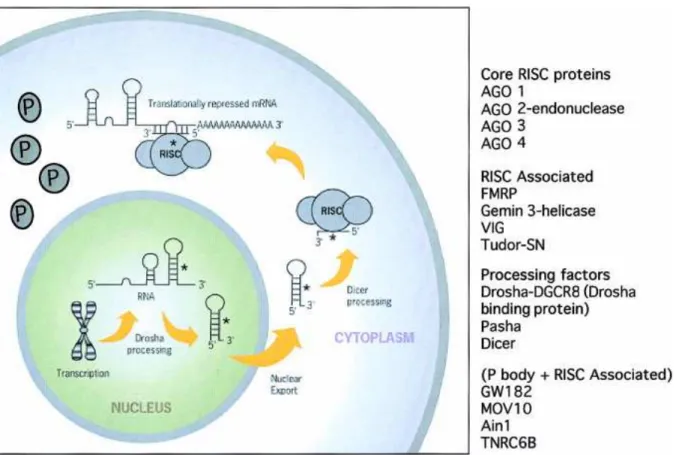
In the cytoplasm, the pre-miRNA is processed into 18-22 nucleotide imperfect double-stranded RNA duplex (miRNA: miRNA*) by the cytoplasmic Ribonuclease III, Dicer, which in humans acts with the transactivator RNA (tar)-binding protein, TRBP (Chendrimada et al. 2005). After cleavage of the mRNA, the miRNA remains intact and can direct the recognition and destruction of additional mRNA (Tang et al., 2003). It was observed that the cooperative action of multiple RISC provides the most efficient translational inhibition (Doench et al., 2003).
Using conserved motifs that did not match any known miRNA, it was possible to predict other miRNA candidates in humans (Xie et al., 2005). Similar approaches have recently been applied to the prediction of miRNAs in A. thaliana (Adai et al., 2005), flies and worms (Chan et al., 2005). This technique has already been successfully applied to identify miRNA expression in mouse embryos (Kloostermam et al., 2006).
It is possible to introduce these vectors into adenovirus or retrovirus (Chen et al., 2004) system and then transfect cultured cells or inject them into mouse tissues in vivo. Harris et al., 2006); the analyzed phenotypes show the important collective functional role of miRNAs in many developing tissues, but cannot provide information about the exact role of individual miRNA. The first indication of the involvement of miRNAs in mammalian limb development came from an in situ hybridization study on mouse embryos (Schulman et al., 2005).
Using microarray analyses, Hornstein et al. 2005) identified a miRNA, miR-196, that is preferentially expressed in the hind legs. Recently, another miRNA, miR-181, was discovered to be upregulated during terminal differentiation of myoblasts ( Naguibneva et al., 2006 ). Several miRNA were preferentially detected in specific hematopoietic cell lineages: miR-181 in differentiated B lymphocytes, miR-142s in B lymphocytes and myeloids, miR-223 in myeloids (Chen et al., 2004).
In another study, Harris et al. 2006) observed that inactivation of Dicer led to dramatic branching effects in the lung.
For example, miR-125b-1 is located in a region that is deleted in a subset of patients with breast, lung, ovarian and cervical cancer (Calin et al., 2004) and has recently also been associated with leukemia. The first indication that miRNAs may function as tumor suppressors came from a report that showed that patients diagnosed with B-cell chronic lymphocytic leukemia (CLL) often had deletions or downregulation of two clustered miRNA genes, miR-15a and miR-16, have. -1 (Calin et al., 2002). In particular, the transcripts of certain let-7 are downregulated in human lung cancer (Takamizawa et al., 2004).
Elegans found that the 3'UTR of Ras genes contains multiple complementary sites for the let-7 family and that let-7 and Ras expression are inversely correlated in tumors (Grosshans et al., 2005; Johnson et al., 2005). The MYC oncogene, which encodes a basic helix-loop-helix transcription factor, is often mutated or amplified in human cancers and has been shown to function as an important regulator of cell growth due to its ability to induce both cell proliferation and apoptosis ( Pelengaris et al., 2002). A recent report (Felli et al., 2005) describes the ability of miR-221 and miR-222 to downregulate the KIT oncogene and future studies will reveal that miRNA function as key regulators of many cancer-related genes such as BCL2, Ras, E2F1. , MYC and KIT.
A recent report by Lu et al. 2005) found that the expression profile of relatively few miRNAs (200) can be sufficient to accurately classify human cancers. AntagomiRs, which are cholesterol-conjugated AMOs, have already been used to inhibit miRNA activity in various organs after injection into mice (Krutzfeldt et al., 2005) and could be promising therapeutic agents.
II The mammary gland
Ia The mammary gland: structure and cellular composition
In general, the epithelial tissue at birth is differentiated into cells that make up ducts, elongated ducts that transport milk, and luminal secretory and myoepithelial cells that together make up the central lumen and the outer layer of the alveoli, the functional secretory structural unit of the mammary gland. Each alveolus has a spherical structure within which a monolayer of epithelial cells secretes milk into the central lumen. The milk is transported into the ducts by the contractile actions of myoepithelial cells and delivered to the body surface through the nipple.
The extensive system of ducts and alveoli is embedded in the stroma, which supports the epithelial tissue and provides nutrition to epithelial cells (see review: Hennighausen and Robinson, 2005). In the mouse, five pairs of mammary glands are located just beneath the skin, extending from the thoracic (three pairs) to the inguinal (two pairs) areas of the animal along what is called the mammary or mammary line (Richert et al., 2000 ). There is a gradient of differentiation between the glands, with the first thoracic gland being the least differentiated and the fifth inguinal gland the most (Bolander, 1990).
Ic The development of mammary gland
While the first is rudimentary (Topper and Freeman, 1980; Russo IH and Russo J, 1996), the stroma is thick and dense around epithelial structures and is composed of eosinophilic fibrous connective tissue and fibroblasts and, in the early stage of development, is filled with large adipocytes. The period of fastest growth occurs during puberty from approx. 3-6 weeks of age in the mouse. The terminal end buds (TEBs) appear after 3 weeks at the tips of growing ducts and are the sites of highest epithelial proliferation in the gland (Richert et al., 2000).
This migration and proliferation lead to both elongation of ducts and invasion of the fat pad; the differentiation in the TEBs is also responsible for branching (Gordon and Bernfield, 1980; Silberstein and Daniel, 1982) and formation of lateral and alveolar buds, which eventually subdivide to form rudimentary alveolar structure in the post-pubertal glands, after 10-12 weeks of age, in response to cyclic secretion of ovarian hormones at each estrous cycle (Andres and Strange, 1999). In early pregnancy, a massive proliferation of ductal branches and the formation of alveolar nodules, as in the postpubertal stage, could be observed. During the second half of pregnancy, the alveolar buds progressively split and differentiate into individual alveoli that fill the majority of the fat pad in late pregnancy.
With the onset of lactation, milk in the lumen of the alveoli is forced into the ducts (Asch HL and Asch BB, 1985; Richardson, 1949; Dulbecco et al., 1986), the fat in the adipocytes is metabolized and the alveoli expand to fill completely. gland (Neville, 1999). On the first day of involution, no major morphological changes are observed, except for the flattening of the epithelium due to the filling of the alveoli with milk.
Id Endocrine control on mammary development
This process is initiated by milk stasis once milk removal has stopped (Quarrie et al., 1996). Studies of knockout mice for ERα have shown that both stromal and epithelial ERα are required for normal ductal elongation and outgrowth during puberty (Bocchinfuso et al., 2000), even though ERα is not required for gestational alveolar expansion (Mueller et al., 2002 ). Recombinant tissue experiment showed that estradiol elicits epithelial mitogenesis indirectly through ER stromal cells (Cunha et al., 1997).
Knockout mice showed that it is the PR-B form responsible for the proliferative effects in the mammary epithelium, especially for the expansion of the alveolar compartment, and only to a small extent for the elongation and branching of the duct (Mulac-Jericevic et al., 2003). A possible candidate is the receptor activator of nuclear factor kB (NF-kB) ligand or RANK-L (Mulac-Jericevic et al., 2003), which belongs to the tumor necrosis factor (TNF) family. In particular, ERBB4 was shown to have a more prominent role in luminal cell function during lactation than does PRL (Long et al., 2003).
Breast development is controlled not only by systemic hormones, such as estrogen, progesterone and PRL, but also by peptides produced in the stromal or epithelial compartment, such as the osteoclast differentiation factor RANKL (Fata et al., 2000), inhibinβB (Robinson et al., 1997) and a member of the TGFβ family (Nguyen and Pollard et al., 2000). Several lines of evidence from knockout mice suggest that RANKL, compared to PRL, induces identical or related developmental programs during pregnancy (Humphreys et al., 1999; Fata et al., 2000).
Ie Role of extracellular matrix on mammary development
MDGF1, TGFα and TGFβ are autocrine and mitogenic factors secreted by epithelial cells to stimulate the production of collagen IV, an essential component of the basement membrane where epithelial cells lie and proliferate in a polarized manner during alveolar development ( Martinez and Houdebine, 1994 , chap. 1). Estrogen indirectly controls the synthesis of collagen IV and the activity of the growth factors through the breakdown of the basement membrane, which supports epithelial cells. The study of the biochemical composition and structure of the MEC (Hassell et al., 1985; Kleinman et al., 1986; Miller and Gay, 1987) shows that this basement membrane is not a passive layer, but rather an active membrane that receives structural and functional messages to control the behavior of stromal and epithelial cells (Bissel and Aggeler, 1981; Bissel and Hall, 1987; Bissel et al., 1982; Hay, 1981; IngBer and Jamieson, 1985; Wicha , 1984. ).
Based on in vitro studies and immunolocalization experiments, it has long been assumed that components of the basal lamina, such as laminin, heparin sulfate proteoglycans and type IV collagen, are all derived from epithelial cells and that components of the reticula lamina, such as collagen types I and III, fibronectin and tenascin, is derived from the stroma (Russell and Vonderhaar, 2002). Recent studies have assessed that the stroma is the primary source of extracellular matrix proteins and that collagen I, IV and laminin also originate from stroma (Keely et al., 1995). Furthermore, the expression of fibronectin from the stroma appears to be regulated by ovarian steroid hormones in association with epithelial-stromal interactions (Woodward et al., 2001).
It is not clear whether this dynamic construction of the basement membrane during mammary gland development is the result or the cause of epithelial morphogenesis. It is clear that several components of this basement membrane regulate the formation and function of epithelial cells and their response to external signals, such as ovarian steroid hormones or growth factors (Woodward et al., 2000).
If The miRNAs in the mammary gland
Objective