Vol-7, Special Issue-Number5-July, 2016, pp215-222 http://www.bipublication.com
Case Report
A Study on the Somatic Embryo Production for Arnebia
Euchroma Micropropagation
Siyamak Mohammadi and Mohammad Zaefizadeh*
1Department of Agricultural Biotechnology,
Ardabil Science and Research Branch, Islamic Azad University, Ardabil, Iran
2
Department of Agricultural Biotechnology, Ardabil Branch, Islamic Azad University, Ardabil, Iran.
*Corresponding Author: Mohammad Zaeifizadeh
ABSTRACT:
Arnebia Euchroma is medicinal plant of boraginacea family. This genus retains annual and perennial herbaceous species with standing stem, simple and alternate leaves, and bell-shaped and flattened flowers. They are scattered in Asia, Europe and Mediterranean and its smoke from burning it or its poultice were used as disinfectant and for wound healing in traditional medicine. Plant roots contain alkannin, shikonin and their derivatives which are extracted by organic solvents. Micropropagation of this plant through somatic embryo has not been popular. In this research, using seed explants, the direct micropropagation of 10 cultivars of Arnebia Euchroma population is studied. Based on the results, Arnebia Euchroma seed shows the first signs of callogenesis on the explants were observed after 7 days of inserting the explants in callus induction medium. The seeds inflated and callus formation began afterwards. After 14 days, the callus was easily observable in explants. The calli derived from seed explant were prepared to be returning after 30 days. Derived calli were cream and crunchy.. Before returning, all samples had 100 percent embryogenesis in MS medium. Only calli diameter was studied as the measured factor. On embryogenesis, the MS medium with hormone level of 2.5MgL IBA and 2.5MgL BA, had the highest callogenesis.
Keywords: Arnebia Euchroma, somatic embryogenesis, callogenesis
INTRODUCTION:
Plants provide the energy and constituents of the body and also the vitamins which regulate the metabolism and the effective materials of the medicines. Using plants for treating diseases has always been a part of human history, boraginacea family is anherbaceous species, and it is hardly found in the form of shrubs and it belongs to the warm and temperate regions. It has coarse hairs and it is one of the best methods of distinguishing this family, from other plant families. It includes 13 genera and 2,200 annual and perennialherbaceous and shrub species. Arnebia Euchroma, also known as royle, is of boraginaceae family. This genus has annual and perennial herbaceous species with standing stem,
which claims that any plant cell includes all genetic data required for converting to a complete plant. Hence, culturing cell, tissue and organ provides the opportunity to form a plant by cultivating one of the aforementioned parts.
Figure 1
METHODOLOGY:
In order to compare plant callogenesis, embryogenesis and regeneration and also the suspension culture, the arnebiaeuchroma cultivar which grows in Kerman Province and Aras River banks was used. In each 10 cm Petri plates, 10 sterilized seeds werecultivated on the solid culture medium under sterile conditions. The medium was the basic MS and the complementary organic materials, without hormone and they were kept at 25±1 °C and 16 hours of light with intensity of 3,500-4,000 lm. After 4 days, with 2 cm seedlings, the non-polluted Petri plates were opened under sterile condition and their leaves and roots were divided into 2 mm pieces by a scalpel and left in Petri plates containing the basic medium and complementary organic materials with four different hormone levels. Around 10 leaf pieces or 10 root pieces were cultivated in each Petri plate and kept at 25±1 °C in the dark. Explants were subcultured in the same medium and under the same conditions after 4 weeks that calli had grown. The measured traits in callogenesisperiod: Since hundred percent callogenesis took place, the statistical studies in the callogenesisstage was carried out on calli sizes only and after the subculture, the callus volume was measured, using Hooker and Nabors standard (Figure 1).
Embryogenesis Medium:
When enough amount calliare generated from arnebiaeuchroma, they were taken to the embryogenesis medium containing basic media of
B5 and MS completed with the following materials:
Medium (1): Without growth regulator materials Medium (2): 1 MgL2-4-D + 0.5 MgL BA Medium (3): 1 MgL2-4-D+ 1MgL BA Medium (4): 1 MgL2-4-D + 2.5 MgL BA Medium (5): 1 MgL2-4-D + 5 MgL BA Medium (6): 2.5MgL2-4-D + 0.5 MgLKin Medium (7): 2.5MgL 2-4-D + 1 MgL BA Medium (8): 2.5 MgL 2-4-D + 2.5MgL BA Medium (9): 2.5 MgL 2-4-D + 5 MgL BA Medium (10): 1 MgLIBA + 0.5 MgL BA Medium (11): 1 MgL IBA+ 1 BA
Medium (12): 1 MgL IBA + 2.5 MgL BA Medium (13): 1 MgLIBA + 5MgL BA Medium (14): 2.5MgLIBA + 0.5 MgL BA Medium (15): 2.5MgLIBA + 1 MgL BA Medium (16): 2.5 MgLIBA + 2.5 MgLBA Medium (17): 2.5 MgLIBA + 5MgL BA For two weeks, calli were kept in media at 25±2 °C for 16 hours of light and 8 hours of darkness and studied based on the embryogenesis rate.
Medium of Converting to Plant:
In this stage, heart-shaped embryos were kept in the germination medium containing basic SH medium along with hormone compounds of 6 MgL 2-4-D and 0.2 MgL Kin.
Suspension Culture Medium:
500 mg of embryogeniccalliwas put in a 100 ml Erlenmeyer flasks containing 20 ml of B5 liquid mediumalong with 1 MgL of 2-4-D and 0.2 MgL of Kin. Erlenmeyer flasks were put on horizontal shakers at 50 rpm in 6/8 hours of light in 5 replications at 27 °C. Subcultures were carried out once each 5 days in the first week and each 10 days during the later subcultures.
After observing the embryo formation in six-week cellular suspension, 1 ml of each medium was deprived by 1 ml sampler and left on the filter and the number of the embryos on the filter in each ml unit was measured.
Statistical Calculations:
and MSTATC software in factorial experiments in completely randomized designs. The correlation coefficient between the data derived from embryogenesis and generation was measured and the interactive effects between various traits were studied and the diagrams were drawn using Excel. Logarithmic transformations were used in all analyses when the inter-treatment variances were not homogenous.
Discussion and Conclusion: Callus Induction Medium:
On leaf and root explants, around 4 days after leaving the explants in callus induction medium, the first signs ofcallogenesiswere observed on the explants. Initially the root inflated and subsequently, the epidermis was layered and the cells under the epidermis layer began splitting and
callogenesis began. After ten days, the callus in explants was easily observed. (Figures 3, 4 and 5) On seed explants, after 7 days of leaving the explants in callus induction medium, the first signs of callogenesison the explants wereobserved. Initially, the seeds inflated and subsequently callogenesis began. After 14 days of the callus in explants was easily observed. After 20 days, callus generated from leaf and root explants were ready for installation and the calli derived from seed explant were read for installation after 30 days. The derived calli were cream and crunchy. (Figures 6 and 7) Before installing, since all samples had 100 percent callogenesis in SH medium, only calli diameter was studied as the measured factor.
Table 1 presents the ANOVA of somatic embryogenesis and the number of regenerations which took place in various hormone treatments. It could be observed that there is a significant difference between replications and treatments at one percent level.
df Ms F
Replication 2 35.35 16.17** Treatment 16 3046.53 1393.48**
E 32 2.18
In this research, callus was prepared from both root and leaf of Arnebia Euchroma. Results are presented in Table 2.
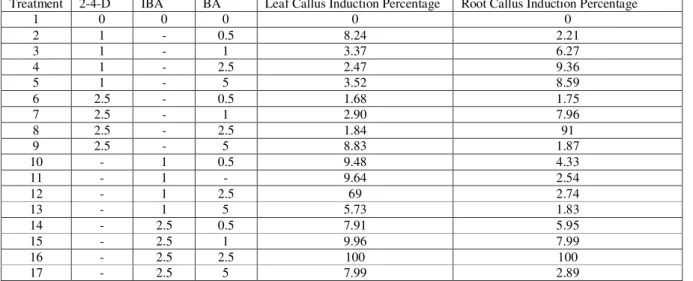
Table 2. Callus Induction in ArnebiaEuchroma from Leaf and Root Explants in various Hormone Compounds Treatment 2-4-D IBA BA Leaf Callus Induction Percentage Root Callus Induction Percentage
1 0 0 0 0 0
2 1 - 0.5 8.24 2.21
3 1 - 1 3.37 6.27
4 1 - 2.5 2.47 9.36
5 1 - 5 3.52 8.59
6 2.5 - 0.5 1.68 1.75
7 2.5 - 1 2.90 7.96
8 2.5 - 2.5 1.84 91
9 2.5 - 5 8.83 1.87
10 - 1 0.5 9.48 4.33
11 - 1 - 9.64 2.54
12 - 1 2.5 69 2.74
13 - 1 5 5.73 1.83
14 - 2.5 0.5 7.91 5.95
15 - 2.5 1 9.96 7.99
16 - 2.5 2.5 100 100
17 - 2.5 5 7.99 2.89
IBA.This situation held true for the leaf; that is, the aforementioned treatment had the height callus rate in leaf.
Figure 2. Cultivating Sterilized Seeds in Culture with Hormone
Figure 3. Sample of Wet Callus and Juicy Callus
Figure 4. Sample of Non-Embryogenic Callus Figure 5, Sample of Embryogenic Callus
Figure 6. Initiation of Callus Formation in Seed Explant
Figure 7. Sample of Calli Derived from Seed Cultivation
Calli generated from the root were light brown and dense, while the calli derived from leaf were light yellow and less dense, comparing to the root. Root et al. (1998), Leo and Gia (1999) and Mangijulaet al. (2005), reached similar results.
Regeneration: Until regeneration signal is not issued, the cultivation medium was changed two times during the four weeks. Regeneration retrieved from leaf calli are green and crunchier.and the regeneration from leaf calli are light brown and denser than the leaf.
Table 3 presents the results from various hormone compounds impact on somatic embryogenesis and shoot organogenesis in arnebiaeuchromaduring around 8 weeks after callogenesis.
Table 3. Results from Various Hormone Compounds Impact on Somatic Embryogenesis and Shoot Organogenesis in ArnebiaEuchroma during around 8 Weeks after Callogenesis
Treatment Somatic Embryogenesis in each Cultivation
Regeneration in each
Cultivation Regenerations
0 - - -
9 - 6.1 6.1
10 2.63 9.7 10.5
11 3.6 12.1 15
12 6.4 11.4 17.8
13 11.1 5.6 16.7
14 13.98 4.4 18.38
15 16.2 3.6 19.8
16 8.8 6.2 11.4
various hormone compounds impact on somatic embryogenesis and shoot organogenesis in arnebiaeuchroma during around 8 weeks after callogenesis. Based on the number of regenerations in each cultivation, the treatment without any hormone (1) and treatment 12 with 11.4 cases and treatment 11 with 12.1 cases had the highest rank. Yu et al. (1997) reached similar results in their studies on one of Lithospermumerithrorhyzon families.
Figure 8. Formation of Spherical Embryo Figure 9. Formation of Semi-Spherical Embryo
Figure 10. Formation of Spear-Shaped Embryo Hormone Level Impact on Embryogenesis:
Considering table 3, it could be observed that the hormone levels of treatment 15 (2.5 MgL IBA along with 1MgL BA) had the highest impact on embryogenesis and it is categorized in group “a” and other hormone levels, except the hormone level of treatment 14 which contained 2.5 MgL IBA along woth 0.5 MgL BA, were in lower groups. These results are in accordance with Mangijulaet al. (2005) results.
Regeneration Environment:
After 10 days, the first signs of nodulation (spherical embryos) appeared on callus medium (Figure 8) and after 20 days, the white special embryos turned green and after several days, the spherical embryos transformed into heart-shaped embryos.
Plant Conversion Medium:
In this stage, embryos had a spear-shaped form initially and then turned to semi-spherical shape. (Figures 10 and 11) Subsequently, semi-spherical embryos germinated and shoot and root were formed. (Figure 12, 13, 14 and 15)
Figure 11. Rooting during Conversion to Seedling Figure 12. Root growth in Plant Conversion Medium
Figure 13. Shooting during Conversion to Seedling Figure 14. Sample of Root and Shoot Formation
In this stage, for each Erlenmeyer flask, 500 mg of grown calli was taken to the suspension cultivation medium for embryogenesis.
In this stage, 500 mg of embryogeniccalli,which were transferred to the 100 ml Erlenmeyer flasks containing 20 ml of liquid B5 medium, containing 1 MgLD-4-2 and 0.2 MgL Kin, was studied. Afer 10 days, the changes in calli were observed, so that the cells which were separated from each other due to the shaker force were inflated and turned in spherical form. (Figure 6) Accordingly, figures were provided from each of the subculture stages and after 6 weeks after the first cultivation, v in cell suspension were taken for counting under microscope.
Figure 15. A Sample of Suspension Cultivation Figure 16. A Sample of Callogenic Embryo in
Suspension Cultivation
Figure 17. germination in SuspensionCultivation Medium
Figure 18. Shooting in SuspensionCultivation Medium
Figure 19. A Sample of the Embryo development in SuspensionCultivation Medium CONCLUSION
On callogenesis, seed and leaf explant had the highest calli rate. On embryogenesis, the MS medium with hormone level of 2.5 MgL IBA and 2.5 MgL BA, had the highest callogenesis.
REFERENCES:
1. Omidi, M. (2000). Study on tissue culture, cytogenetic and barley proteins. Thesis PhD in Plant Breeding (molecular genetics and genetic engineering) Faculty of Agriculture, Tehran University
2. Bagheri. A. (1996). Principles of plant tissue culture. Ferdowsi University of Mashhad page, 406.
3. Tores, K. (1994).tissue culture techniques to plants and gardening Khoshkhoy. M, (Translator) First Edition Shiraz University Publication Center page (1-103)
4. Abdmishany. S andBushehriA.A.SH. (1987). Plant Breeding completed (First pub) plant biotechnology.
5. Fazelzadeh, A. (2003). Effect of heat treatment for the regeneration of callus culture immature embryos atmosphere. Master's thesis Plant Breeding Department of Tehran University. 6. Kouchaki, A. translator. (1994). cultivation in
arid regions. Publications Jihad Mashhad University. 202 Page.
plant using four common methods, agricultural knowledge, Vol. 1 (2) 1 -13.
8. Mehrabi, A. (2002). investigated callus induction and regeneration in canola and evaluation of salt tolerance of plants using tissue culture technique. Crop master's thesis. Faculty of Agriculture, Tehran University. 9. Nouri-Gonbalani, Gh. Translator (1992).
Experiments in plant tissue culture, Tabriz University Press. No 323, Page 411.
10.valizadeh, M and Kamya, T. (1993). Evaluation of callus, callus growth and callus protein in alfalfa species (Medicaago) CROP SCIENCES Congress. Mashhad, Iran.
11.Agrawal. L.R, (1998) Fundamenteds of plant breeding in hybrid seeds.
12.Alexander I.K, Debchev P.D, Attanassov A.I. & Scrag A.H (1994) Alfalfa embryo production in airlift vessels via Domatic embryigenetsis, Plant cell, Tissue and Organ cutture 38:19-23
13.Allen, S.G., A.K. Dobrenz, and P.G. Bartels. 1986. Physiological response of Salt-tolerant and non tolerant alfalfa to salinity during germination. Crop Scince 26:1004-1008 14.Al-Niemi, T.S. W.F. camphell, and M.D.
Rumbaugh. 1992. Response of alfalfa cultivars to salinity during germination and post germination growth. Crop Scince 32:976-980 15.Arcyones, Davey.M.R, Santos. A.V. P and
Cockyng. E. (1982) Somatic embryogenesis in tissue from mesophyl and cell suspension protoplast of Medicage caerulea and M.glytirosa. Plant Physiology: 3:105-110 16.Atanassov, A. and D. C. W. Brown (1984)
Plant regeneration Medicage sativa L. plant cell tissue and organ culture 3:149-162
17.Ayaydin F, Vissi E, Meszaros T, Miskolczi P, Kovacs Y, Feher A, Dombradi V, Erdodi F, Gergely P and Dudits D(2000). Inhibitation of serin / threonine specific protein phosphat ases causes premature activation of cdc2 MSF Kinase at G2/M transition and early mitotic microtubule Organisation in aifaalfa. Plant J. 23:85-86.
18.Bhoywani. S.S. and M. K. Razdan. (1990.) Plant tissue culture: Theory and Practice, Elsever.
19.Binarova.P., Nedelnik.J, Fellner. M and NedbaLkova .B . (1990) Selection for resistance to filtrates of Fasarium SPP. In embryogenic cell suspension culture of
Medicage Sativa L. Plant Cell Tissue and Organ Culture. 22:191-196
20.Bingham E.T, Hurley L.V, Kaatz D.M and Sanders JW (1975) Breeding Alfaifa wich Regenerates from Callus Tissue in culture Crop SCi 5:719-721
21.Bingham E.T., McCoy T.J. and Walker K.A. (1988), Alfalfa tissue culture. pp.903-929. In Alfalfa and alfalfa improvements. Eds., Hanson, A.A. Madison , Wisconsis, USA. 22.Boger. L., Calderini. O, Binarova. P, Mattauch.
M, Till. S, Kiegerl. S, Jonak. C., Barker. P, Huskisson. N. S etal (1999). A MAP Kinase is activated late in plant mitosis and become localized to the plane of cell division. Plant Cell 11:101-113
23.Brown. DCW, Frost L.A. and Koehl. E. M. (1983) The effect of germplasm source in the invitro embryogenesis is response of cultivated alfalfa. The Genetis Soc. 14: Abstr, NO. D5 24.Brown. D. C. W. (1988) Germplasm
Determination of Invitro somatic Embryogenesis in Alfalfa. HORTSCIENCE: 26-28
25.Brown. D. C. W., Nagarajon. P and Walton. P. D. (1989). Embryogenic Invitro responses in creeping and noncreeping rooted selection from M. Sativa. J. Genet. & Breed. 43: 73-76 26.Carneiro J.P.B.G., Varennes.A, Serrao. M.G,
Vaz. F, Pires F.P, Oliveria. A and Sousa M.I.M.T (1997) Productividade das luzernas anuais em alguns solos de portugal. Past. Forr. 18: 35-47
27.cloutier. S. and Londry B.S. (1994) Molecular markers applied to plant tissue culture. Invitro Cell & Develop Biol. Plant. 30:32-39
28.Compton. M.E and Veillex. R. E. (1991) Variation for genetic recombination among tomato plants regenerated from three tissue culture systems. Genome. 34: 810-817
29.Compton, M. E. (1994). Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell, Tissue and Organ Culture 367:242-317
30.Crawford EJ, Lake AWH & Boyce KG (1989) Breeding annual Medicages species for Semiarid conditions in solution Australia. Agr. 42:399-437
33.Croughan T.P., Chu O.R., (1991). Rice (Oryza sativa L.): Establishment of callus cultures and Regeneration of Plants. Biotechnology in Agriculture and Forestry, Vol. 4, Ric (ed. By Y.P.S. Bajaj): 19-36
34.das Naves LO, Duque SRL, de Almeida Js, Fevereiro PS (1999) Repetitive somatic embryogenesis in Medicago truncatula spp. Narboneniss and M. truncatula Gaerth CV. Yem along. Plant Cell Rep 18:398-405
35.Davletova S, Mesiaros T, Miskolczi P, Oberschall A, Torok K, Magyar Z, DuditsD, Deak M (2001) Auxin and heat Shoke activation of a novel member of the calmadolin like domain protein kinase gene family in cultured alfalfa cell. J EXP Bot 52: 215-221 36.Dencher PD, Velcheva M, Dragijska R,
Kulklin AI & Attanassov AI (1990) Someatic embryogenesis in Medicago. Bioteknol. Biotekh. 52:66-70
37.Dencher PD, Velcheva M and Attanassov (1991) A new approach to direct someatic embryogenesis in Medicago 10:338-341 38.Dencher PD.et al., (1993). Kinetic studies of
embryo development and nutrient utilization in an alfalfa somatic embryogenesis system. Plant Cell, Tissue and Organ Culture 33:67-73 39.Dijak M & BROW DCW (1987) Patterns of
direct and indirect embryogenesis from mesophyll protoplast of Medicago sativa. Plant Cell Tissue and Organ Culture 9:121-130. 40.Dixon R.A (1994). Isolation and maintenance
of callus and cell suspension culture. PP. 1-20. In: Plant Cell Culture a practical approach. Eds., Dixon, R.A and R.A. Gonzales. Second edition, Oxford University Press.
41.Dole J and Binrova P (1989) The effects of colchcine on ploidy level, morphology and embryogenic capacity of alfalfa suspension culture. Plant Sci. 64: 213-219.
42.Dudits D, Bogre L, Gyorgyey J (1991) Molecular and Cellular approaches to the analogs of plant embryo development from somatic cells invitro. J Cell Sci. 99:475-487. 43.Dudits D, Gyorgyey J, Bogre L and Boka L
(1995) Molecular biology of somatic embryogenesis In TA Thorp, Invitro embryogenesis in plants. Kluwer Academic publishes, Netherland 6:267-308
44.Dung T.N, Buivanle A and Van T.T (2000) Somatic embryogenesis and direct shoot regeneration pf rice (Oryza sativa L.) using
thin cell layer culture of aplical meristemic tissue. Jornal of Plant Physiology 157. P: 559-565
45.Feher A, Pasternak T, Dudits D (2002) Activation of embryogenic cell division in leaf protoplastderived alfalfa cells: The role of auxin and stress. Plant Physiology.13-14 .www.sci-u-szeged hu/ABS.
46.Finer J.J., 1996. Plant regeneration Via Embryogenicf Suspension cultures. Plant Cell Culture. (ed. R.A. Dixon and R.A. Gonzales) 6:99-125
47.Fintad k, Brown DCW & Joy k (1993) Charactrization of compentence during induction of somatic embryogenesis in alfalfa tissue culture. Plant Cell Tiss. Org. Cult. 34:125-132
48.Frankin C.J., Dixon R.A., (1996). Initiation and maintenance of callus and cell suspension cultures. Plant cell culture: (ed. R.A. Dixion and R.A. Gonzales): 1-25
49.Gallego N, Hita O , Villalobos N, Dorado A, Martin L & Guerra H (2000) Somatic emkbryogenesis1 and plant regeneration in Medicage arbora.L- Invitro Plant Physiol. 50.Groose.R.W and E.T.Bingham (1984).
Variation in plants regenerated from tissue culture of tetraploid alfalfa heterozygous several traits. Crop Science 24: 655-658 51.Gupta, P.K. (1998.) Genetics and