Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2 Supplement 1
S188
The role of angiotensin II and TGF-beta on the
progression of chronic allograft nephropathy
Matthew R Weir, Chiming WeiKeywords: chronic allograft nephropathy, angiotensin II, transforming growth factor-beta
Division of Nephrology, Department of Medicine, Division of
Cardiovascular Surgery, Department of Surgery, University of Maryland Medical Systems, 22 S. Greene Street, Room N3W143, Baltimore, MD 21201, USA
Correspondence to: Dr Matthew Weir Division of Nephrology, Department of Medicine, Division of
Cardiovascular Surgery, Department of Surgery, University of Maryland Medical Systems, 22 S. Greene Street, Room N3W143, Baltimore, MD 21201, USA
Tel: +1 410 328 5720 Fax: +1 410 328 5685 E-mail: mweir@ medicine.umaryland.edu
JRAAS2001;2 (suppl 1):S188-S190
Abstract
Chronic allograft nephropathy is the most prevalent cause of graft dysfunction and failure. Its pathogenesis and treatment remains poorly defined. The calcineurin inhibitors, cyclosporine and tacrolimus, may play a role in the progressive loss of renal function in patients with chronic allograft nephropathy. This effect may be either related to the direct stimulation of profibrogenic cytokines such as transforming growth factor (TGF-β) or indirect mechanisms, through increases in blood pressure or alterations in either carbohydrate or lipid metabolism. Experimental studies have demonstrated that
angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin II receptor blockers (ARBs) can attenuate cyclosporine-mediated increases in TGF-βproduction in renal tissue.
Clinical studies have demonstrated that either cyclosporine or tacrolimus dose reduction may help reduce the rate of loss of renal function in patients with chronic allograft nephropathy. Moreover, other studies have demonstrated that a chronic reduction in the dose of cyclosporine in transplant patients can reduce serum TGF-βlevels. Treatment with an ARB can normalise the plasma levels of TGF-βin renal transplant patients receiving cyclosporine. All these observations suggest that there may be a role of cyclosporine, and possibly tacrolimus, in worsening chronic allograft nephropathy through their effects on the renin-angiotensin-aldosterone system (RAAS) and TGF-βproduction.
Introduction
Cyclosporine (CSA) is a critical part of our current immunosuppressive approach in solid organ transplant patients.1 Unfortunately, this drug has
well-recognised nephrotoxic effects and there is evidence that chronic use of CSA may be associated with development of renal tubular atrophy and striped interstitial fibrosis, coupled with progressive renal dysfunction.2,3
The aetiology of calcineurin inhibitor-mediated nephrotoxicity is not fully understood. It may be due in part to both direct and indirect mechanisms. The calcineurin inhibitors may have direct vasculopathic and tubulopathic effects leading to irreversible renal injury.4,5 CSA, through a variety of
mechanisms, may directly or indirectly stimulate vasoconstriction, interfere with renal blood flow and glomerular filtration rate, and stimulate the development of hypertension, which may cause renal injury.4,5 One of the leading possibilities
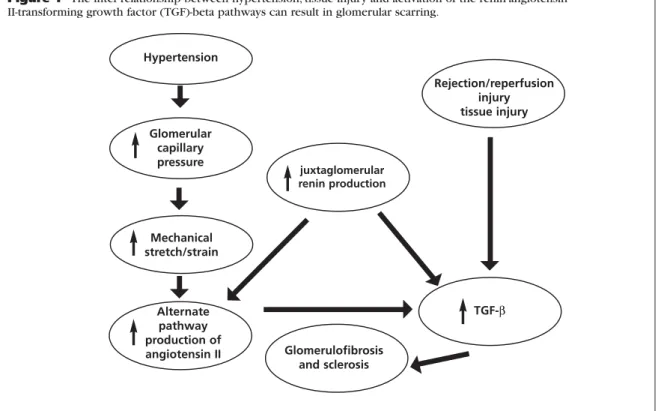
implicated in causing renal injury is the effect of the CSA on the renin angiotensin-transforming growth
factor (TGF-β) axis (Figure 1).
Substantial experimental and clinical evidence supports the concept that calcineurin inhibitors may induce nephrotoxicity via activation of this pathway. Experimental studies have demonstrated that drugs which block the renin-aldosterone system (RAAS) such as angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin II receptor blockers (ARBs) can attenuate CSA-mediated tissue production of TGF-β.6
Likewise, ARBs can normalise plasma levels of TGF-β in renal transplant patients receiving CSA.7 Similarly,
CSA reduction, coupled with the use of other non-nephrotoxic immuno-suppressive medications, has been associated with the reduction in serum TGF-β levels.8 As described herein, chronic reduction in
CSA drug levels is associated with a reduction in renal proximal tubule cell immunohistochemical staining for angiotensin II (Ang II), the Ang II type 1 (AT1)-receptor and TGF-β. This change is associated
with improvement in renal function and renal pathological scores.9,10
Methods Patients
Fifteen patients with renal transplants, with deteriorating kidney function and biopsies which indicate chronic allograft nephropathy, participated in a clinical study to progressively reduce or withdraw CSA while the patients’ immuno-suppression was supplemented with mycopheno-late mofetil, 1 gram p.o. b.i.d., and low dose corticosteroids, 0.1 mg/kg/day. All patients gave written informed consent prior to participation. The consent was approved by the University of Maryland Human Volunteers Research Committee. Patients were enrolled a mean of 2.1 years post-transplantation. Mean serum creatinine at the time of intervention was 3.5±0.4 mg/dl.
These renal transplant recipients undergoing CSA reduction had kidney biopsies before and after the intervention (mean 15.8±0.8 months), in order to assess the impact of CSA dose reduction (approximately 50%) on renal function and intragraft production of Ang II, AT1-receptors, and
the three isoforms of TGF-β (measured with immunohistochemical staining).
Patients received standard clinical care as would be appropriate for any change in immuno-suppression, with careful monitoring of renal function and clinical course.Mycophenolate mofetil, 1 gram p.o. b.i.d., and prednisone, 0.1 mg/kg/day
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2 Supplement 1
were given to maintain immuno-suppression during CSA reduction. Blood pressure was maintained at approximately 140/90 mmHg and no patients received ACE-I or ARBs.
Immunohistochemical staining
The presence of Ang II, AT1-receptor, and TGF-β
isoforms 1, 2, and 3 was documented using a specific immunohistochemical staining tech-nique.11,12 The sections were reviewed by
two trained pathologists without knowledge of the clinical course of the patients. The presence of Ang II, AT1-receptors, and the three isoforms of TGF-β were assessed by microscopic examination and quantified with the degree of immunohistochemical staining score ranging from 0 to 4: no staining (0), minimal (1), mild (2), moderate (3), maximal density staining (4). The positive staining area was also assessed.
Histopathological evaluation
Biopsy specimens were also classified, using the Banff working classification criteria, for the evidence of interstitial fibrosis, tubular atrophy, vascular sclerosis, transplant glomerulopathy, and vascular hyalinosis. Statistical comparisons between groups were performed by using factorial ANOVA followed by Fischer’s least significance difference test of repeated measures. Significance was accepted for p<0.05.
Results
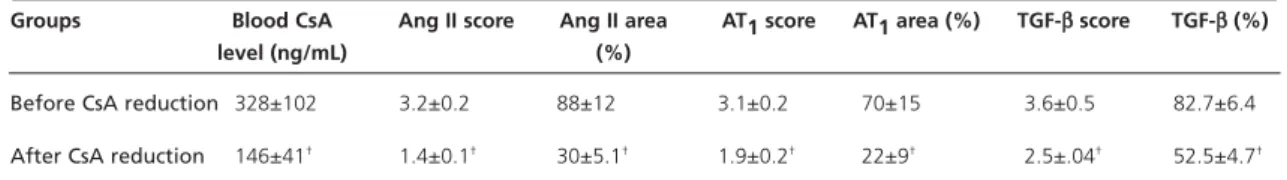
Fifteen renal transplant recipients with biopsy-proven chronic allograft nephropathy had their CSA dose reduced to achieve an approximate 50% reduction in whole blood twelve-hour trough (TDX assay) levels. CSA levels were 328±102
ng/ml pre-intervention, and after dose reduction fell to 146±41 ng/ml.
Immunohistochemical staining
The positive staining of Ang II, AT1-receptors and
TGF-β was primarily localised in renal tubular epithelial cells and was not evident in normal control human biopsy specimens. As shown in Tables 1 and 2, chronic CSA dose reduction was associated with a substantial improvement in renal histology, which correlated closely with reduction in Ang II,AT1-receptor staining, and TGF-βstaining.
Discussion
Chronic CSA dose reduction in patients with chronic allograft nephropathy resulted in an improvement in renal histology and function, associated with an attenuation of AT1-receptor,
Ang II and TGF-β staining within the allograft. These observations strongly suggest the involvement of this axis in the pathogenesis of chronic CSA nephropathy, which may be a major cause of chronic allograft nephropathy. Although this disease process is multifactorial, these studies indicate that either chronic CSA reduction and/or use of pharmacological antagonists of the RAAS may be a therapeutic strategy to consider in patients with biopsy-proven chronic allograft nephropathy and declining kidney function.
The clinical study demonstrating that CSA reduction is associated with reduced plasma TGF-β levels and correlates with reduced human allograft fibrosis adds to the understanding implicating CSA and the profibrogenic cytokine TGF-β in the development of chronic allograft nephropathy.13 Moreover, a subsequent study
comparing the effects of losartan, an ARB, and
S189
PAPER
Hypertension
Glomerular capillary pressure
Mechanical stretch/strain
juxtaglomerular renin production
Glomerulofibrosis and sclerosis
Rejection/reperfusion injury tissue injury
TGF-β
Figure 1 The inter-relationship between hypertension, tissue injury and activation of the renin-angiotensin II-transforming growth factor (TGF)-beta pathways can result in glomerular scarring.
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2 Supplement 1
S190
amlodipine, a calcium channel blocker, on plasma levels of TGF-β in CSA-treated renal transplant recipients indicated an important differential effect: the Ang II receptor blocker reduced plasma TGF-β levels, whereas the calcium channel blocker did not.8Thus, pharmacological targeting
of the RAAS may be an important strategy in reducing graft exposure to CSA-induced production of TGF-β. This study is of interest since hypertension, which can also stimulate TGF-β production, was similarly reduced with both therapies, yet only the ARB was capable of reducing plasma TGF-βlevels.
Although TGF-βis a non-specific profibrogenic cytokine, which may be stimulated by a variety of different pathways, including abnormalities in carbohydrate and lipid metabolism and drugs such as CSA, the evidence presented herein strongly suggests that it has a relationship with the RAAS in the development of CSA nephropathy. Moreover, there is important rationale from experimental studies for the concomitant use of CSA reduction and pharmacological blockade of the RAAS in order to attenuate chronic CSA nephropathy.14
We have started a prospective, randomised controlled clinical trial in patients with chronic allograft nephropathy who have undergone calcineurin inhibitor reduction (CSA or FK) and who were randomised to receive either candesartan, 16 to 32 mg once-daily, or amlodi-pine 2.5 to 10 mg once-daily, plus additional antihypertensive therapy to control systolic blood pressure to <130 mmHg. Patients will have yearly kidney graft biopsies in order to assess the impact of these therapies on graft function and immunohistochemical staining for Ang II, AT1
-receptors and TGF-β. Patients will have their glomerular filtration rate monitored with radioisotopic techniques. It is our expectation that this trial will provide confirmatory evidence that reducing calcineurin inhibitors and employing specific blockade of the RAAS as part
of an antihypertensive regimen may be an optimal strategy for delaying progression of chronic allograft nephropathy.
References
1. Cohen DJ, Loerscher R, Rubin MF et al. Cyclosporine: a new immunosuppressive agent for organ transplantation.Ann Intern Med1984;101:667-82.
2. Paller MS. Cyclosporine nephrotoxicity and the role of cyclosporine in living-related donor transplantation. Am J Kidney Dis1990;16:414-6.
3. Bantle JP, Paller MS, Boudreau RJ et al. Long-term effects of cyclosporine on renal function in organ transplant recipients.J Lab Clin Med1990;114:233-40.
4. Remuzzi G, Perico N. Cyclosporine-induced renal dysfunction in experimental animals and humans.Kidney Int
1995;48: S70.
5. Bennett WM, DeMattos A, Mayer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles’ heel of immunosuppressive therapy.Kidney Int1996;50:1089.
6. Shihab FS, Bennett WM,Tanner AM,Andoh TF. Angiotensin II blockade decreases TGFβ1 and matrix proteins in cyclosporine nephropathy.Kidney Int1997;52:660-73. 7. Inigo PJ, Campistol JM, Lario S, Bescos M, Oppenheimer F. Losartan normalizes the plasma levels of TGFβ1 in a random, crossover study (losartan vs amlodipine) in renal transplant patients treated with cyclosporine. J Am Soc Nephrol
1999;10:759A.
8. Inigo PJ, Campistol JM, Lario S, Bescos M, Oppenheimer F. Relationship between TGFβ1 plasma levels and immuno-suppressive therapy in renal transplant recipients J Am Soc Nephrol 1999;10:758A.
9. Song H, Seta K, Kinjo M et al. Decreasing expression of transforming growth factor-beta in human renal biopsies with improvement of renal pathological score and renal function following chronic cyclosporine reduction. Transplantation
1999;67:S236.
10. Wei C, Song H, Drachenburg C et al. Decreasing expression of angiotensin II and AT1 receptors in sequential human transplant renal biopsies with improvement of renal pathological score and renal function following chronic cyclosporine reduction.J Am Soc Nephrol1999;10:92A. 11. Wei C, Heublein DM, McKinley L et al. Natriuretic peptide system in human heart failure.Circulation1993;88:1004-09. 12. Wei C, Lerman A, Rodeheffer RJ et al. Endothelin in human congestive heart failure.Circulation1994;89:1580-6. 13. Cuhaci B, Kiman MSA, Bloom RD et al.Transforming growth factor-beta levels in human allograft chronic fibrosis correlate with rate of decline in renal function.Transplantation1999;
68:785-90.
14. Burdmann EA, Andoh TF, Nast CC. Prevention of experimental cyclosporin-induced interstitial fibrosis by losartan and enalapril.Am J Physiol 1995;269:F491-F499.
PAPER
Table 1 Chronic cyclosporine reduction: impact on IHCS score and area*.
Groups Blood CsA Ang II score Ang II area AT1 score AT1 area (%) TGF-ββscore TGF-ββ(%) level (ng/mL) (%)
Before CsA reduction 328±102 3.2±0.2 88±12 3.1±0.2 70±15 3.6±0.5 82.7±6.4
After CsA reduction 146±41† 1.4±0.1† 30±5.1† 1.9±0.2† 22±9† 2.5±.04† 52.5±4.7†
*Mean ±SE. IHCS score = immunohistochemical staining score (0-4) area; % = positive staining area; CsA = cyclosporine † p<0.05 vs.before CsA reduction
Ang II = angiotensin II; AT1= angiotensin II type 1 receptor; TGF-β= transforming growth factor-beta
Table 2 Chronic cyclosporine reduction: impact on renal pathology and function*.
Groups FIB (%) VS (%) TG (%) VH (%) BUN (mg/dl) CR (mg/dl)
Before cyclosporine reduction 55±5 26±3 40±4 18±2 88±9 3.5±0.4
After cyclosporine reduction 26±4† 5±1† 14±2† 2±0.5† 40±4† 2.5±.02†
*Mean ±SE
† p<0.05 vs.before cyclosporine reduction