Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
Evidence for a cyclic AMP-dependent pathway in
angiotensin AT1-receptor activation of human omental
arteries
Hoa Ytterberg, Lars Edvinsson
Keywords: Ang II,
omental arteries, cAMP,
candesartan, losartan, U46619, forskolin, irbesartan, PD 123319, RO31-8220, H89, RT-PCR, AT1- and AT2 -receptors
Department of Internal Medicine,
University Hospital, Lund,
Sweden
Correspondence to: Dr Hoa Ytterberg Division of
Experimental Vascular Research,
Wallenberg Neurocentrum plan 3, Lund University, SE-221 85 Lund, Sweden
Tel: +46 46 22 20617 Fax: +46 46 22 20616 E-mail: hoa@ ytterberg.com
JRAAS2001;2
(suppl 1):S42-S47
Abstract
Enhanced responses to vasoconstriction induced by neuropeptide Y and α2-adrenoceptor agonists have been
seen following pharmacological activation of the adenylyl cyclase (AC) system. Since preliminary studies revealed only minor responses to angiotensin II (Ang II) in human omental arteries, we have investigated whether enhanced activity of AC may unravel further functional Ang II receptors. Human omental arteries were obtained in conjunction with elective gut surgery. After dissection of the vessel, the endothelium was removed by 10 sec of Triton X-100 treatment. Ring segments (1–2 mm long) were mounted on a myograph and studied. Ang II produced small contractions, 27±5% relative to the response elicited by 60 mM K+. However, enhanced Ang II (105±10%, p<0.001) responses were seen during AC activation by forskolin (0.1–1 µM). This enhanced contractile response to Ang II was not inhibited by the angiotensin II type 2 (AT2-receptor antagonist
PD 123319 (0.1 µM), but was blocked in an insurmountable way by the angiotensin II type 1 (AT1)-receptor antagonist candesartan (1 nM) and in a
surmountable manner by losartan (0.1 µM) and irbesartan (0.1 µM). Pertussis toxin (a Gi-protein blocker) and the protein kinase C inhibitor, RO31–8220 (0.01, 0.1 and 1 µM), markedly reduced this response, while the protein kinase A inhibitor, H89 (1, 10 µM), had no effect. RT-PCR provided evidence for the presence of mRNA for both AT1- and AT2-receptors.
The results suggest that both a cAMP-dependent and a cAMP-independent mechanism are involved in the contractile responses to Ang II in human omental arteries and that both responses are mediated via the AT1-receptor.
Introduction
In several cardiovascular disorders, blockade of the renin-angiotensin-aldosterone system (RAAS) by angiotensin-converting enzyme inhibitors (ACE-I) has been found to prevent or delay disease progression.1 Owing to side-effects of ACE-I, and to provide more specific and efficacious treatment, angiotensin II type 1 (AT1)-receptor blockers were
developed.2 At present, only two angiotensin receptors have been identified in man, the AT1
-and AT2-receptors, which are members of the G
protein-coupled, seven transmembrane-domain receptor superfamily. Experimental studies have revealed that angiotensin II (Ang II) produces vasoconstriction via AT1-receptors, while the AT2
-receptor can induce cell differentiation, tissue
repair and antiproliferation.1,3 All the well-known vasomotor effects of Ang II are mediated by AT1 -receptors.4,5 Thus, blockade of AT
1-receptors may
leave the AT2-receptor untouched and able to mediate potentially beneficial effects on the vas-culature.
Ang II is generally considered to induce vaso-constriction through a direct mechanism.4,6 However, in preliminary studies it produced only a weak, if any, constrictor response in human omental arteries. The possibility that the capacity of the AT1-receptor is underestimated under these assay conditions was therefore examined. Recent pharmacological studies have demonstrated that enhanced contractile responses to neuropeptide Y7or to α2-adrenoceptor agonists8can be obtained following prior pharmacological manipulation of the adenylyl cyclase (AC) system.9
The present study was designed to investigate the putative involvement of AC in response to Ang II in human omental arteries, to characterise the angiotensin receptor subtype involved and possi-ble messenger pathways activated. In addition, the expression of mRNA for AT1- and AT2-receptors was studied by reverse transcription-polymerase chain reaction (RT-PCR).
Methods
Tissue collection
Omental arteries were removed from patients (n=15, 14 males and one female, aged 34–85 years) undergoing elective gut surgery. The Human Ethics Committee of the Lund University approved the study. All subjects were informed and agreed to participate in the study.
The vessels were placed in buffer solution aerated with 95% O2/5% CO2and transported to
displace-Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
ment device.11The position of the holder could be changed by means of a movable unit, allowing fine adjustments of the vascular tension by varying the distance between the metal prongs. The experi-ments were continuously recorded by the soft-ware program Chart V 3.5 (MacLab, AD Instruments UK). The specimens were immersed in temperature-controlled (37°C) tissue baths, contain-ing a buffer solution of the followcontain-ing composition (mM): NaCl 119; NaHCO3 15; KCL 4.6; MgCl2 1.2;
NaH2PO4 1.2; CaCl2,1.5; glucose 5.5. The solution
was continuously aerated with 95% O2/5% CO2,
resulting in a pH of 7.4. Depending on the vessel size (0.5–0.8 mm outer diameter, 1–2 mm long), a tension of 1.5–2.5 mN was applied. Owing to initial spontaneous relaxation of the vessel segments, it was necessary to make several adjustments of tone with the movable holder, in order to maintain a stable resting tension. After approximately 1 hour, when the tension had stabilised at the desired level, each vessel segment was exposed to a buffer solu-tion, containing 60 mM KCl, obtained by substitut-ing equimolar concentrations of NaCl for KCl in the buffer solution described above. Only those seg-ments which responded with a potassium-induced contraction (>1 mN), that was reproducible a second time after washout with standard buffer solution, were used in subsequent experiments.
Experiment protocol
After another rest for 1 hour, some of the ments were used as controls, while other seg-ments were pretreated with antagonists for 60 minutes. All those segments were exposed to the thromboxane-mimetic U46619 (0.1 mM) and relaxed back to baseline with the AC-activator forskolin (0.1–1 µM), before the cumulative appli-cation of Ang II. Some tests were also performed with Ang II alone, without precontraction and forskolin treatment. All experiments were run in parallel. Each vessel segment was only exposed to Ang II on one occasion. After this, the segment was discarded, in order to avoid any possible tachyphylatic interference. Some tests were done
to compose the concentration-response curve using cumulative and fractionate exposures; however, there was no difference (data not shown).
Drugs
Candesartan, irbesartan and losartan (generous gifts from Dr P Morsing,Astra Zeneca, Sweden), H89 and RO31-8220 (Gibco, BRL), PD 123319, U46619, forskolin, acetylcholine, Ang II and pertussis toxin (Sigma, USA) were dissolved and diluted in saline. Stock solutions were kept at -40°C until use.
Calculation and statistics
Data are expressed as mean values± standard error of the mean; n refers to the number of patients from which the vessel segments were obtained. Contractile responses in each segment were expressed as the percentage of the contraction induced in that segment by 60 mM K+, and the maximum contractile effects of an agonist were indicated as Emaxvalues. The pD2, the negative
log-arithm of the molar concentration that produced half-maximum contraction, was calculated from the straight-line equation between the concentra-tion above and below the midpoint of the con-centration-response curve. The material was tested by one-way ANOVA for differences between groups. Differences were considered significant at p values <0.05. The dissociation constant, pKB,
was calculated as described by Tallarida et al.12
RNA preparation
Total RNA from human omental arteries was isolated, using the TRIzol reagent (Life Technologies, Gaithersburg, USA) following the supplier's protocol. The cells were kept in a medium containing 10% HS and 10% FCS until confluence and then homogenised in TRIzol. The RNA was precipitated with isopropanol, washed with 75% ice-cold ethanol, air-dried and finally redissolved in 20 µL diethyl-pyro-carbonate (DEPC)-treated water. The RNA concen-tration was determined spectrophotometrically. Estimating the ratio of 18S/28S RNA on a denaturing gel assessed the quality of the RNA preparation.
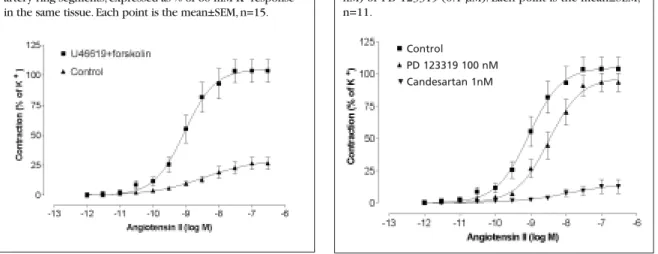
Figure 2 Concentration-response curve to Ang II after pre-contraction with U46619 (0.1 µM) and relaxation with forskolin in the absence and presence of candesartan (0.1 nM) or PD 123319 (0.1 µM). Each point is the mean±SEM, n=11.
Figure 1 Concentration-response curve for Ang II alone (control), and after pre-contraction with U46619 (0.1 µM) and relaxation with forskolin (0.1–1 µM) in human omental artery ring segments, expressed as % of 60 mM K+response
in the same tissue. Each point is the mean±SEM, n=15.
PD 123319 100 nM
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
Reverse transcription-polymerase chain reaction (RT-PCR)
The reverse transcription of total RNA to cDNA and subsequent PCR were carried out with the GeneAmp RNA PCR kit (Perkin Elmer, Foster City, USA) in a Perkin Elmer DNA Thermal Cycler. First Strand cDNA was synthesised from 1 µg total RNA in a 20 µL reaction volume, using random hexamers as primers.The reaction mixture was incubated at 42°C for 15 minutes, heated to 99°C for 5 minutes, then chilled to 5°C for 5 minutes. A 5 µL portion of the resultant cDNA was amplified by PCR in a final volume of 50 µL, following a standard PCR protocol. AmpliTaq DNA polymerase (Perkin Elmer) was used as the thermostable enzyme. The PCR was carried out with the following profile: 2 minutes at 95°C for one cycle; 1 minute at 72°C for one cycle; incubation at 4°C for 5 minutes and PCR without the RT-step (data not shown) were included in all experiments.
The following primers, which were designed, based on the published gene sequences,13,14 were used for amplification of human AT1- and AT2-receptors:
AT1:Forward5'-GATGATTGTCCCAAAGCTG, Reverse 5'-TAGGRAARRGCCAAAGGCC, generating a 255 bp product. AT2: Forward 5'-AAGAAGAAATC-CCTGGCAAGC, Reverse 5'-CTTGGTCACGGGT-TATCCTGT, generating a 302 bp product.
Results
Effect of Ang II in forskolin-treated vessels Ang II (1 pM–0.3 µM) caused only a minor contrac-tion of human omental arteries at resting tone, which was 27±5% (n=12) of the maximal response to 60 mM K+. However, a significantly enhanced Ang II response was obtained when the vessel segment was first precontracted with 0.1 µM U46619, and relaxed back to baseline with 0.1–1 µM forskolin, prior to the cumulative addition of Ang II (105±10%, n=15) (p<0.001) (Figure 1).
Effect of candesartan and PD 123319 on Ang II-induced contraction
Pretreatment of the arteries with candesartan, (1 nM) for 1 hour, almost fully suppressed the Ang II-induced contractions to 13±5% (n=8, p<0.001 vs. control), but there was no effect with the AT2-
recep-tor blocker PD 123319, 0.1 µM (98±7%, n=11) (Figure 2). In addition, the weak Ang II responses in untreated vessels were antagonised by candesartan (1 nM) (5±1%) (p<0.001).
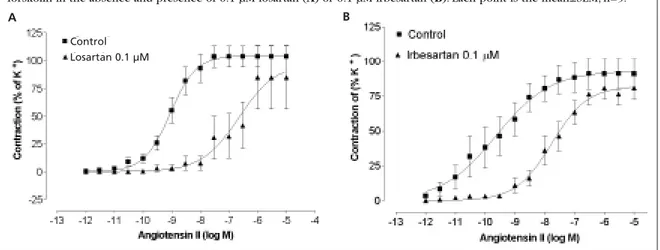
Effect of losartan and irbesartan on Ang II responses
Losartan (0.1 µM) and irbesartan (0.1 µM), two com-petitive AT1-receptor antagonists, caused parallel
rightward shifts of the concentration-response curves to Ang II (Emax=93±7%, pD2=7.28±0.37, n=5
and Emax=99±10%, pD2=7.07±0.18, n=5;
respective-ly). The maximum contraction to Ang II was not sig-nificantly changed by either antagonist, compared with control (Emax=105±10%, pD2=8.92±0.18)
(Figure 2).The dissociation constant pKBfor losartan
was calculated as 9.46±0.01 and for irbesartan as 8.45±0.01 (Figure 3).
Effect of pertussis toxin
In segments preincubated with pertussis toxin (100 ng/ml) for 1 hour, the Ang II-induced contractions were significantly reduced.These contractions were 22±7% (n=4;p<0.005) vs.control (105±10%) (n=15) without any difference in pD2 (8.92±0.18 vs.
9.27±0.05; p>0.05) (Figure 4).
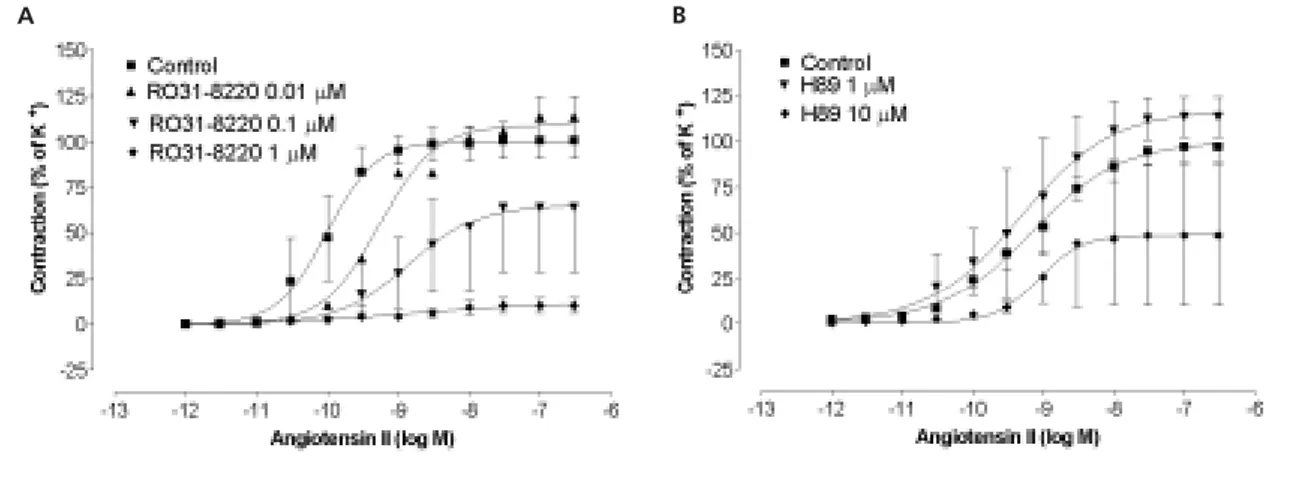
Effect of the specific PKC inhibitor, RO31-8220
The effect of RO31-8220 (1, 0.1, 0.01 µM) on Ang II-induced contractile responses in human omental arteries is shown in Figure 5A. Increasing concentra-tions of RO31-8220 resulted in a progressive decrease in the maximum contractile response to Ang II, which was significant at both 10-7and 10-6M (p<0.05).
Effect of the specific PKA inhibitor, H89 Ang II responses were not significantly different from control following treatment with H89 (1 and 10 µM) (Figure 5B).
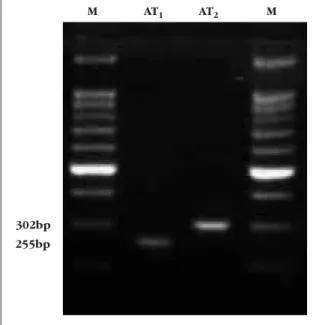
AT1- and AT2-receptor expression
Agarose gel electrophoresis of the RT-PCR prod-ucts from human omental arteries demonstrated products of the expected size, corresponding to Figure 3 Concentration-response curve to Ang II after pre-contraction with U46619 (0.1 µM) and relaxation with
forskolin in the absence and presence of 0.1 µM losartan (A) or 0.1 µM irbesartan (B). Each point is the mean±SEM, n=5.
A B
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
mRNA encoding the human AT1-receptors (255
bp) and AT2-receptors (302 bp). No bands were
detected in negative controls where the reverse transcriptase enzyme was omitted in the first strand cDNA reaction.Thus, we can conclude that mRNA for both angiotensin AT1- and AT2-receptors
are present in human omental arteries (Figure 6).
Discussion
The vasoconstrictor response to Ang II is very vari-able in human vessels, largely dependent on the source of the blood vessels.The responses in sub-cutaneous arteries or omental arteries is unaffect-ed by AT2-receptor blockade15 while a slightly
increased contraction can be seen in pregnant rat uterine artery.16 The selective AT
2-receptor
antag-onist, PD 123319, did not, in the present study, modify the contraction to Ang II in forskolin-treated omental arteries, suggesting that the AT2
-receptor was not present to a functionally signifi-cant degree. This is in contrast to the RT-PCR results, which demonstrated the presence of mRNA for both AT1- and AT2-receptors. Any
inter-ference by the endothelium and a putative AT2
-receptor in the present study was avoided by
removal of the endothelium. As revealed by others,15,17 mechanical removal of the endotheli-um, or preincubation with a nitric oxide synthase inhibitor, the prostaglandin blocker indomethacin or an endothelin receptor antagonist, does not modify the Ang II contractile response. Thus, it is suggested that the contractile response of isolated human arteries to Ang II involves predominantly AT1-receptor activation, with no significant influ-ence of endothelial factors. This agrees well with the observations in the present study, in which Ang II-induced contraction was blocked by can-desartan in the de-endothelialised arteries.The RT-PCR results would imply that only the mRNA for the AT1-receptor is translated into functional receptors in vessel walls.
Recently, a mechanism was described for α2 -adrenoceptors and neuropeptide Y Y1 receptors,
that may also be implicated also for angiotensin receptors.8,18 This involves cross-talk between receptor and effector systems and could represent an obligatory requirement for these receptors to produce contraction via a reduction in the levels of cAMP. This response was not affected by manip-ulation of the endothelium. For the contractile α2 -adrenoceptor and the enhancement by neuropep-tide Y, evidence for a cyclic AMP-dependent mech-anism was provided by a set of studies in which forskolin was used to directly activate AC.This was verified by direct measurement of a correlation between relaxation to forskolin and tissue eleva-tion of cAMP.7,8,18 In the present study, we used this protocol after adjustment for human tissue sensi-tivity.The experiments were performed after first inducing a stable contraction with U46619 and subsequently a relaxation to baseline by activating AC with forskolin.19 Thereafter, cumulative appli-cation of Ang II resulted in a strong concentration-dependent contraction, which reached the same level of contraction as the initial U46619 activa-tion.This effect can be explained by the ability of Ang II to reduce cyclic AMP levels, thereby per-mitting the contractile response to U46619 to return to baseline (Figure 1). The Ang II-induced response under these conditions was
charac-Figure 5 Contractile effects Ang II after pre-contraction with U46619 (0.1 µM) and relaxation with forskolin in the absence and presence of (A) R031–8220 (0.01-1.0 µM) and (B) H89 (1 or 10 µM). Each point is the mean±SEM, n=4.
A B
Figure 4 Concentration-response curve for Ang II after pre-contraction with U46619 (0.1 µM) and relaxation with forskolin in the absence and presence of 100 ng/ml pertussis toxin (PTX). Each point is the mean±SEM, n=4.
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
terised using surmountable (losartan and irbesar-tan) and insurmountable (candesarirbesar-tan) competi-tive AT1-receptor antagonists.20,21 The results appear to conform well with published data and strongly support activation of AT1-receptors. Losartan and irbesartan shifted the concentration-response curve of Ang II to the right, in a compet-itive manner and with dissociation constants that are in agreement with those published.21 For can-desartan, the pEC50was not altered but the Emax
markedly reduced, agreeing well with the insur-mountable type of antagonism previously shown for this agent.20 Thus, since the AT
2-antagonist
PD 123319 was without effect, we conclude that the contraction was mediated via the AT1-receptor subtype. Also, the weak direct contraction at resting tone was antagonised by candesartan, thus supporting the presence of an AT1-receptor also
mediating this type of response. Since the con-traction was weak and not in the scope of this study, no further experiments were carried out. However, we have shown in subcutaneous arteries that this is mediated by the AT1-receptor, acting via another G protein and involving elevation of intra-cellular Ca2+.22
In order to characterise the receptor-coupling mechanisms in some detail, we used the Gi-protein inhibitor, pertussis toxin. Pertussis toxin caused a significant depression of the Ang II con-centration-response curve (Figure 4). The marked inhibition of the Ang II response suggests that the Gi-protein may be coupled to further intracellular mechanisms.23 In the cascade leading to regula-tion of intracellular calcium ion homeostasis, protein kinases play a pivotal role.24 In vascular smooth muscle, the contraction operates via the
two major protein kinase A and C systems.25,26 In our study, we used RO31-8220 as a selective protein kinase C inhibitor27and H89, as a selective protein kinase inhibitor.28RO31-8220, but not H89, caused significant inhibition of the Ang II response. Our results therefore suggest that, under the present conditions, AT1-receptor activation in the omental
artery is linked to an intracellular mechanism via a protein kinase C (PKC)-dependent pathway.
In conclusion, the present data suggest that the response to Ang II in human omental arteries is mediated by AT1-receptors. At resting tone, the Ang II response is weak, but in conjunction with elevat-ed tone and activation of AC, a strong AT1-mediated contractile response is uncovered, which acts via a Gi-protein and PKC.
Acknowledgements
We would like to extend special thanks to Dr P Morsing of AstraZeneca, Sweden for the generous gifts of the non-peptide Ang II receptor ligends and Dr MingYan Hou for assistance with the RT-PCR. Supported by the Swedish Medical Research Council (Grant no. 5958).
References
1. Timmermans PBM, Wong PC, Chiu AT, Herblin WF. Nonpeptide angiotensin II receptor antagonists. Trends Pharmacol Sci1992;12:55-62.
2. Hollenberg NK, Sever PS. The past, present and future of hypertension management: a potential role for AT1-receptor antagonists.JRAAS2000;1:5-10.
3. Capponi AM. Distribution and signal transduction of angiotensin II AT1and AT2-receptor.Blood Pressure 1996;5 (Suppl 2):41-6.
4. Brown L, Sernia C. Angiotensin receptors in cardiovascu-lar diseases.Clin Exp Pharmacol Physiol1994;21:811-18. 5. Hirsch AT, Tallness CE, Schunkert H, Paul M, Dzau VJ. Tissue specific activation of cardiac angiotensin converting enzyme in experimental heart failure.Circ Res 1991;69:475-82. 6. Vraamark T, Valdemamar G, Strandgaard S, Paulson O. Angiotesin II receptor antagonist CV-11974 and cerebral blood flow autoregulation.J Hypertens1995;13:755-61.
7. Roberts RE, Kendall DA, Wilson VG. A study of NPY-medi-ated contraction of the porcine isolNPY-medi-ated ear artery. Br J Pharmacol1999;127:284-90.
8. Roberts RE, Tomlinson AE, Kendall DA, Wilson VG. Alfa2-Adrenoceptor-mediated contractions of the porcine insolated ear artery: evidence for a cyclic AMP-dependent and a cyclic AMP-independent mechanism.Br J Pharmacol 1998;124:1107-14.
9. Seamon KB, Daly JW. Forskolin: Its biological and chemical properties. Adv Cyclic Nucleotide Res1986;20:1-150. 10. Hamel E, Assumel-Lundin C, Evindsson L, Fage D, MacKenzie ET. Neuronal versus endothelial origin of vasoac-tive actylcholine in pial vessels. Brain Res
1987;15:420(2):391-6.
11. Högestätt ED, Andersson KE, Edvinsson L. Mechanical properties of rat cerebral arteries as studied by a sensitive device for recording of mechanical activity in isolated small vessels.Acta Physiol Scand1983;117:449-61.
12. Tallarida RJ, Cowen A, Adler MV. pA2 and receptor differ-entiation: a statistical analysis of competitive antagonism. Life Sci1979;20;25(8):637-54. Review.
13. Bergsma D, Ellis C, Kumar C et al.Cloning and character-ization of a human angiotensin II type I receptor. Biochem Biophys Res Commun1992;183:989-95.
14. Martin M, Su B, Elton T. Molecular cloning of the human angiotensin II type 2 receptor cDNA.Biochem Biophys Res
Commun1994;205:645-651.
15. Garcha RS, Sever PS, Hughes AD. Action of antagonists on angiotensin II-induced tone in human isolated subcutaneous arteries.Br J Pharmacol 1999;127:1876-82.
16. Zwart AS, Davis EA,Widdop RE. Modulation of AT1 recep-tor-mediated contraction of rat uterine artery by AT2 receptor.
Br J Pharma1998;125:1429-36.
Figure 6 Gel electrophoresis of RT-PCR products demonstrating the presence of mRNA for angiotensin AT1
-and AT2-receptors in human omental arteries. Single bands
of the expected sizes (255 bp for AT1, 302 bp for AT2)
were observed. A 100 bp DNA ladder (Promega) was run to confirm the molecular size of the amplification products (lanes M).
M
255bp 302bp
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
17. Holmgren A, Pantev E, Erlinge D, Edvinsson L. Inhibition of angiotensin II-induced contraction by losartan in human coro-nary arteries.J Cardiovasc Pharmacol1998;32: 662-4. 18. Prieto D, Buus CL, Mulvany MJ, Nilsson H. Neuropeptide Y regulates intracellular calcium through different signaling path-ways linked to a Y1-receptor in rat mesenteric small arteries.Br J Pharmacol 2000;129:1689-99.
19. Thomas EA, Elhlert FJ. Pertussis toxin blocks M2 mus-carinic receptor-mediated effects on contraction and cyclic AMP in guinea pig ileum, but not M3-mediated contractions and phosphoinositide hydrolysis.JPET1994;271:1042-51. 20. Morsing P, Abrahamson T. Mechanistic differences of various AT1-receptor blockers in isolated vessels of different origin.Hypertension1999;33:1406-13.
21. Vanderheyden PML,Vauquelin G. Distinction between sur-mountable and insursur-mountable selective AT1 receptor antago-nists by use of CHO-K1 cells expressing human angiotensin II AT1 receptors.Br J Pharmacol 1999;126:1057-65.
22. Ytterberg H, Edvinsson L. Characterisation of angiotensin II receptors in isolated human subcutaneous resistance arter-ies. JRAAS 2001;2(suppl 1);S37-S41.
23. Jackson EK. Pertussis toxin normalizes enhanced
renavas-cular responses to angiotensin II in spontaneously hyperten-sive rats.Life Sci 1994;54(24):PL445-50.
24. Holzmeiste J, Graf K,Warnecke C, Fleck E, Regitz-Zagrosek, V. Protein kinase C-dependent regulation of the human AT1 promoter in vascular smooth muscle cells.Am J Physiol 1997; 273(2Pt 2):H655-64
25. Heaslip RJ, Sickels BD. Evidence that prostaglandin can contract the rat aorta via a novel protein kinese C-dependent mechanism.J Pharmacol1989;Exp Ther 250:41-51.
26. Murray MA, Faraci FM, Heistad DD. Role of protein kinases C in constrictor responses of rat basilar artery in vivo.J Physiol
1992;445:169-79.
27. Morreale A, Mallon B, Beale G,Watson J, Rumsby M. Ro31-8220 inhibits protein kinase C to block the phorbol ester-stim-ulated release of choline and ethanolamine metabolites from C6 glioma cell: p70 S6 kinase and MAPKAP kinase-1beta do not function downstream of PKC in activating PLD. FEBS lett
1997;417(1):38-42.