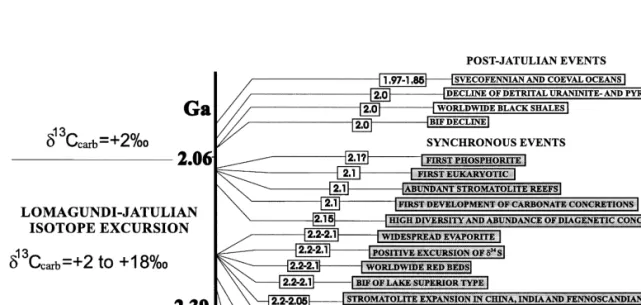
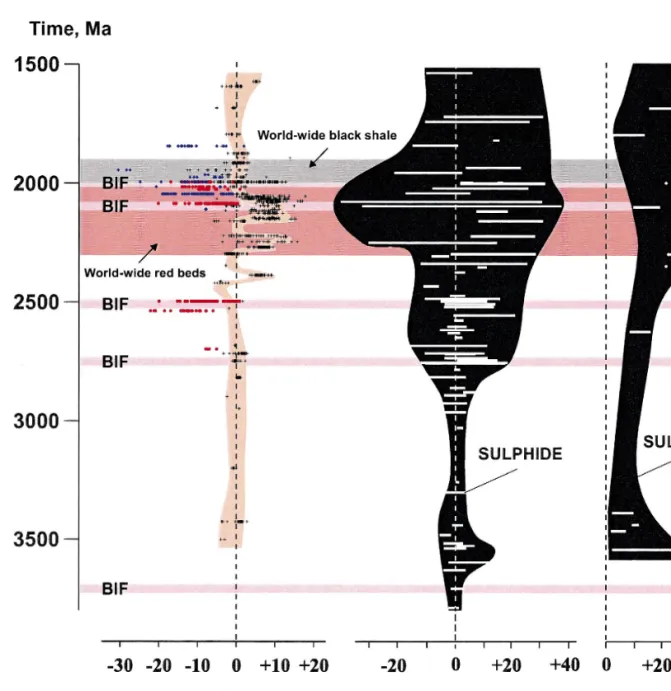
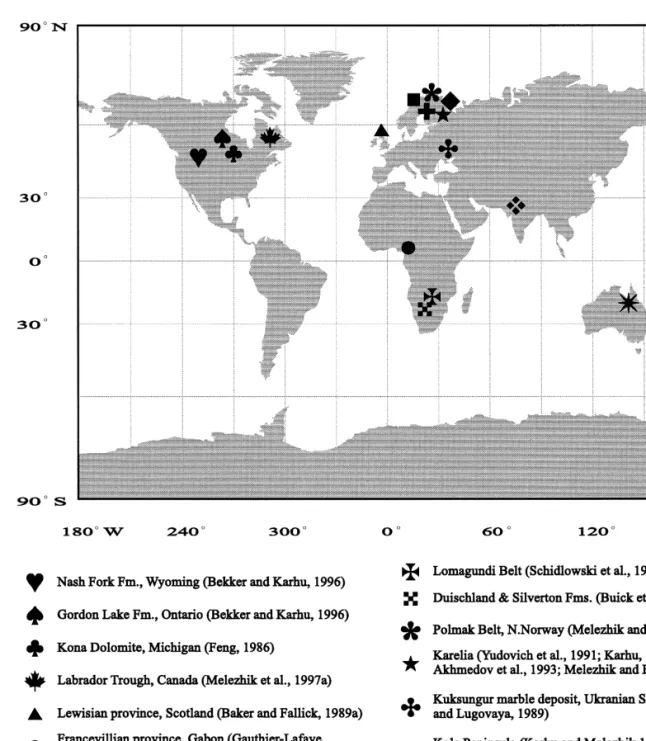
It has been suggested that the required high Corg burial was driven by high sedimentation rates and high productivity, which in turn was attributed to seawater anoxia, orogenic events, sea-level fluctuations, or combinations of these factors, e.g. ., Jenkyns, 1980; Magaritz et al., 1983; Worldwide distribution of the Paleoproterozoic 2.40–2.06 Ga C-rich carbonate rocks modified from Melezhik et al., 1997a. The Lomagundi event was attributed to locally enhanced burial of organic material in a confined basin Schid-Žlowski et al., 1976, two new discoveries of ca.
Major phenomena related to the Palaeoproterozoic Lomagundi-Jatulian isotopic event changed from Melezhik et al., 1997c. Limestone Veizer et al., 1992a; 12 Timeball Hill Formation Buick et al Kuetsjarvi Sedimentary Formation Melezhik and¨.
Current problems
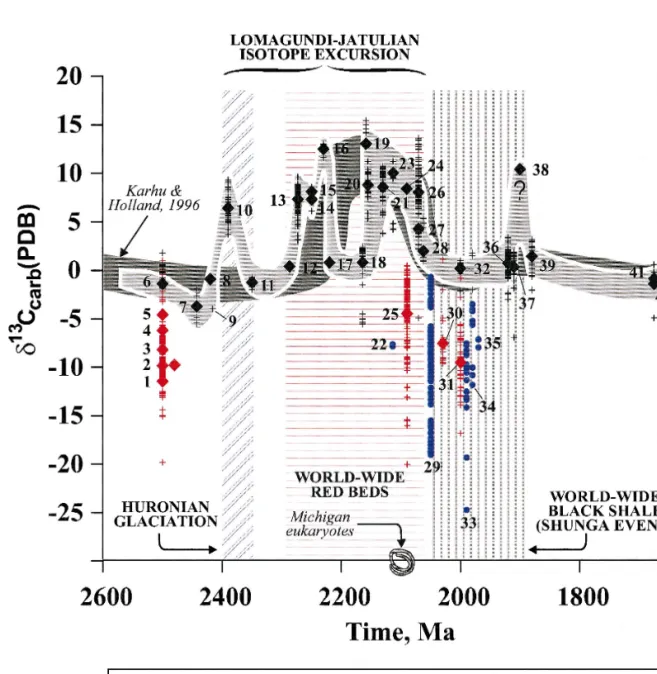
Kolasjoki returns to 0‰, and 3 that the total duration of the Paleoproterozoic isotopic events may exceed 300 Ma. It is noteworthy that the first red beds indicating free atmospheric oxygen appear to have appeared almost 200 Ma later than the first positive carbon isotope. Therefore, there need not be a direct link between the earliest positive carbon isotope shift, the global development of red beds, and the formation of oxygen-rich atmospheres, as it did.
Significance of the Tulomozerskay Formation carbonates in a global context
Gunflint Formation is accurately constrained, then it should be equivalent to the 2.09 Ga Jouttiaapa carbonates which average d13Ccarb of q8.4‰ ŽKarhu, 1993; Karhu and Holland, 1996. We must accept that a 'cause-and-effect' relationship between the excursion and a series of related phenomena is still not fully understood, and global d13Ccarb values are not constrained.
Analytical methods
Instrumental accuracy and precision of Philips PW 1480 X-ray spectrometer shown by six runs on synthetic standard ZGI-KH. Dual analyzes were performed to assess the accuracy of Sr measurements using a Philips PW 1480 X-ray spectrometer. Deviation from Sr ppm measurements using a Philips PW 1480 X-ray spectrometer and solid state mass spectrometry on a Finnigan MAT 261.
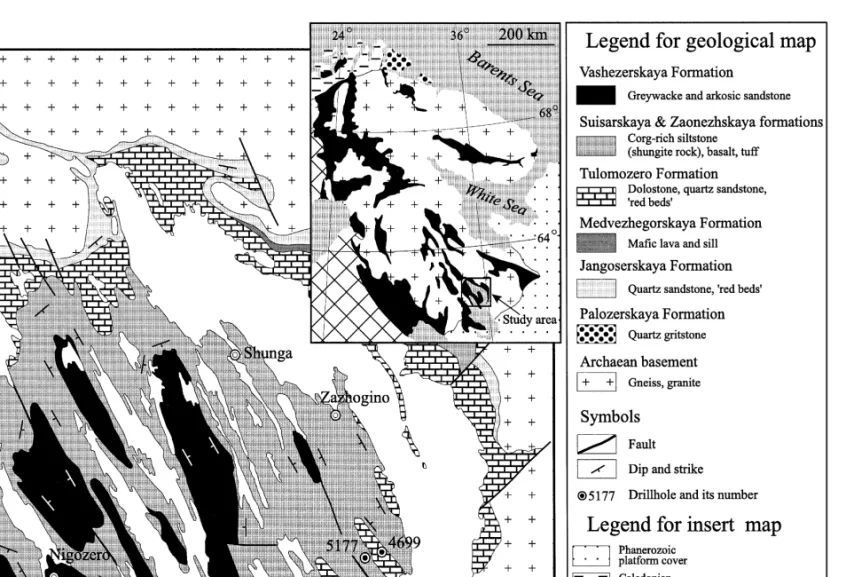
Geological background
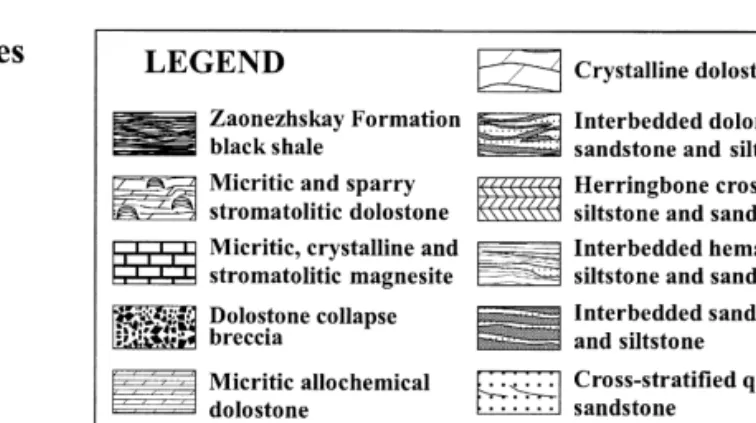
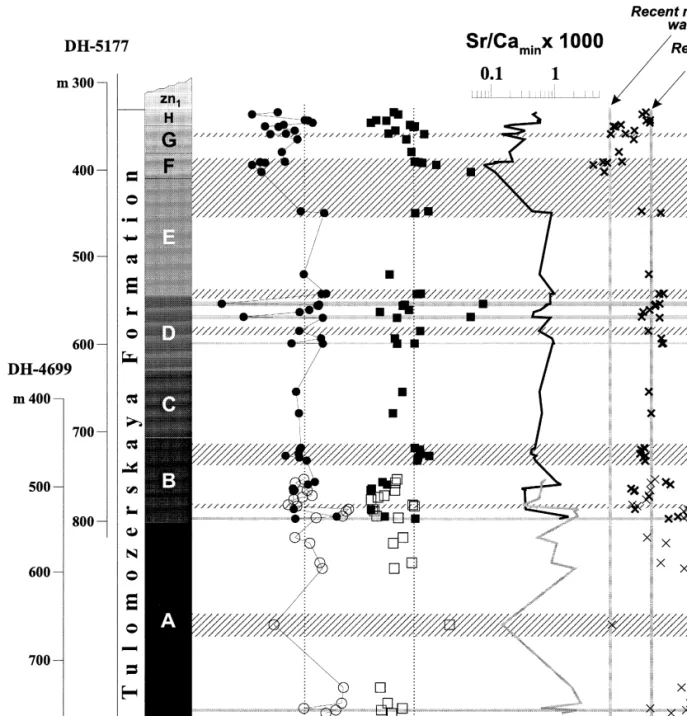
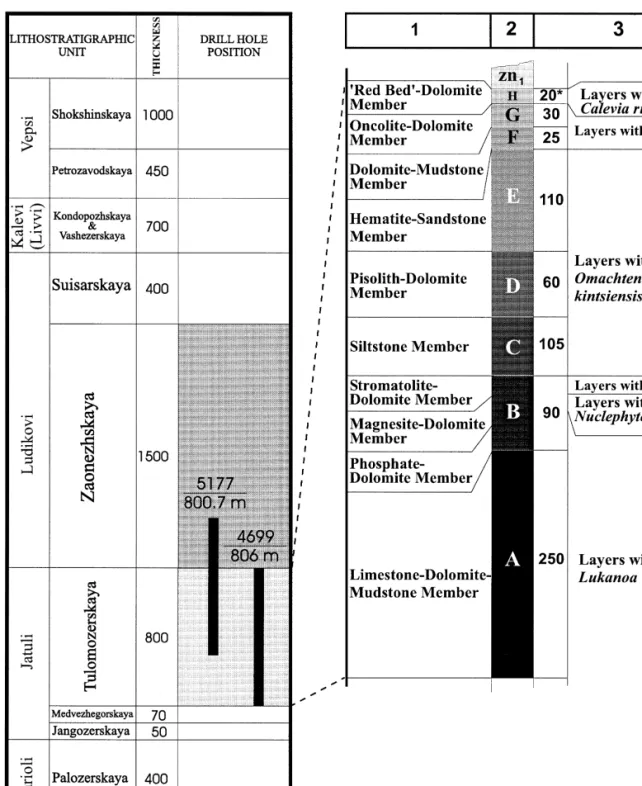
On the basis of lithology and mineralogical composition, the Tulomozerska Formation was divided into several "piles" Akhmedov et al., Ž. Lithostratigraphic features of the Tulomozerskaya Formation based on Melezhik et al., 1999a; cast member Thickness Lithofacies Rock assemblage Color Morphology of stromatolite. X Micrite and sparry Red, beige, white laminated Unpreserved Tepee, breccias associated with tepees See Table 4.
IX Micritic, crystalline, White, pale yellow Massive, laminated Not preserved – Playa, sabkha environment stromatolitic magnesite. V Crystalline dolostones Beige, pink Structureless, indistinct Not preserved Desiccation cracks, dolomite Upper intertidal zones of parallel-laminated pseudomorphed gypsum, sheltered bays and lagoons. Drill core logging has revealed a range of lithofacies and their frequent variation in the stratigraphic column.
The Tulomozerskaya lithofacies assemblage includes several groups, namely siliciclastic-dominated, mixed siliciclastic clastic-carbonate lithofacies and carbonate-dominated. The overall paleoenvironmental interpretation of the Tulomozerskaya sequence based on the lithofacies analysis together with stromatolite morphologies Žin detail see Melezhik et al., 1999a, has been presented. Although part of the carbonate rocks are formed in peritidal shallow marine environments, the dolostones of Members A and C can be attributed to a limited evaporite environment developed in ephemeral ponds in the upper intertidal zones of a carbonate flat or in sabkhas and playa- lakes on the coast. .
Most of the Tulomozerskaya carbonates, which are believed to have formed in shallow marine conditions, show characteristics of lower energy deposits, accumulated in protected environments.
Petrographic characteristics of carbonate rocks
Geochemical results
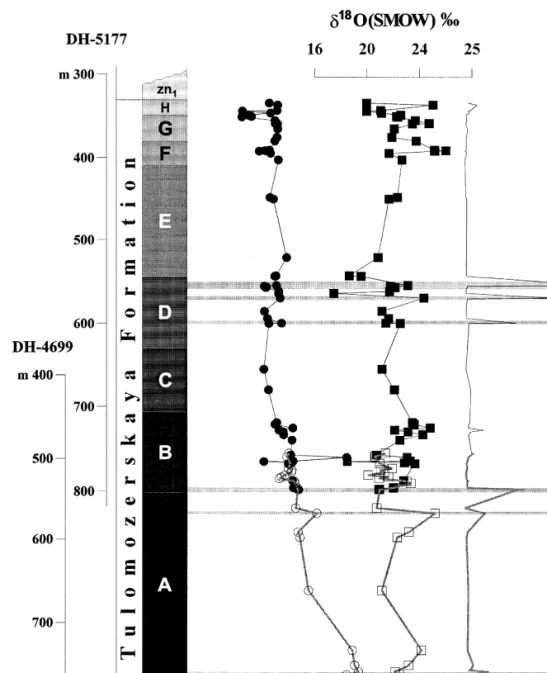
The whole-rock analyzes show that the Sr content exhibits considerable variation with a range of. The histogram of the carbon isotope data reveals two modes: a strong one at and a weaker one. Four of the samples from the second population are dolostones highly enriched in 13C which in the.
The carbon isotope values show an erratic decrease upward in the range from q17.2‰ in the lower part of member A to q8.0‰ in . less variable and fall within the range of q7.3 to q10.1‰. The only exception to this pattern are the four samples from the lower part of Mem-.
Evaluation of diagenesis
Whole-rock analyzes show that the Sr content shows considerable variation ranging from. average values for the carbonates of the three highest members, F, G and H 26, 54 and 64 ppm respectively,Ž. The dispersion of Sr content in dolostones is also independent of MgrCa. ratio rs0.18, although certain magnesite-bearing rocks show depletion of Sr as the MgrCa ratio. Four samples from the second population are highly 13C-enriched dolomites located in the. the lowest part of the succession Article A , and. one sample comes from member B, fig.
Higher up in the overall sequence, the observed d13C values of members D–H are less variable and fall within the range of q7.3 to q10.1‰. The only exception to this sample are the four samples from the lower part of Mem-. d13C, d18O values and MgrCa ratio of carbonate rocks as a function of depth for the entire sequence. Concentration of Sr, MnrSr, SrrCadol in rocks and SrrCas ratios in solution as a function of depth for the entire sequence of the Tulomozerskaya Formation in the northern area of Lake One. The shaded horizontal lines are magnesite layers.
1992 reported that Mn and Sr can serve as a tool to calibrate the relative diagenetic rank of sequences. If post-depositional Sr reset can be quantified, the Sr content of carbonates can help constrain the nature of the dolomitizing fluid. Under equilibrium conditions, the amount of Sr in the mineral relative to the amount from the parent fluid of the mineral is a distribution function.
Given the Sr content of a dolomite, one can infer the Sr chemistry of the parent fluid, but we are limited by the large uncertainty in DSr values for dolomite, and unknown SrrCa for Paleoproterozoic seawater.
Comparison with other contemporaneous
The timing of dolomitization is a very important factor in determining dolomite Sr content TuckerŽ and Wright, 1990. Rather, low-Sr diagenetic calcite will be replaced by highly Sr-depleted dolomite. This has been exemplified by the chemistry of modern dolomite from the Arabian Gulf, Bahamas and Florida Bays, which contains about 600 ppm Sr BehrensŽ and Land, 1972; Land and braces, 1973.
The highest Sr values of 406 and 491 ppm from the least altered Members A and B dolostones may reflect that they formed via diagenetic dolomitization of a Sr-rich CaCO3 precursor, such as aragonite, e.g. Mirota and Veizer, 1994. If aragonite was not a CaCO precursor, one can alternatively suggest that the dolomite precipitated from a solution with enriched SrrCas conditions relative to this. This is consistent with the sedimentological data and facies interpretations indicating upper subtidal for sheltered bay and lagoon environments for members A and B dolostones.
12, can be attributed to a mixing zone where the meteoric component was high in Ca but lower in Sr. The Tulomozerskaya Formation carbonates have higher d13C than all other isotopically anomalous Paleoproterozoic carbonates reported. The maximum d O value is also higher than those reported for isotopically anomalous carbonates by Baker and Fallick 1989a;Ž.
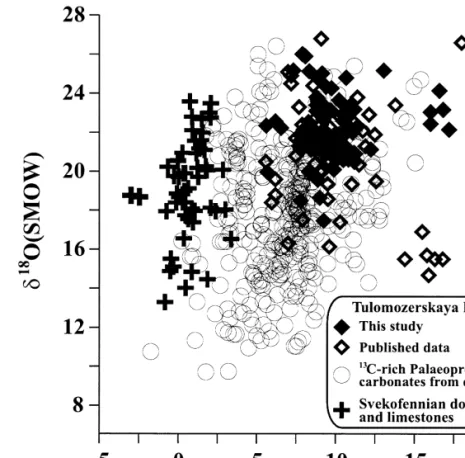
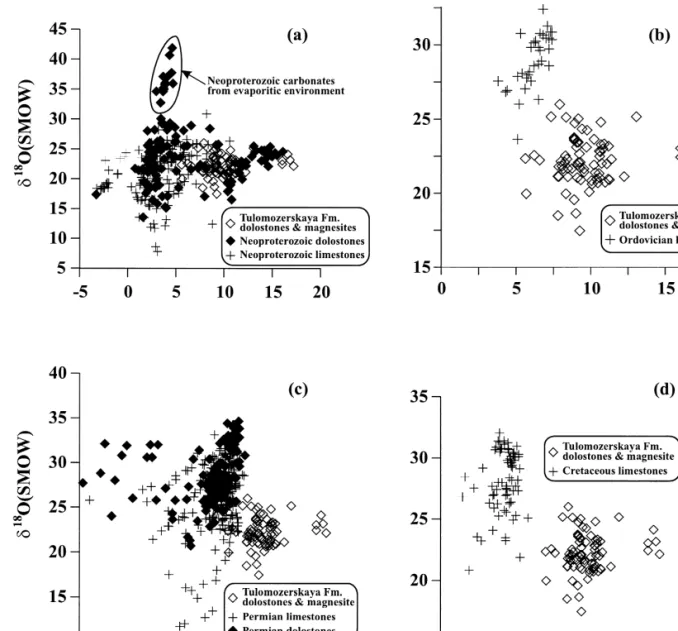
Comparison with 13 C-rich carbonates of other ages
Interpretation of 13 C enrichment
The latter is on average 3‰ higher than the d18O of the Dead Sea due to a higher rate of evaporation Katz et al.,Ž 1977. Calcium and magnesium carbonate sediments formed in the highly evaporating coastal sabka of Abu Dhabi , on the Arabian side of the Persian Gulf Mc-Ž. Thus, it is very likely that increased evaporation is one of the factors leading to the extreme 13C enrichment of Member A carbonates.
The extent of this effect can be global or basin-wide, and evaluation of these alternatives is key to explaining the carbon isotope variation. Carbonates rich in 13C were formed during low water levels in those parts of the lake that were isolated from the main lake and its waters. As a result, the whole process could only lead to a local enrichment of Tulomozerskaya carbonates in 13C, although it should contribute.
Anaerobic e.g. sulfate reduction and aerobic respiration, fermentation and methanogenesis are common processes leading to the breakdown of the microbial biomass produced. We believe that the stromatolite basin model is a potential target that can explain some of the statements and discussions presented above. Based on modern analogues, we hypothesize that the Onega stromatolites may have contributed about q5‰ to the 13C of the Tulomozerskaya carbonates.
Simplified model illustrating enhanced enrichment of the Tulomozerskaya carbonates in 13C compared to inferred global.
Conclusions 1. General
The Pokrovsky Geological Institute of the Russian Academy of Sciences in Moscow donated unpublished isotopic data on the shark. Application of trace element and strontium isotope geochemistry to studies of carbonate diagenesis. Facies and evolution of Precambrian carbonate depositional systems: emergence of a modern platform archetype.
Highly 13C-enriched carbonate and organic matter in the Neoproterozoic sediments of the Bambui Group, Brazil. Isotope shift in the Late Permian of the Delaware Basin, Texas, precisely timed by varied sediments. Geochemistry of carbonate rocks in Phosphoria and related formations of the western phosphate field.
Isotopic composition of strontium, oxygen and carbon in the Upper Precambrian carbonates from the western slope of the Anabar Uplift KotuikanŽ River. A review of the paleohydrological interpretation of carbon and oxygen isotope ratios in primary lacustrin. Late Cenozoic Dolomites of the Bahamas: Metastable Analogues for the Formation of Ancient Platform Dolomites.
Stable isotope geochemistry of early Proterozoic carbonate concretions in the Animikie Group of the Lake Superior region: evidence for bacterial processes.