Article
Expression of Thyroid Stimulating Hormone Receptor
mRNA in Mouse C2C12 Skeletal Muscle Cells
Jung Hun Ohn1, Sun Kyoung Han2, Do Joon Park1, Kyong Soo Park1, Young Joo Park1
1
Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University College of Medicine;
2
Biomedical Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
Background: We analyzed whether thyroid stimulating hormone receptor (TSH-R) is expressed in a skeletal muscle cell line and if TSH has influence on the differentiation of muscle cells or on the determination of muscle fiber types.
Methods: TSH-R gene expression was detected with nested real-time polymerase chain reaction (RT-PCR) in C2C12, a mouse skel-etal muscle cell line. The effect of TSH on myotube differentiation was assessed by microscopic examination of myotube formation and through the measurement of expression of muscle differentiation markers, i.e., myogenin and myoD, and muscle type-specific genes, i.e., MyHC1, MyHC2a, and MyHC2b, with quantitative RT-PCR before and after incubation of C2C12 myotube with TSH. Results: TSH-R was expressed in the mouse skeletal muscle cell line. However, treatment with TSH had little effect on the dif-ferentiation of muscle cells, although the expression of the muscle differention marker myogenin was significantly increased after TSH treatment. Treatment of TSH did not affect the expression of muscle type-specific genes.
Conclusion: TSH-R is expressed in a mouse skeletal muscle cell line, but the role of TSH receptor signaling in skeletal muscle needs further investigation.
Keywords: Receptors, thyrotropin; Muscle, skeletal; Muscle differentiation
INTRODUCTION
Thyrotropin or thyroid stimulating hormone (TSH) regulates thyroid hormone biosynthesis and secretion in thyroid follicular cells through TSH receptor (TSH-R) [1]. TSH-R is a G-protein-coupled receptor with seven membrane spanning segments [2]. Hyperthyroid or hypothyroid states affect multiple body systems, including muscle function and mass, and it has been thought that those changes were from alternations of serum thyroid hormone levels. However, serum TSH levels are also decreased or increased, respectively, in these states; thus, it might be possible that TSH-R has a role in muscle tissues,
in-dependent of serum thyroid hormone. Recently, extrathyroidal expression and the role of TSH-R, independent of thyroid hor-monal status, have been the subject of intense research. It has been reported that TSH-R is expressed in tissues like lympho-cytes, pituitary gland, thymus, testes, kidney, adipose tissue, and osteoblasts [3-5]. TSH-R in preadipocytes is thought to induce lipolysis and thermogenesis [6,7]. TSH-R in osteoblasts or osteoclast precursors is known to be involved in skeletal re-modeling, bone formation, and bone resorption [8].
TSH-R has also been reported to be expressed in extraocu-lar muscles and cardiomyocytes, although contradictory results exist [9,10]. Clinically, muscle function is affected by altered
Received: 8 March 2013, Accepted: 8 May 2013
Corresponding author: Young Joo Park
Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea
Tel: +82-2-2072-4183, Fax: +82-2-762-2199, E-mail: yjparkmd@snu.ac.kr
Copyright © 2013 Korean Endocrine Society
thyroid function. Hyperthyroid patients often experience skel-etal muscle wasting and weakness, while hypothyroid patients suffer from myalgia, muscle weakness, and exercise intoler-ance. Therefore, it is necessary to explore whether TSH-R is present in skeletal muscle and is involved in the pathogenesis of muscle disorders.
In the present study, we demonstrate the expression of TSH-R mRNA in the C2C12 mouse muscle cell line by the nested real-time polymerase chain reaction (RT-PCR) method and direct sequencing and investigate whether TSH affects myogenic differentiation and fiber type determination through quantita-tion of muscle differentiaquantita-tion markers and myosin heavy chain (MHC) isoforms before and after incubation of C2C12 myo-tubes with TSH.
METHODS
Materials
Triiodothyronine (T3, thyroid hormone, 3,5,3’-triiodo-L-thy-ronine) and bovine TSH were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Cell culture
C2C12 myoblasts were maintained at 37ºC in Dulbecco’s mod-ified Eagle’s medium (DMEM) containing 10% fetal bovine
serum in an atmosphere of 10% CO2. At 80% confluence,
dif-ferentiation was induced by treatment with DMEM
supple-mented with 2% horse serum at 37ºC in 10% CO2, and cells
were maintained in this medium postdifferentiation. Fresh me-dia was changed every other day. Myotubes were used for ex-periments 5 days after differentiation.
Mice
C57BL/6J mice were used. The mice were housed in groups of four or five in plastic microisolator cages at 22ºC with a 12-hour light/12-12-hour dark cycle and fed a laboratory chow diet (Purina irradiated laboratory chow 38057, Purina Korea, Seoul,
Korea) and water ad libitum. Animals were sacrificed after
fast-ing for 6 hours from 6:00 AM. Mice were anesthetized by in-traperitoneal injection of zoletil (Virvac, Carros, France). The thyroid, skeletal muscle (gastrocnemius), heart, hypothalamus, and small intestine were quickly removed, frozen in liquid ni-trogen and used for RNA extraction. All procedures followed the guidelines of the Seoul National University Bundang Hos-pital Animal Policy and Welfare Committee.
RNA extraction
Total RNAs of C2C12 myotubes, myoblasts, and mouse hypo-thalamus were isolated with Trizol (Invitrogen, Carlsbad, CA, USA) and purified with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instruction. cDNA was generated with reverse transcription, which was performed with 1 µg of total RNA and random primers by SuperScript II reverse transcriptase (Invitrogen).
RT-PCR, nested RT-PCR, and sequencing
RT-PCR was conducted using a 40-cycle, two step PCR in an ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA) using SYBR Green Master Mix (ABI) and 200 nM primers. Reactions were performed in duplicate. Primer sequences for RT-PCR are available upon request.
TSH-R cDNA was amplified by nested PCR using primers designed according to the NCBI reference sequence NC 000078. The first round of amplification was done with a for-ward primer (peptide position 41 to 47) spanning exon 1 to exon 6, 5′-CAAGGAGCTCCACCGAATCC-3′, and a reverse primer (peptide position 168 to 173), 5′-GCATAGGCCCTG -GAATGCGT-3′. PCR was performed in 25 μL of reaction mixture using 1 μL of the cDNA and Platinum Taq DNA poly -merase (Invitrogen). The PCR was performed as follows: in-cubation at 95ºC for 5 minutes, followed by 46 amplification cycles of 95ºC for 40 seconds, 58ºC for 40 seconds, and 72ºC for 40 seconds, and a final extension at 72ºC for 10 minutes. The second round of PCR was performed with 2 μL of the first-round PCR product, nested primer sets, and the Platinum Taq DNA polymerase. The nested primer set for TSH-R spanned exons 3 to 6 with the forward primer (peptide position 89 to 95), 5′-GCAGCGGCTGGAACCACATT-3′ and the reverse primer (peptide position 171 to 177), 5′-GGAATGCGTTT- TCAGGGACC-3′. This PCR, which produced one possible product (215 bp), was performed as follows: incubation at 95ºC for 5 minutes, followed by 30 amplification cycles of 95ºC for 40 seconds, 58ºC for 40 seconds, and 72ºC for 40 seconds, and a final extension at 72ºC for 10 minutes. The amplified PCR products were recovered from the agarose gel using a gel ex-traction kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions and directly sequenced with Ap-plied Biosystems 3730xl DNA Analyzer (ApAp-plied Biosystems).
Statistical analysis
The means were compared by the nonparametric Wilcoxon
de-noted as statistically significant. All analyses were performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The expression of TSH-R in C2C12 skeletal muscle cells
TSH-R expression was analyzed with RT-PCR in thyroid and
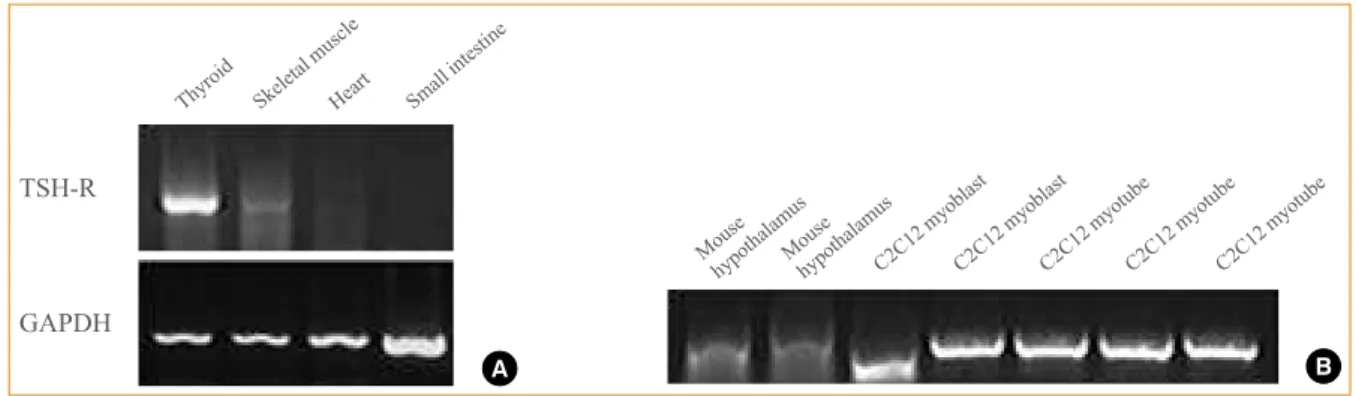
Fig. 1. (A) Expression of thyroid stimulating hormone receptor (TSH-R) in mouse thyroid (positive control), skeletal muscle (gastrocne-mius), heart, and small intestine (negative control) using real-time polymerase chain reaction (RT-PCR) detected with shorter primer sets used in nested RT-PCR (see Methods). TSH-R is expressed in skeletal muscle (gastrocnemius). (B) Expression of TSH-R in mouse hy-pothalamus (positive control) and C2C12 myoblast and myotube, detected with nested RT-PCR. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Mouse
hypothalamusMousehypothalamusC2C12 myoblastC2C12 myoblastC2C12 myotubeC2C12 myotubeC2C12 myotube
B
Thyroid Skeletal muscleHeart Small intestine
TSH-R
GAPDH
A
Fig. 2. Light microscopic images of C2C12 myotube after induction of differentiation with 2% horse serum without thyroid stimulating hormone (TSH) (A) or with TSH at concentrations of (B) 0.001, (C) 0.01, (D) 0.1, (E) 1, and (F) 10 mU/mL (×200).
A B C
hypothalamus tissues as positive controls and in the intestine as a negative control (Fig. 1A). The expression of TSH-R in thyroid tissue was very strong, while that in the heart and small intestine was almost negligible. However, expression was ob-served in skeletal muscle, although it was very weak. We also verified the expression of TSH-R in the mouse muscle cell line, C2C12. To minimize false-positive results of RT-PCR and to exclude nonspecific binding of primers, nested RT-PCR was performed using two sets of primers specific for the TSH-R cDNA (see Methods). Primers were designed to span exons to exclude nonspecific genomic amplification. This method showed amplification of PCR fragments of the expected size in C2C12 myoblasts and myotubes (Fig. 1B). PCR fragments were ex-tracted from the agarose gel and sequenced to confirm the expected PCR product of TSH-R. TSH-R was expressed as
abundantly in C2C12 myoblasts and myotubes as in the posi-tive control from the hypothalamic tissue of C57BL/6J mice (Fig. 1B).
The effects of TSH on the differentiation of muscle cells
After induction of C2C12 myotube differentiation with 2% horse serum, we incubated C2C12 myotubes with or without TSH at different concentrations (0.001, 0.01, 0.1, 1, and 10 mU/ mL) and observed the cells daily for 5 days. However, under light microscopic examination, we could not find any signifi-cant differences in myotube formation at various concentra-tions at once daily time points for 5 days (Fig. 2).
The expression of master regulators of muscle differentia-tion like myogenin and myoD was determined by RT-PCR be-fore and after treatment with TSH (10 mU/mL) and T3 at a
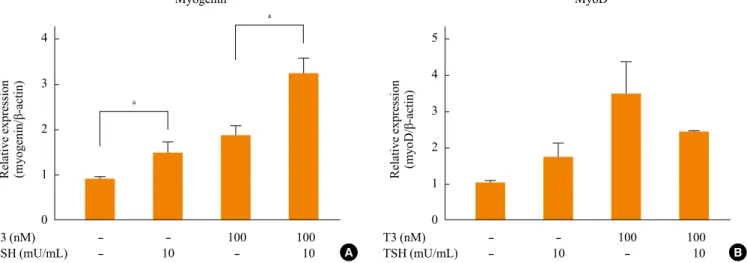
Fig. 3. Expression of muscle differentiation markers, (A) myogenin and (B) myoD, in C2C12 myotube before and after treatment with thyroid stimulating hormone (TSH) and T3 for 24 hours at postdifferentiation day 5. The results are from three independent experiments. TSH and T3 significantly increase the expression of myogenin. aP<0.05.
4
3
2
1
0
5
4
3
2
1
0
- - 100 100
- 10 - 10
- - 100 100
- 10 - 10
T3 (nM) TSH (mU/mL)
T3 (nM) TSH (mU/mL)
Relative expression (myogenin/β-actin) Relative expression (myoD/β-actin)
Myogenin MyoD
a
a
Fig. 4. Expression of myosin heavy chain isoforms, (A) MyHC1, (B) MyHC2a, and (C) MyHC2b, in C2C12 myotube before and after treatment with thyroid stimulating hormone (TSH) and T3 for 24 hours at post-differentiation day 5. The result is from three independent experiments.
2.0
1.5
1.0
0.5
0.0
- - 1 1 100 100 - 10 - 10 - 10 T3 (nM)
TSH (mU/mL)
Relative expression (MyHC1/β-actin)
MyHC1
2.0
1.5
1.0
0.5
0.0
- - 1 1 100 100 - 10 - 10 - 10 T3 (nM)
TSH (mU/mL)
Relative expression (MyHC2a/β-actin)
MyHC2a
1.5
1.0
0.5
0.0
- - 1 1 100 100 - 10 - 10 - 10 T3 (nM)
TSH (mU/mL)
Relative expression (MyHC2b/β-actin)
MyHC2b
A
A B C
concentration of 100 µM for 24 hours at postdifferentiation day 5 (Fig. 3). At this physiologic condition, TSH and T3 con-centrations are around 0.4 to 4 mU/mL and 1.0 to 2.0 nM, re-spectively. Since horse serum, which is essential for myogenic differentiation, contains physiologic concentrations of TSH and T3, we incubated with higher than physiologic doses of TSH and T3 at the respective concentrations of 10 mU/mL and 100 nM. Incubation with TSH increased the expression of myo-genin and myoD by 50% (Fig. 3). As previously reported, T3 significantly induced the expression of myogenin and myoD by 2- to 3-fold [9]. TSH further significantly increased the ex-pression of myogenin induced by T3 (Fig. 3).
The effects of TSH on the myofiber type determination
We further investigated the expression of myofiber-type spe-cific genes (MyHC1, MyHC2a, and MyHC2b) with RT-PCR before and after treatment with TSH (10 mU/mL) and T3 at concentrations of 1 and 100 nM respectively for 24 hours at postdifferentiation day 5. TSH at 10 mU/mL did not induce or repress the expression of MyHC1, MyHC2a, or MyHC2b (Fig. 4). While 1 nM of T3 marginally (nonsignificantly) induced the expression of all MHC isoforms, 100 nM of T3 reduced the expression of MyHC2a and MyHC2b but not MyHC1. This pattern of T3 effect on MHC isoforms was not modified by TSH cotreatment.
DISCUSSION
Hyperthyroid patients frequently suffer from muscle wasting and weakness. In these patients TSH is suppressed and thyroid hormones are increased, which led us to investigate the role of TSH in skeletal muscle tissue. In the present study, we demon-strate the expression of TSH-R in C2C12 myoblast and myo-tube with nested RT-PCR and sequencing. TSH treatment did not affect muscle differentiation, but the expression of genes related to muscle differentiation, myogenin, and myoD, were slightly increased after TSH treatments. The expression of fi-ber type determination-related genes in C2C12 myotube did not change after TSH treatment.
We provide the first evidence that TSH-R is expressed in a skeletal muscle cell line. As for cardiomyocytes, contradictory results exist regarding the presence of TSH-R in cardiomyo-cytes from tissue specimens [10,11], which is thought to result from fibroblast-like cells between myocytes that express TSH-R [10]. Since the cell line and muscle tissues used in our study could have also been contaminated by fibroblasts, we excluded
the possibility of contamination by cells other than myocytes that express TSH-R. Unlike a study of Busuttil and Frauman [10] that failed to detect mRNA in mouse abdominal skeletal muscle tissue, we applied the nested RT-PCR technique, which is more sensitive and specific than conventional RT-PCR. The inherent problem with PCR is its sensitivity, allowing the amplification of target mRNA present at very low levels. However, TSH-R was expressed in a similar amount compared to the expression level in mouse hypothalamic tissue, where TSH-R is reported to be present [12]. Also, we confirmed that the PCR bands were the true TSH-R using a direct sequencing method after DNA extraction from the expected bands. Of course, the presence of a transcript product does not necessari-ly mean functional protein, and we need to further confirm the presence of TSH-R protein in C2C12 myotube.
In the present study, we failed to show the functional signif-icance of TSH-R signaling in skeletal muscle cell differentia-tion by morphologic analysis. However, the finding that TSH mildly elevated the expression of myogenin at 10 mU/mL sug-gests that TSH could contribute to the myogenic differentia-tion process. At 100 nM of T3, which is 50- to 100-fold higher than the physiologic concentration of T3, myogenin was sig-nificantly elevated by 2- to 3-fold, and this increase in myo-genin expression is further enhanced by TSH. However, we could only conclude that overall TSH does not have a major role in myogenic differentiation, although the possibility of some minor role still remains.
As muscular weakness in a thyrotoxicosis state could result from muscle fiber-type switching [13], and suppressed TSH could play a role in this process, we investigated muscle fiber type-related gene expression at high T3 concentrations. T3 treatment at 1 nM concentration increased the expression of all MHC isoforms, and 100 nM T3 repressed the expression of MyHC2a and MyHC2b. However, incubation with TSH alone or in combination with T3 did not significantly affect the pat-tern of the T3 effect on muscle fiber type-related genes. We conclude that TSH receptor signaling does not affect the mus-cle type determination process.
been proposed to share a common autoimmune pathogenesis, and the presence of a common antigen has been suggested. Stringent PCR of extraocular muscle tissue from normal sub-jects revealed the expression of TSH-R in extraocular muscles, and TSH-R is hypothesized to function as a shared antigen with the thyroid and contribute to the occurrence of Graves ophthal-mopathy [10]. Although contradictory data exists as to the ex-pression of TSH-R in cardiomyocytes, a case of a 25-year-old man with Graves disease who suffered from severe cardiomy-opathy reported expression of TSH-R in a myocardial biopsy [14]. This also suggests the existence of a common antigen between the thyroid and heart, contributing to the occurrence of cardiomyopathy in Graves disease. These hypotheses on the pathogenesis of extrathyroidal manifestations in Graves dis-ease propose that skeletal muscle may also be the subject of autoimmune attack, given the skeletal muscle weakness and atrophy observed in patients with Graves disease. Future stud-ies should focus on the autoimmune destruction of skeletal muscle in Graves disease.
In conclusion, we confirmed the expression of TSH-R in a mouse skeletal muscle cell line using nested RT-PCR. Overall, TSH-R seems to have little effect on skeletal muscle differen-tiation or fiber type determination, warranting further study to investigate its functional significance in skeletal muscle.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was re-ported.
REFERENCES
1. Vassart G, Dumont JE. The thyrotropin receptor and the
regulation of thyrocyte function and growth. Endocr Rev 1992;13:596-611.
2. Farid NR, Szkudlinski MW. Minireview: structural and
functional evolution of the thyrotropin receptor. Endocri-nology 2004;145:4048-57.
3. Davies T, Marians R, Latif R. The TSH receptor reveals
it-self. J Clin Invest 2002;110:161-4.
4. Williams GR. Extrathyroidal expression of TSH receptor.
Ann Endocrinol (Paris) 2011;72:68-73.
5. Crisanti P, Omri B, Hughes E, Meduri G, Hery C, Clauser
E, Jacquemin C, Saunier B. The expression of thyrotropin receptor in the brain. Endocrinology 2001;142:812-22.
6. Haraguchi K, Shimura H, Lin L, Endo T, Onaya T.
Differ-entiation of rat preadipocytes is accompanied by expres-sion of thyrotropin receptors. Endocrinology 1996;137: 3200-5.
7. Roselli-Rehfuss L, Robbins LS, Cone RD. Thyrotropin
re-ceptor messenger ribonucleic acid is expressed in most brown and white adipose tissues in the guinea pig. Endo-crinology 1992;130:1857-61.
8. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J,
Eldeiry L, Rajendren G, Blair HC, Davies TF, Zaidi M. TSH is a negative regulator of skeletal remodeling. Cell 2003;115:151-62.
9. Carnac G, Albagli-Curiel O, Vandromme M, Pinset C,
Montarras D, Laudet V, Bonnieu A. 3,5,3’-Triiodothyro-nine positively regulates both MyoD1 gene transcription and terminal differentiation in C2 myoblasts. Mol Endocri-nol 1992;6:1185-94.
10. Busuttil BE, Frauman AG. Extrathyroidal manifestations
of Graves’ disease: the thyrotropin receptor is expressed in extraocular, but not cardiac, muscle tissues. J Clin Endo-crinol Metab 2001;86:2315-9.
11. Drvota V, Janson A, Norman C, Sylven C, Haggblad J,
Bronnegard M, Marcus C. Evidence for the presence of functional thyrotropin receptor in cardiac muscle. Biochem Biophys Res Commun 1995;211:426-31.
12. Kloprogge SJ, Busuttil BE, Frauman AG. TSH receptor
protein is selectively expressed in normal human extraocu-lar muscle. Muscle Nerve 2005;32:95-8.
13. Vadaszova A, Zacharova G, Machacova K, Jirmanova I,
Soukup T. Influence of thyroid status on the differentiation of slow and fast muscle phenotypes. Physiol Res 2004;53 Suppl 1:S57-61.
14. Koshiyama H, Sellitti DF, Akamizu T, Doi SQ, Takeuchi Y,