RESEARCH ARTICLE
Stromal Expression of Heat-Shock Protein 27
Is Associated with Worse Clinical Outcome in
Patients with Colorectal Cancer Lung
Metastases
Thomas Schweiger1,2, Christoph Nikolowsky1,2, Patrick Starlinger3, Denise Traxler1,2, Matthias Zimmermann2, Peter Birner4,7, Balazs Hegedüs1,5,6,7,10, Balazs Dome1,7,8,9,10, Michael Bergmann3, Michael Mildner11, Walter Klepetko1, Konrad Hoetzenecker1,2, Hendrik Jan Ankersmit1,2*
1Department of Thoracic Surgery, Medical University of Vienna, Vienna, Austria,2Christian Doppler Laboratory for Cardiac and Thoracic Diagnosis and Regeneration, Medical University of Vienna, Vienna, Austria,3Department of General Surgery, Medical University of Vienna, Vienna, Austria,4Clinical Institute of Pathology, Medical University of Vienna, Vienna, Austria,52nd Department of Pathology, Semmelweis University, Budapest, Hungary,6Molecular Oncology Research Group, Hungarian Academy of Sciences-Semmelweis University, Budapest, Hungary,7Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria,8National Koranyi Institute of Pulmonology, Budapest, Hungary,9Department of Thoracic Surgery, National Institute of Oncology-Semmelweis University, Budapest, Hungary,
10Department of Biomedical Imaging and Image-guided Therapy, Division of Molecular and Gender Imaging, Medical University of Vienna, Vienna, Austria,11 Department of Dermatology, Medical University of Vienna, Vienna, Austria
*hendrik.ankersmit@meduniwien.ac.at
Abstract
Background
Pulmonary metastases are common in patients with primary colorectal cancer (CRC). Heat-shock protein 27 (Hsp27) is upregulated in activated fibroblasts during wound healing and systemically elevated in various diseases. Cancer-associated fibroblasts (CAFs) are also thought to play a role as prognostic and predictive markers in various malignancies includ-ing CRC. Surprisinclud-ingly, the expression of Hsp27 has never been assessed in CAFs. There-fore we aimed to investigate the expression level of Hsp27 in CAFs and its clinical
implications in patients with CRC lung metastases.
Methods
FFPE tissue samples from 51 pulmonary metastases (PMs) and 33 paired primary tumors were evaluated for alpha-SMA, CD31, Hsp27 and vimentin expression by immunohis-tochemistry and correlated with clinicopathological variables. 25 liver metastases served as control group. Moreover, serum samples (n=10) before and after pulmonary metastasect-omy were assessed for circulating phospho-Hsp27 and total Hsp27 by ELISA.
PLOS ONE | DOI:10.1371/journal.pone.0120724 March 20, 2015 1 / 15
a11111
OPEN ACCESS
Citation:Schweiger T, Nikolowsky C, Starlinger P, Traxler D, Zimmermann M, Birner P, et al. (2015) Stromal Expression of Heat-Shock Protein 27 Is Associated with Worse Clinical Outcome in Patients with Colorectal Cancer Lung Metastases. PLoS ONE 10(3): e0120724. doi:10.1371/journal.pone.0120724
Academic Editor:Gabriele Multhoff, Technische Universitaet Muenchen, GERMANY
Received:August 17, 2014
Accepted:January 26, 2015
Published:March 20, 2015
Copyright:© 2015 Schweiger et al. This is an open access article distributed under the terms of the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability Statement:All relevant data are within the paper and its Supporting Information files.
Funding:This study was supported by the Christian Doppler Laboratory for Cardiac and Thoracic Diagnosis and Regeneration and by the Medical University of Vienna. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Stromal expression of Hsp27 was observed in all PM and showed strong correlation with alpha-SMA (P<0.001) and vimentin (P<0.001). Strong stromal Hsp27 was associated with higher microvessel density in primary CRC and PM. Moreover, high stromal Hsp27 and αSMA expression were associated with decreased recurrence-free survival after pulmonary metastasectomy (P=0.018 andP=0.008, respectively) and overall survival (P=0.031 and
P=0.017, respectively). Serum levels of phospho- and total Hsp27 dropped after metasta-sectomy to levels comparable to healthy controls.
Conclusions
Herein we describe for the first time that Hsp27 is highly expressed in tumor stroma of CRC. Stromalα-SMA and Hsp27 expressions correlate with the clinical outcome after pulmonary metastasectomy. Moreover, serum Hsp27 might pose a future marker for metastatic dis-ease in CRC.
Introduction
Colorectal cancer (CRC) is, after lung cancer, the second most common cause of cancer-related death in Europe [1]. More than one fifth of the patients with CRC present with metastases al-ready at time of diagnosis of the primary cancer and the same proportion will develop metasta-ses during the course of disease [1,2]. The lungs are the second most common site of distant metastasis, making pulmonary metastases (PM) an essential contributor to the high mortality of CRC.
Besides cancer cells themselves, a tumor comprises stromal cells. The interactions of stromal and cancer cells is thought to be a major determinant of the tumor behavior and response to therapy [3,4]. Cellular components of the stroma are fibroblasts, endothelial cells, immune cells and pericytes [5]. Cancer-associated fibroblasts (CAF), and especially activated fibroblasts, play a major role in the tumor-stroma network, similar to dermal fibroblasts in wound healing. This contributed to the description of tumors as“wounds that do not heal”by Dvoraket al. in
the late 80’s [6]. CAF contribute to various tumor-promoting characteristics like extra-cellular matrix turnover, tumor growth, angiogenesis and metastasis [7,8]. Due to the expression ofα
-smooth muscle actin (α-SMA), activated CAF are often described as myofibroblasts. They have
also been shown to be positive for fibroblast-activation protein-α/seprase, palladin and
vimen-tin [9–12]. Recently, efforts have been made to characterize the“signature”of these fibroblasts by proteome and gene expression profiling [13,14].
In the context of benign diseases, wound healing and keloid formation it is well known that activated fibroblasts express high levels of heat-shock protein 27 (Hsp27), which is crucial for fibroblast adhesion, contractility and motility [15–17]. The TGF-beta induced p38-MAPK pathway is the key regulator in the induction of Hsp27 in smooth muscle cells and myofibro-blasts [18,19]. Once synthesized, two main functions of Hsp27 are critical in wound healing process: promoting myofibroblast motility and angiogenesis. Hsp27 is involved in the stabiliza-tion of actin filaments and of SNAIL, an inducer of epithelial-mesenchymal transistabiliza-tion. Both mechanisms contribute to the induction of the myofibroblastic phenotype [20,21]. Another function of Hsp27 executed in a paracrine manner is enhancing angiogenesis. It was demon-strated that extracellular Hsp27 leads to NfκB activation and subsequent expression of the Medical University of Vienna. Preliminary results with
proangiogenic factors VEGF and interleukin-8 (IL-8) in endothelial cells [22]. Together with others, our group could show that an overexpression of Hsp27 is strongly linked to several be-nign and malign pathologies of the lung associated with fibroblast activation, including emphy-sema/chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis and non-small cell lung cancer [21,23–26].
Given the fact that activated fibroblasts play an important role in the progression of malig-nant disease as well as in various non-maligmalig-nant diseases of the lung, we aimed to investigate the prevalence of Hsp27 positive tumor stroma in CRC lung metastases and corresponding pri-mary tumors. Furthermore, we sought to describe the implications on the clinical outcome after metastasectomy and the presence of cellular and secreted Hsp27 in these patients.
Materials and Methods
Study population
From April 2009 to November 2013, all consecutive cases of pulmonary metastasectomy from primary CRC and appropriate tissue samples were included in this study. Patients received di-agnostic work-up including thoracic and abdominal computed tomography (CT). If patients had undergone pulmonary metastasectomy before, specimen of the first metastasectomy was also examined and the date of the first metastasectomy was used for outcome calculation. Lung metastasis free survival (LMFS) was defined as the time between diagnosis of the primary tumor and diagnosis of the metastatic spreading to the lung. R0 resection was achieved in all patients. Of 33 patients, specimens of the corresponding primary tumor could be obtained. Fol-low-up examinations were carried out in 3 to 6 months intervals. Recurrence-free survival (RFS) was defined as the period from the first pulmonary metastasectomy to evidence of recur-rent disease at any site verified by CT scans. In 10 consecutive cases, serum samples obtained before metastasectomy and during follow-up were available. Follow-up serum samples were collected 3 to 6 months after surgery. Additionally, serum samples from age-, gender- and smoking status- matched healthy volunteers were collected. All patients gave their written in-formed consent prior to blood collection and participation. A study cohort of 25 consecutive patients with resected CRC liver metastases served as additional control group. The study was approved by the ethics committee of the Medical University of Vienna (EK 91/2006, EK1194/ 2011 and EK1044/2012) and was conducted according to the declaration of Helsinki. The cur-rent study cohort is based on previous published works by our group.[27,28]
Immunohistochemistry and immunofluorescence
Immunohistochemical staining was performed according to a standard protocol. Shortly, for-malin-fixed, paraffin-embedded tissue specimens were cut in 4-μm thick sections and deparaf-finized. Heat-mediated antigen retrieval was performed. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide. Sections were incubated with the appropriate primary antibody for 1h at room temperature. For immunohistochemistry, as secondary step, the poly-mer- based ImmPRESS kit (Vector Laboratories, Burlingame, California) was used according to the manufacturer’s protocol. 3,3’-Diaminobenzidine (DAB) was used as substrate (Vector Laboratories, Burlingame, California). Finally, the sections were counterstained with hematox-ylin. Vimentin staining was performed in a Ventana ES Immunostainer System (Ventana Med-ical Systems Inc., Tucson, AZ, USA). For immunofluorescence, appropriate fluorescent secondary antibodies were used and cell nuclei were counterstained with DAPI (Sigma Aldrich, St. Louis, MO, USA). As negative controls, the primary antibody was omitted. Positive controls were tissue samples with known presence of the respective target protein. A detailed list of anti-bodies and dilutions is provided as supplementary information (S1 Table).
Hsp27 in CRC Lung Metastases
Scoring of stained tumor cells. Immunohistochemistry staining score (IHC score) was calculated as described previously [29]. The percentage of positive tumor cells could reach val-ues between 0 and 100%, and were multiplied by the staining intensity (0 to 3). Thus, IHC scores could range from 0 to 300. Two blinded observers rated the staining. In case that the two ratings differed, the slide was discussed and re-evaluated. The continuous IHC score was di-chotomized by applying the median score of metastases or primary tumors as cut-off value.
Scoring of stained stromal cells. The sections were scored semiquantitatively as described previously [10]. Briefly, the slides were screened at low magnification and evaluated for their staining intensity in the stromal cells in the tumor center. The stromal staining was assessed as grade 0 (negative,<1% positive stromal cells), grade 1+ (low,1–10% positive stromal cells), grade 2+ (intermediate,>10–50% positive stromal cells) and grade 3+ (strong,>50% positive stromal cells) by two blinded observers. In case that the two ratings differed, the slide was dis-cussed, re-evaluated and a consensus was reached on all slides.
Determination of microvessel density. Microvessel density (MVD) was measured using the“hotspot”method, as published elsewhere [30]. In brief, the slides were screened at low magnification to identify the area with the greatest number of CD31-positive microvessels (“hotspot”). MVD was determined by counting all microvessels at 200x magnification (corre-sponding to 0.95 mm2). Mean values were calculated from two independently counted densi-ties. In case of strong inter-observer discrepancy, the slide was reevaluated.
Enzyme-linked immunosorbent assay (ELISA)
Serum samples were assessed by commercially available human Hsp27, phospho-Hsp27 and interleukin-8 ELISA kits (all R&D Systems, Minneapolis, USA). Measurement was conducted according to the manufacturer’s instructions. The samples were assayed in duplicates. The ab-sorbance was measured at 450nm using a plate reader (PerkinElmer, Waltham, USA), com-pared to a standard curve with known protein content and converted to pg/mL.
Statistical analysis
All obtained data was evaluated statistically using SPSS 19 (SPSS Inc., Chicago, USA) and GraphPad Prism 6 (GraphPad Software Inc., California, USA). Student’s t-test was used to compare means of two independent groups, paired t-test for dependent groups and expressed as mean±standard deviation (SD). Kaplan-Meier curves and log-rank test were used to com-pare survival functions. Chi-square test and Fisher’s exact test were used to compare binominal variables. All tests were two-sided. P-values equal or below 0.05 were considered as
statistically significant.
Results
Tissue specimens of PM from a total of 51 consecutive patients were available. Median age at metastasectomy was 63 years (range 33–83). 29 (56.9.1%) male and 22 (43.1%) female patients were included. The clinicopathological characteristics of the included patients are summarized inTable 1.
Hsp27 is highly expressed in cancer-associated stroma of lung
metastases
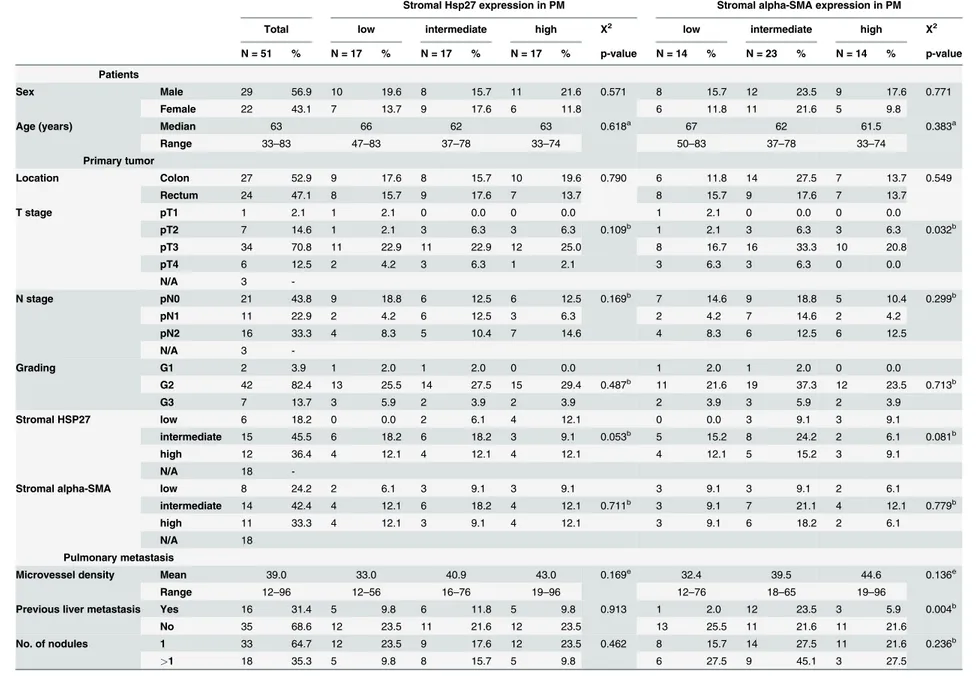
Table 1. Descriptive data on patient, primary tumor and metastases characteristics stratified by stromal Hsp27 andα-SMA expression (N = 51).
Stromal Hsp27 expression in PM Stromal alpha-SMA expression in PM
Total low intermediate high X2 low intermediate high X2
N = 51 % N = 17 % N = 17 % N = 17 % p-value N = 14 % N = 23 % N = 14 % p-value
Patients
Sex Male 29 56.9 10 19.6 8 15.7 11 21.6 0.571 8 15.7 12 23.5 9 17.6 0.771
Female 22 43.1 7 13.7 9 17.6 6 11.8 6 11.8 11 21.6 5 9.8
Age (years) Median 63 66 62 63 0.618a 67 62 61.5 0.383a
Range 33–83 47–83 37–78 33–74 50–83 37–78 33–74
Primary tumor
Location Colon 27 52.9 9 17.6 8 15.7 10 19.6 0.790 6 11.8 14 27.5 7 13.7 0.549
Rectum 24 47.1 8 15.7 9 17.6 7 13.7 8 15.7 9 17.6 7 13.7
T stage pT1 1 2.1 1 2.1 0 0.0 0 0.0 1 2.1 0 0.0 0 0.0
pT2 7 14.6 1 2.1 3 6.3 3 6.3 0.109b 1 2.1 3 6.3 3 6.3 0.032b
pT3 34 70.8 11 22.9 11 22.9 12 25.0 8 16.7 16 33.3 10 20.8
pT4 6 12.5 2 4.2 3 6.3 1 2.1 3 6.3 3 6.3 0 0.0
N/A 3
-N stage pN0 21 43.8 9 18.8 6 12.5 6 12.5 0.169b 7 14.6 9 18.8 5 10.4 0.299b
pN1 11 22.9 2 4.2 6 12.5 3 6.3 2 4.2 7 14.6 2 4.2
pN2 16 33.3 4 8.3 5 10.4 7 14.6 4 8.3 6 12.5 6 12.5
N/A 3
-Grading G1 2 3.9 1 2.0 1 2.0 0 0.0 1 2.0 1 2.0 0 0.0
G2 42 82.4 13 25.5 14 27.5 15 29.4 0.487b 11 21.6 19 37.3 12 23.5 0.713b
G3 7 13.7 3 5.9 2 3.9 2 3.9 2 3.9 3 5.9 2 3.9
Stromal HSP27 low 6 18.2 0 0.0 2 6.1 4 12.1 0 0.0 3 9.1 3 9.1
intermediate 15 45.5 6 18.2 6 18.2 3 9.1 0.053b 5 15.2 8 24.2 2 6.1 0.081b
high 12 36.4 4 12.1 4 12.1 4 12.1 4 12.1 5 15.2 3 9.1
N/A 18
-Stromal alpha-SMA low 8 24.2 2 6.1 3 9.1 3 9.1 3 9.1 3 9.1 2 6.1
intermediate 14 42.4 4 12.1 6 18.2 4 12.1 0.711b 3 9.1 7 21.1 4 12.1 0.779b
high 11 33.3 4 12.1 3 9.1 4 12.1 3 9.1 6 18.2 2 6.1
N/A 18
Pulmonary metastasis
Microvessel density Mean 39.0 33.0 40.9 43.0 0.169e 32.4 39.5 44.6 0.136e
Range 12–96 12–56 16–76 19–96 12–76 18–65 19–96
Previous liver metastasis Yes 16 31.4 5 9.8 6 11.8 5 9.8 0.913 1 2.0 12 23.5 3 5.9 0.004b
No 35 68.6 12 23.5 11 21.6 12 23.5 13 25.5 11 21.6 11 21.6
No. of nodules 1 33 64.7 12 23.5 9 17.6 12 23.5 0.462 8 15.7 14 27.5 11 21.6 0.236b
>1 18 35.3 5 9.8 8 15.7 5 9.8 6 27.5 9 45.1 3 27.5
aKruskal-Wallis test;
bFisher
’s exact test;
eOneway-ANOVA; LMFS: Lung-metastasis free survival after primary tumor
doi:10.1371/journal.pone.0120724.t001
Hsp27
in
CRC
Lung
Metastases
PLOS
ONE
|DOI:10.137
1/journal.p
one.0120724
March
20,
2015
5/1
correlation of the stromal Hsp27 staining with patient and tumor characteristics is depicted in
Table 1. Staining intensity of the tumor cells was determined separately (S1A Fig). 23 (45%) metastases were scored as Hsp27 highly positive and 28 (55%) metastases as low/negative (IHC score range 0–160; median/cut off 30). There was no correlation between tumor and stromal Hsp27 expression in pulmonary metastases (Chi square test;P= 0.237). 33 corresponding
pri-mary tumors were available. The stromal Hsp27 expression in the pripri-mary tumors 0 (0%), 6 (18%), 15 (46%) and 12 (36%) were scored as 0, 1+, 2+ and 3+, respectively. Determining the Hsp27 staining in the tumor cells in the primary tumor tissue, 14 (42%) of the cases were scored as Hsp27 positive and 19 (58%) as low/negative (IHC score range 5–100; median/cut off 70).
Hsp27 is co-expressed with
α
-SMA and vimentin in the stroma of PMs
Activated tumor stroma has been described as highlyα-SMA positive in primary and
metastat-ic CRC [12,31]. Therefore, the specimens were stained forα-SMA. The expression ofα-SMA
in the stroma was rated in the same semiquantitative manner as the Hsp27 staining. 0 (0%), 13 (25.5%), 24 (51%) and 13 (25.5%) cases were scored as 0, 1+, 2+ and 3+, respectively. The stain-ing score distribution accordstain-ing to the clinicopathological variables is depicted inTable 1.
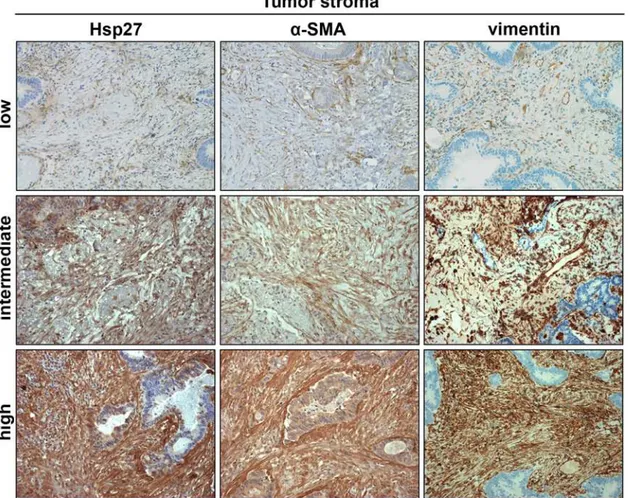
Fig 1. Representative images showing pulmonary metastases with low, intermediate and high intensity of positive tumor stroma stained for Hsp27 andα-SMA.Stromal fibroblasts were further identified by vimentin staining. (DAB substrate, same tumor specimen per row, 200x magnification).
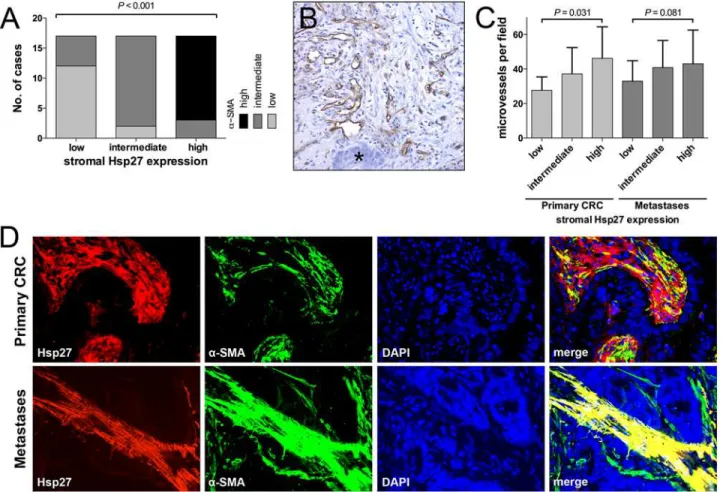
Expression levels ofα-SMA correlated significantly with Hsp27 expression in PMs (P<0.001,
Fig. 2A). This was also observed in liver metastases (S2 Fig). To further assess the stromal co-expression ofα-SMA and Hsp27, representative slides of primary and metastatic tumors were
co-labeled forα-SMA and Hsp27 by immunofluorescence. A strong co-expression could be
ob-served especially in PMs (Fig. 2D). Tumor stroma with strong Hsp27 andα-SMA staining was
also highly positive for vimentin (S3 Table).
MVD is increased in tissue samples with Hsp27-positive tumor stroma
Recently it was shown that Hsp27 mediates angiogenesis [22]. We therefore determined the MVD by CD31 staining and correlated it with the Hsp27 expressions (Fig. 2B and 2C). In PM, we found a trend towards higher MVD in samples with high Hsp27 levels. MVD was 33.0±2.9, 40.1±3.8 and 43.0±4.7 for 1+, 2+ and 3+ Hsp27 intensity (mean±SD). However, this trend did not reach the level of significance (P =0.081). Similarly, in primary CRC with high stroma
lev-els of Hsp27, significantly more microvesslev-els could be found (27.7±3.2, 37.2±4.0 and 46.4±5.2 for 1+, 2+ and 3+ Hsp27 intensity, respectively;P =0.031). A detailed description of MVD
stratified by clinicopathological characteristics is provided inS2 Table.
Fig 2. The degree of Hsp27 expression score in the tumor stroma correlated significantly with the expression of stromalα-SMA (A).CD31-positive microvessels surrounded by tumor stroma next to tumor cells (asterisk) (B). MVD was significantly increased in primary tumors and metastases with strong stromal Hsp27 expression (C). Immunofluorescence showed a co-expression of stromal Hsp27 andα-SMA especially in PM (400x magnification) (D).
doi:10.1371/journal.pone.0120724.g002
Hsp27 in CRC Lung Metastases
Stromal Hsp27 is associated with poor outcome after pulmonary
metastasectomy
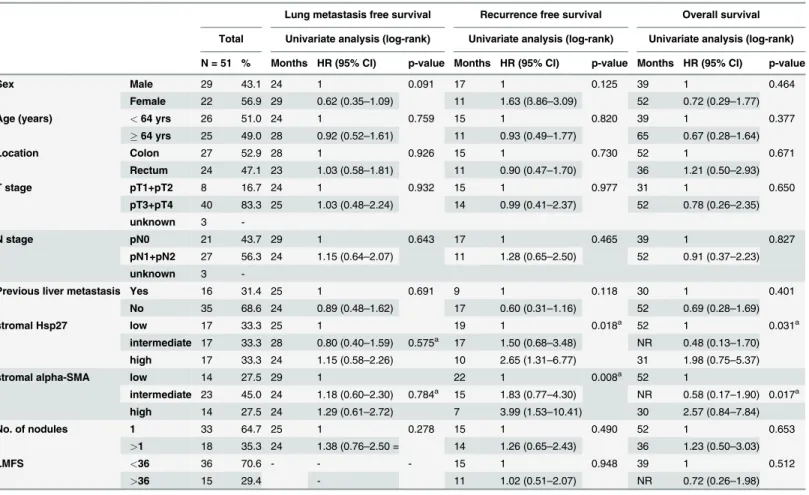
We assessed the association of relevant clinicopathological variables with LMFS, RFS and OS after metastasectomy (Table 2). No variable had significant impact on the LMFS. Female pa-tients had a strong trend towards a shorter time to PMs (P =0.091). The stromal expression of
neitherα-SMA nor Hsp27 was associated with a significantly decreased LMFS. However, both
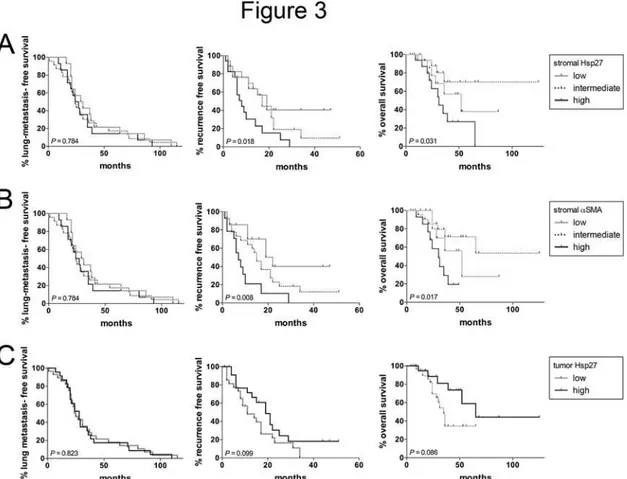
histological markers had a significant prognostic impact on the RFS after pulmonary metasta-sectomy (P =0.018 andP =0.008 for stromal Hsp27 andα-SMA, respectively) (Fig. 3). Again,
female patients showed a strong trend towards decreased RFS. Moreover, patients with a histo-ry of previous liver metastases had a decreased RFS compared to patients without liver metasta-ses in the history. However, both variables, sex and the history of previous liver metastametasta-ses, had no significant influence on the RFS. Additionally to that, extensive stromal Hsp27 stromal Hsp27 andα-SMA were associated with a decreased overall survival after metastasectomy
(P =0.031 andP =0.017 for stromal Hsp27 andα-SMA, respectively). Due to the high rate of
concordance, stromal Hsp27 andα-SMA were no independent factors in the outcome analysis
when adding both variables into a multivariate analysis (data not shown).
Table 2. Univariate analysis assessing clinicopathological variables and lung metastasis free survival, recurrence free survival after metastasect-omy and overall survival of patients (N = 51) with CRC metastasizing to the lung.
Lung metastasis free survival Recurrence free survival Overall survival
Total Univariate analysis (log-rank) Univariate analysis (log-rank) Univariate analysis (log-rank)
N = 51 % Months HR (95% CI) p-value Months HR (95% CI) p-value Months HR (95% CI) p-value
Sex Male 29 43.1 24 1 0.091 17 1 0.125 39 1 0.464
Female 22 56.9 29 0.62 (0.35–1.09) 11 1.63 (ß.86–3.09) 52 0.72 (0.29–1.77)
Age (years) <64 yrs 26 51.0 24 1 0.759 15 1 0.820 39 1 0.377
64 yrs 25 49.0 28 0.92 (0.52–1.61) 11 0.93 (0.49–1.77) 65 0.67 (0.28–1.64)
Location Colon 27 52.9 28 1 0.926 15 1 0.730 52 1 0.671
Rectum 24 47.1 23 1.03 (0.58–1.81) 11 0.90 (0.47–1.70) 36 1.21 (0.50–2.93)
T stage pT1+pT2 8 16.7 24 1 0.932 15 1 0.977 31 1 0.650
pT3+pT4 40 83.3 25 1.03 (0.48–2.24) 14 0.99 (0.41–2.37) 52 0.78 (0.26–2.35)
unknown 3
-N stage pN0 21 43.7 29 1 0.643 17 1 0.465 39 1 0.827
pN1+pN2 27 56.3 24 1.15 (0.64–2.07) 11 1.28 (0.65–2.50) 52 0.91 (0.37–2.23)
unknown 3
-Previous liver metastasis Yes 16 31.4 25 1 0.691 9 1 0.118 30 1 0.401
No 35 68.6 24 0.89 (0.48–1.62) 17 0.60 (0.31–1.16) 52 0.69 (0.28–1.69)
stromal Hsp27 low 17 33.3 25 1 19 1 0.018a 52 1 0.031a
intermediate 17 33.3 28 0.80 (0.40–1.59) 0.575a 17 1.50 (0.68
–3.48) NR 0.48 (0.13–1.70) high 17 33.3 24 1.15 (0.58–2.26) 10 2.65 (1.31–6.77) 31 1.98 (0.75–5.37)
stromal alpha-SMA low 14 27.5 29 1 22 1 0.008a 52 1
intermediate 23 45.0 24 1.18 (0.60–2.30) 0.784a 15 1.83 (0.77
–4.30) NR 0.58 (0.17–1.90) 0.017a
high 14 27.5 24 1.29 (0.61–2.72) 7 3.99 (1.53–10.41) 30 2.57 (0.84–7.84)
No. of nodules 1 33 64.7 25 1 0.278 15 1 0.490 52 1 0.653
>1 18 35.3 24 1.38 (0.76–2.50 = 14 1.26 (0.65–2.43) 36 1.23 (0.50–3.03)
LMFS <36 36 70.6 - - - 15 1 0.948 39 1 0.512
>36 15 29.4 - 11 1.02 (0.51–2.07) NR 0.72 (0.26–1.98)
Soluble Hsp27 is systemically increased in patients before pulmonary
metastasectomy
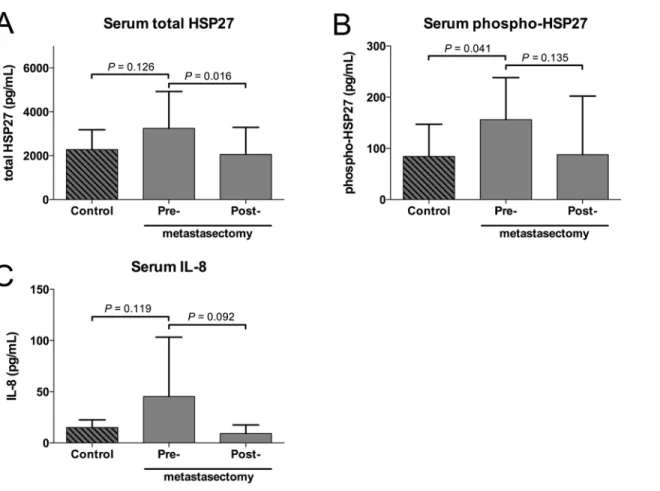
Elevated levels of soluble Hsp27 can be detected systemically in various malignant and non-malignant diseases. In a subset of 10 of our patients paired serum samples (pre- and post-metastasectomy) were available (Fig. 4). We compared the serum level of total Hsp27 and phospho-Hsp27 to matched healthy volunteers (S3 Table). Serum total Hsp27 levels were 2276 ±905, 3245±1684 and 2064±1226 pg/mL (mean±SD) for control, pre- and post-metastasect-omy samples, respectively. Total Hsp27 decreased significantly after metastasectpost-metastasect-omy
(P= 0.016). Serum phospho-Hsp27 levels were 84±63, 156±82 and 88±114 pg/mL (mean±SD)
for control, pre- and post-metastasectomy samples, respectively. A significant difference was found between healthy controls and pre-metastasectomy samples (P= 0.041). Although the
levels dropped after metastasectomy, the difference was not significant. IL-8, a proangiogenic cytokine involved in the Hsp27 signaling, was detected at very low concentrations of 15±8, 45 ±58 and 9±8 pg/mL (mean±SD) in the serum samples. The differences of systemic IL-8 levels between the three groups did not differ significantly from each other. No correlation was found between the expression level of stromal Hsp27, tumor Hsp27 and systemic levels (data not shown). Moreover, no significant correlation between total or phospho-Hsp27 and standard inflammatory markers (CRP and fibrinogen) was found (S1B and S1C Fig).
Fig 3. Kaplan-Meier plots showing the lung-metastasis free survival, recurrence free survival and overall survival after metastasectomy dependent on stromal Hsp27 (A), stromalα-SMA (B) and tumor Hsp27 (C) scoring.
doi:10.1371/journal.pone.0120724.g003
Hsp27 in CRC Lung Metastases
Stromal Hsp27 expression in pulmonary metastases is significantly
increased compared to liver metastases
We further evaluated the stromal expression of stromal Hsp27 andα-SMA in liver metastases
of 25 patients. Regarding the stromal Hsp27 expression 0 (0%), 12 (48.0%), 11 (44.0%) and 2 (8.0%) cases were scored as 0, 1+, 2+ and 3+, respectively. Stromalα-SMA expression was
rated as expression 0 (0%), 8 (32.0%), 11 (44%) and 6 (24.0%) cases were scored as 0, 1+, 2 + and 3+, respectively. We found a strong correlation between stromal Hsp27 andα-SMA
(P<0.001). Furthermore, stromal Hsp27 positivity was significantly more often found in pul-monary metastases compared to liver metastases (P = 0.014), whereas this difference was not significant forα-SMA (P = 0.625). Moreover, expression of Hsp27 in tumor cells (median 120
(range 0–270)) was significantly increased in liver metastases compared to pulmonary metasta-ses (P = 0.037). We further analyzed the overall survival of the patients with liver metastametasta-ses depending on their stromal Hsp27,α-SMA and tumor Hsp27 expression, but significant
differ-ences were not found (S3 Fig).
Discussion
Distant metastases are the main cause of cancer-related mortality. Today, the tumor microen-vironment and especially fibroblasts become of growing interest, providing additional informa-tion on tumor behavior and potential therapeutic targets [32]. Myofibroblasts can be found in
Fig 4. Total Hsp27 (A), phospho-Hsp27 (B) and IL-8 (C) were measured in serum samples of healthy volunteers, patients with CRC lung metastases before and 3 months after metastasectomy (each n = 10; whiskers indicate standard deviation).
the microenvironment of various tumors and extensive fibroblast-activation could be linked to rapid disease progression in malignant disease [32].
Herein we could demonstrate in a well-defined study cohort that vimentin+αSMA+stromal
cells are highly present in pulmonary metastases of primary CRC. Wikberget al. outlined that
myofibroblasts in the tumor center are a better prognosticator for poor prognosis than fibro-blasts at the tumor margin [33]. We, therefore, restricted the scoring of the putative fibroblast activation to the central portion of the tumor. Compared to Henryet al., who identified
myofi-broblasts by FAP-staining in primary colon cancer, we found a similar distribution of the stro-mal scoring (0%, 20%, 38% and 35% for negative, low, intermediate and strong staining, respectively). Additionally, they described the association of high amounts of activated fibro-blasts with a decreased overall survival in patients with colon cancer, especially in the metasta-sized situation [10]. This goes in line with our findings showing a decreased RFS after
pulmonary metastasectomy.
Interestingly,α-SMA positive phenotype of tumor-associated stroma was accompanied by
the expression of Hsp27. On the one hand, this small heat-shock protein has been examined in CRC tumor cells themselves [34–36]. An increased expression of Hsp27 in tumor cells of pri-mary CRC was described as negative prognosticator for survival [37,38]. On the other hand, Hsp27 expressing fibroblasts were examined in the context of wound healing and fibrosis. To the best of our knowledge, stromal Hsp27 has not been described as a potential prognostic marker in CRC. Interestingly, strong stromal Hsp27 expression was associated with high MVD in the primary tumors (P = 0.031) and in PM (P = 0.081). Due to the strong correlation of Hsp27 withα-SMA as an established marker for fibroblast activation, the over-expression of
Hsp27 was associated with significantly worse clinical outcome after pulmonary metastasect-omy (median RFS 19, 17 and 8 months for 1+, 2+ and 3+, respectively), comparable to the prognostic impact of stromalα-SMA expression. The decrease RFS was also translated in a
sig-nificantly different OS between the low, intermediate and high stromal Hsp27 orα-SMA,
re-spectively (Fig. 3). Similarly, Tsujinoet al. describedα-SMA positive fibroblasts as being
capable to predict disease recurrence in a cohort of patients with stage II and III primary CRC [12]. Kahlertet al. demonstrated by microdissection of primary CRC, lung and liver metastases
that a panel of pro-angiogenic factors are differentially expressed in tumor cells and the stromal compartment. The stromal expression of angiopoietin-2 in pulmonary metastases was an inde-pendent prognosticator for poor survival after surgery in multivariate analysis (P= 0.044),
even in a rather small study cohort (n = 25) [39]. These findings support the results of our work, in which we could describe Hsp27 as a further proangiogenic, stromal marker with prog-nostic impact after metastasectomy. It is also important to mention, that Satoet al. described
high stromal expression of the description factors ETS1 as a predictor of CRC lung metastases [40]. Interestingly, ETS1 is strongly interacting with the small heat-shock protein network [41].
The analysis of blood samples in a subgroup of patients revealed that metastatic CRC is a relevant inducer even of systemic Hsp27 expressions. The level of soluble total Hsp27 and phospho-Hsp27, a polymerized form of Hsp27, significantly decreased after complete removal of the PMs. Moreover, compared to a matched control group of healthy volunteers, pre-operative total Hsp27 and phospho-Hsp27 levels were increased and dropped after surgery to comparable low levels. Similar to our findings, Zhaoet al. could demonstrate increased serum
levels of Hsp27 in ovarian cancer with peritoneal metastases. In the same work it was also shown that the levels dropped after chemotherapy [42]. IL-8, which is thought to mediate local-ly the proangiogenic effect of Hsp27, was also elevated before metastasectomy without reaching significance [22]. Nontheless, the circulating levels of Hsp27 did not correlate with the stromal or tumor expression. Furthermore it did not correlate with the systemic levels of CRP or
Hsp27 in CRC Lung Metastases
fibrinogen. Thus, the circulating Hsp27 might be influenced by other factors like secretion, degradation and elimination. Based on these preliminary results, further studies with adequate cohort sizes will be necessary to further clarify the possible prognostic and predictive role of circulating Hsp27 levels in patients with CRC.
In patients with CRC liver metastases the tumor stroma Hsp27+vimentin+αSMA+
fibro-blasts were less evident than in pulmonary metastases. In contrast to this, the Hsp27 expression in tumor cells was significantly higher in resected liver metastases compared to pulmonary me-tastases. This goes in line with the observation that the p38 MAPK signaling, a pivotal kinase for Hsp27 phosphorylation and activation, is downregulated in CRC pulmonary metastases compared to liver metastases [43].
The findings of this work are of translational relevance because of two aspects: first, Hsp27, systemically, or confined to the tumor stroma, might possess potential prognostic and predic-tive value. Pulmonary metastasectomy is a widely offered treatment option in patients with CRC PM. However, the identification of patients who will benefit from surgery alone or an op-tional adjuvant chemotherapy is a matter of current research [44]. Biomarkers like Hsp27 might help to identify patients with high risk of early recurrence of disease after surgery. Adju-vant chemotherapy and a stringent follow-up could be offered to these patients. During the fol-low-up of patients with malignant disease, serum markers, e.g. CEA, CA19–9 and beta-HCG, are routinely used nowadays and additional markers might potentiate the accuracy of these es-tablished markers. A limitation to this study is, that CEA and CA19–9 levels were not routinely determined before metastasectomy. Correlation of these established tumor markers with serum Hsp27 will be addressed in a future study.
Secondly, Hsp27 is a promising drug target. Apatorsen (OGX-427, Oncogenex, Bothell, Washington, USA), a modified antisense oligonucleotide, binds to the Hsp27 mRNA tran-script and therefore inhibits the Hsp27 expression [45]. Currently ongoing Phase II
studies are recruiting patients with advanced prostate, bladder, pancreatic and non-small cell lung cancer [46]. The elevated expression levels of Hsp27 in the tumor-associated stroma provide a rationale for the use of Hsp27 also in patients with primary CRC. Given that not only CRC lung metastases, but also liver and lymph node metastases exhibit strong myofibro-blast recruitment, especially patients with metastatic disease might benefit from Hsp27 inhibition.
In conclusion, we could demonstrate that Hsp27 is co-expressed withαSMA in the tumor
stroma of CRC lung metastases and, moreover, that its over-expression is associated with worse clinical outcome after metastasectomy. Of note, soluble Hsp27 was also systemically measurable, making serum Hsp27 a potential future serum marker in CRC.
Supporting Information
S1 Table. Antibodies and dilutions used for immunohistochemistry and immunofluores-cence.
(DOCX)
S2 Table. Descriptive data on patient, primary tumor and metastases characteristics strati-fied by MVD and vimentin (N = 51).
(DOCX)
S3 Table. Characteristics of matched patients and healthy controls. (DOCX)
fibrinogen (n = 10) (B and C). Neither total Hsp27 (B), nor phospho-Hsp27 (C) correlated sig-nificantly with CRP or fibrinogen.
(TIF)
S2 Fig. In CRC liver metastases stromal Hsp27 andα-SMA scoring correlated significantly
(n = 25) (A). (TIF)
S3 Fig. The distribution of stromal Hsp27 (A) differed significantly in liver metastases (n = 25) compared to lung metasatses (n = 51).This difference did not reach significance for stromalα-SMA (B). Hsp27 expression in tumor cells was significantly higher in liver
metasta-ses compared to lung metastametasta-ses (C). No significant differences were observed between the subgroups regarding overall survival (D-F).
(TIF)
Author Contributions
Conceived and designed the experiments: MM PB BD WK KH HJA. Performed the experi-ments: TS CN DT MZ BH. Analyzed the data: TS DT KH HJA. Contributed reagents/materi-als/analysis tools: PS MB MZ PB BH BD MM WK KH HJA. Wrote the paper: TS PS MB MZ PB BH BD MM WK KH HJA.
References
1. La Vecchia C, Bosetti C, Lucchini F, Bertuccio P, Negri E, et al. Cancer mortality in Europe, 2000–
2004, and an overview of trends since 1975. Ann Oncol 2010; 21: 1323–1360. doi:10.1093/annonc/ mdp530PMID:19948741
2. Malvezzi M, Arfe A, Bertuccio P, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2011. Ann Oncol 2011; 22: 947–956. doi:10.1093/annonc/mdq774PMID:21303801 3. Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell
Bio-chem 2007; 101: 805–815. PMID:17226777
4. Smith NR, Baker D, Farren M, Pommier A, Swann R, et al. Tumor stromal architecture can define the in-trinsic tumor response to VEGF-targeted therapy. Clin Cancer Res 2013; 19: 6943–6956. doi:10. 1158/1078-0432.CCR-13-1637PMID:24030704
5. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvi-ronment. Cancer Cell 2012; 21: 309–322. doi:10.1016/j.ccr.2012.02.022PMID:22439926
6. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–1659. PMID:3537791
7. Orimo A, Tomioka Y, Shimizu Y, Sato M, Oigawa S, et al. Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. Clin Cancer Res 2001; 7: 3097–3105. PMID:11595701
8. Nakagawa H, Liyanarachchi S, Davuluri RV, Auer H, Martin EW Jr, et al. Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene 2004; 23: 7366–7377. PMID:15326482
9. Brentnall TA, Lai LA, Coleman J, Bronner MP, Pan S, et al. Arousal of cancer-associated stroma: over-expression of palladin activates fibroblasts to promote tumor invasion. PLoS One 2012; 7: e30219. doi:
10.1371/journal.pone.0030219PMID:22291919
10. Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res 2007; 13: 1736–1741. PMID:17363526 11. Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, et al. Quantitative evaluation of vimentin
ex-pression in tumour stroma of colorectal cancer. Br J Cancer 2007; 96: 986–992. PMID:17325702 12. Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, et al. Stromal myofibroblasts predict disease
recurrence for colorectal cancer. Clin Cancer Res 2007; 13: 2082–2090. PMID:17404090
13. Torres S, Bartolome RA, Mendes M, Barderas R, Fernandez-Acenero MJ, et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for
Hsp27 in CRC Lung Metastases
colorectal cancer. Clin Cancer Res 2013; 19: 6006–6019. doi:10.1158/1078-0432.CCR-13-1130
PMID:24025712
14. Herrera M, Islam AB, Herrera A, Martin P, Garcia V, et al. Functional heterogeneity of cancer-associat-ed fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin Cancer Res 2013; 19: 5914–5926. doi:10.1158/1078-0432.CCR-13-0694PMID:24052018 15. Hirano S, Rees RS, Gilmont RR. MAP kinase pathways involving hsp27 regulate fibroblast-mediated
wound contraction. J Surg Res 2002; 102: 77–84. PMID:11796002
16. Hirano S, Shelden EA, Gilmont RR. HSP27 regulates fibroblast adhesion, motility, and matrix contrac-tion. Cell Stress Chaperones 2004; 9: 29–37. PMID:15270075
17. Suarez E, Syed F, Alonso-Rasgado T, Mandal P, Bayat A. Up-regulation of tension-related proteins in keloids: knockdown of Hsp27, alpha2beta1-integrin, and PAI-2 shows convincing reduction of extracel-lular matrix production. Plastic and reconstructive surgery 2013; 131: 158e–173e. doi:10.1097/PRS. 0b013e3182789b2bPMID:23358011
18. Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, et al. A role for p38(MAPK)/HSP27 path-way in smooth muscle cell migration. J Biol Chem 1999; 274: 24211–24219. PMID:10446196 19. Meyer-Ter-Vehn T, Gebhardt S, Sebald W, Buttmann M, Grehn F, et al. p38 inhibitors prevent
TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Investigative ophthalmology & visual science 2006; 47: 1500–1509.
20. Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regu-lates endothelial cell migration. FASEB journal: official publication of the Federation of American Socie-ties for Experimental Biology 1998; 12: 1481–1490.
21. Wettstein G, Bellaye PS, Kolb M, Hammann A, Crestani B, et al. Inhibition of HSP27 blocks fibrosis de-velopment and EMT features by promoting Snail degradation. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2013; 27: 1549–1560. doi: 10.1096/fj.12-220053PMID:23288928
22. Thuringer D, Jego G, Wettstein G, Terrier O, Cronier L, et al. Extracellular HSP27 mediates angiogene-sis through Toll-like receptor 3. FASEB journal: official publication of the Federation of American Socie-ties for Experimental Biology 2013; 27: 4169–4183. doi:10.1096/fj.12-226977PMID:23804239 23. Lofdahl M, Kaarteenaho R, Lappi-Blanco E, Tornling G, Skold MC. Tenascin-C and alpha-smooth
mus-cle actin positive cells are increased in the large airways in patients with COPD. Respir Res 2011; 12: 48. doi:10.1186/1465-9921-12-48PMID:21496259
24. Jan Ankersmit H, Nickl S, Hoeltl E, Toepker M, Lambers C, et al. Increased serum levels of HSP27 as a marker for incipient chronic obstructive pulmonary disease in young smokers. Respiration 2012; 83: 391–399. doi:10.1159/000336557PMID:22469636
25. Hacker S, Lambers C, Hoetzenecker K, Pollreisz A, Aigner C, et al. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue de-struction. Clinical laboratory 2009; 55: 31–40. PMID:19350847
26. Zimmermann M, Nickl S, Lambers C, Hacker S, Mitterbauer A, et al. Discrimination of clinical stages in non-small cell lung cancer patients by serum HSP27 and HSP70: a multi-institutional case-control study. Clin Chim Acta 2012; 413: 1115–1120. doi:10.1016/j.cca.2012.03.008PMID:22465083 27. Schweiger T, Hegedus B, Nikolowsky C, Hegedus Z, Szirtes I, et al. EGFR, BRAF and KRAS status in
patients undergoing pulmonary metastasectomy from primary colorectal carcinoma: a prospective fol-low-up study. Ann Surg Oncol 2014; 21: 946–954. doi:10.1245/s10434-013-3386-7PMID:24281417 28. Schweiger T, Kollmann D, Nikolowsky C, Traxler D, Guenova E, et al. Carbonic anhydrase IX is
associ-ated with early pulmonary spreading of primary colorectal carcinoma and tobacco smoking. Eur J Car-diothorac Surg 2013.
29. Driessen A, Landuyt W, Pastorekova S, Moons J, Goethals L, et al. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-an-giogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcino-mas. Ann Surg 2006; 243: 334–340. PMID:16495697
30. Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992; 84: 1875–1887. PMID:1281237
31. Yeung TM, Buskens C, Wang LM, Mortensen NJ, Bodmer WF. Myofibroblast activation in colorectal cancer lymph node metastases. Br J Cancer 2013; 108: 2106–2115. doi:10.1038/bjc.2013.209PMID:
23652304
33. Wikberg ML, Edin S, Lundberg IV, Van Guelpen B, Dahlin AM, et al. High intratumoral expression of fi-broblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumour Biol 2013; 34: 1013–1020. doi:10.1007/s13277-012-0638-2PMID:23328994
34. Yoshimura T, Nagahara M, Kuo C, Turner RR, Soon-Shiong P, et al. Lymphovascular invasion of colo-rectal cancer is correlated to SPARC expression in the tumor stromal microenvironment. Epigenetics: official journal of the DNA Methylation Society 2011; 6: 1001–1011. doi:10.4161/epi.6.8.16063PMID:
21725199
35. Tweedle EM, Khattak I, Ang CW, Nedjadi T, Jenkins R, et al. Low molecular weight heat shock protein HSP27 is a prognostic indicator in rectal cancer but not colon cancer. Gut 2010; 59: 1501–1510. doi:
10.1136/gut.2009.196626PMID:20947885
36. Ghosh A, Lai C, McDonald S, Suraweera N, Sengupta N, et al. HSP27 expression in primary colorectal cancers is dependent on mutation of KRAS and PI3K/AKT activation status and is independent of TP53. Exp Mol Pathol 2013; 94: 103–108. doi:10.1016/j.yexmp.2012.09.001PMID:22982087 37. Wang F, Zhang P, Shi C, Yang Y, Qin H. Immunohistochemical detection of HSP27 and hnRNP K as
prognostic and predictive biomarkers for colorectal cancer. Med Oncol 2012; 29: 1780–1788. doi:10. 1007/s12032-011-0037-3PMID:21861207
38. Yu Z, Zhi J, Peng X, Zhong X, Xu A. Clinical significance of HSP27 expression in colorectal cancer. Mo-lecular medicine reports 2010; 3: 953–958. doi:10.3892/mmr.2010.372PMID:21472339
39. Kahlert C, Pecqueux M, Halama N, Dienemann H, Muley T, et al. Tumour-site-dependent expression profile of angiogenic factors in tumour-associated stroma of primary colorectal cancer and metastases. Br J Cancer 2014; 110: 441–449. doi:10.1038/bjc.2013.745PMID:24292449
40. Sato T, Miwa A. Ets-1 and integrin beta3 for lung metastasis from colorectal cancer. Apmis 2002; 110: 347–353. PMID:12076271
41. Bosman JD, Yehiely F, Evans JR, Cryns VL. Regulation of alphaB-crystallin gene expression by the transcription factor Ets1 in breast cancer. Breast Cancer Res Treat 2010; 119: 63–70. doi:10.1007/ s10549-009-0330-4PMID:19205872
42. Zhao M, Ding JX, Zeng K, Zhao J, Shen F, et al. Heat shock protein 27: a potential biomarker of perito-neal metastasis in epithelial ovarian cancer? Tumour Biol 2014; 35: 1051–1056. doi: 10.1007/s13277-013-1139-7PMID:24061637
43. Urosevic J, Garcia-Albeniz X, Planet E, Real S, Cespedes MV, et al. Colon cancer cells colonize the lung from established liver metastases through p38 MAPK signalling and PTHLH. Nature cell biology 2014; 16: 685–694. doi:10.1038/ncb2977PMID:24880666
44. Schweiger T, Lang G, Klepetko W, Hoetzenecker K. Prognostic factors in pulmonary metastasectomy: spotlight on molecular and radiological markers. Eur J Cardiothorac Surg 2014; 45: 408–416. doi:10. 1093/ejcts/ezt288PMID:23729747
45. Hadaschik BA, Jackson J, Fazli L, Zoubeidi A, Burt HM, et al. Intravesically administered antisense oli-gonucleotides targeting heat-shock protein-27 inhibit the growth of non-muscle-invasive bladder can-cer. BJU Int 2008; 102: 610–616. doi:10.1111/j.1464-410X.2008.07669.xPMID:18384625
46. Oncogenex Corp. Apatorsen (OGX-427). 2014. Available: http://www.oncogenex.com/apatorsen-ogx-427.
Hsp27 in CRC Lung Metastases