The resurrection of
Neohattoria
Kamim. (Jubulaceae,
Marchantiophyta): a six decade systematic conflict
resolved through a molecular perspective
Juan Larraín1, Benjamin Carter2, Blanka Shaw2, Jörn Hentschel3, Lynika S. Strozier1, Tatsuwo Furuki4, Jochen Heinrichs5, Barbara Crandall-Stotler6, John Engel1, Matt von Konrat1
1 Science & Education, he Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605-2496, U.S.A.
2 Department of Biology, Duke University, Durham, NC 27708-0338, U.S.A. 3 Department of Systematic Botany with Herbarium Haussknecht and Botanical Garden, Friedrich Schiller University, Fürstengraben 1, 07743 Jena, Germany 4 Natural History Museum & Institute, 955-2 Aoba-cho, Chuo-ku, Chiba-shi, Chiba 260-8682, Japan 5 Ludwig-Maximilians-Universität München, Department für Biologie I, Systematische Botanik und Mykologie, GeoBio-Center, Menzinger Straße 67, 80638 München, Germany 6 Southern Illinois University, Department of Plant Biology, Mail Code 6509, wCarbondale IL 62901-6509, U.S.A.
Corresponding author:Juan Larraín (musgoschiloe@gmail.com)
Academic editor:E. Cooper | Received 20 March 2015 | Accepted 16 April 2015 | Published 16 June 2015
Citation: Larraín J, Carter B, Shaw B, Hentschel J, Strozier LS, Furuki T, Heinrichs J, Crandall-Stotler B, Engel J, von Konrat M (2015) he resurrection of Neohattoria Kamim. (Jubulaceae, Marchantiophyta): a six decade systematic conlict resolved through a molecular perspective. PhytoKeys 50: 101–122. doi: 10.3897/phytokeys.50.4940
Abstract
he systematic placement of Frullania herzogii has been contentious since its description six decades ago. Over the years it has been interpreted as either a member of the genus Frullania or segregated into its own genus, Neohattoria, due to morphological similarities with both Frullania and Jubula. Here we provide molecular evidence that supports the recognition of the genus Neohattoria and its inclusion within the Jubulaceae, together with Jubula and Nipponolejeunea. Jubulaceae are placed sister to Lejeuneaceae rather than to the monogeneric Frullaniaceae.
Keywords
DNA sequence data, Frullania, Frullaniaceae, Japan, Jubula, Jubulaceae, Lejeuneaceae, liverwort,
Nipponolejeunea
http://phytokeys.pensoft.net
Copyright Juan Larraín et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
he liverwort Frullania herzogii S.Hatt. was originally described by Hattori (1955) from a poor, sterile specimen collected on Mt. Hayachine in Iwate Prefecture, north-ern Honshu, Japan. Since that time the generic and even familial placement of the species has remained controversial. he species also has remained poorly known par-tially because of its seemingly limited distribution in the subalpine coniferous forest zones of Honshu and Hokkaido, Japan, and the Kuril Islands (Inoue et al. 1981, Stotler and Crandall-Stotler 1987). Hattori (1955) remarked that the leaf morphol-ogy, with acute teeth along the margin, difered from all the other Japanese Frul-lania Raddi species known by him. A few years later, in his monograph of Japanese Frullaniaceae, Kamimura (1961) erected the new genus Hattoria Kamim. to separate this taxon from other Frullania species. He stated that although his new genus su-pericially resembled species of Cololejeunea (Spruce) Schifn. or Frullania, there was an important similarity between the branching patterns of Hattoria herzogii (S.Hatt) Kamim. and species in the genus Jubula Dumort. In both Jubula and Hattoria, the branches replace the lobule of the leaf at the point of insertion, and the leaf lobes are attached to both the main stem and to the branch. Although Kamimura (1961) noted the similarity of cell shape between Hattoria and Frullania, he considered the combination of branching architecture and leaf denticulation suicient to recognize
Hattoria as a distinct genus. A year later he had to give a new name, Neohattoria
Kamim., to his recently described genus (Kamimura 1962), because of the almost simultaneous although earlier description of Hattoria by Schuster for a liverwort in the Lophoziaceae (Schuster 1961).
Later Schuster (1963), in a key for the Southern Hemisphere genera of liver-worts, expanded the circumscription of Neohattoria to include two more species,
(as Neohattoria caledonica R.M.Schust.). Of these, F. hodgsoniae is now considered a member of F. subg. Diastaloba Spruce sect. Inconditum von Konrat, Hentschel & Heinrichs (von Konrat et al. 2010), while the rest of the taxa are currently in-cluded in Frullania subg. Microfrullania (R.M.Schust.) R.M.Schust. he current taxonomic placement of these taxa is based on both morphological (Hattori and Mizutani 1982, Schuster 1992) and molecular evidence (Hentschel et al. 2009, von Konrat et al. 2012).
Asakawa et al. (1979) demonstrated, based on chemical compound diferences, that Jubulaceae sensu lato should be divided into three families, i.e. Jubulaceae, Frullani-aceae and LejeuneFrullani-aceae. his view has been conirmed by most molecular phylogenies published to date (e.g., Forrest et al. 2006, Heinrichs et al. 2005, 2007). Asakawa et al. (1979) listed 11 morphological characters that support the separation of Frullaniaceae and Jubulaceae, and placed Neohattoria together with Jubula in the Jubulaceae. Hat-tori (1982, 1984, 1986) and HatHat-tori and Mizutani (1982) also accepted the separation between Jubulaceae and Frullaniaceae and argued that Amphijubula R.M.Schust., a genus formerly considered by Schuster (1970, 1980) as intermediate between Jubula
and Frullania, should be placed within Frullania. his view was irst held by Engel (1978), who had earlier reduced Amphijubula to a synonym of Frullania.
In 1987, Stotler and Crandall-Stotler published a thorough treatise of the taxo-nomic history of Neohattoria herzogii (S.Hatt.) Kamim. in the context of a detailed re-evaluation of its morphology, including the discovery of immature female inlo-rescences. In that contribution they came to the conclusion that this taxon should be considered within the circumscription of Frullania, although in its own subgenus, F.
subg. Dentatilobi Stotler & Crand.-Stot. heir conclusion was based on both vegeta-tive and reproducvegeta-tive characters, including the morphology of the bracts surrounding the female gametangia, lobule anatomy, leaf cell pattern, and the morphology of re-generants. Although they recognized that leaf-lobe insertion, branch morphology, and morphology of stylus are more similar to Jubula than to Frullania, they concluded that on the basis of the Frullania-like inlorescences and regenerants, Neohattoria should be synonomized with Frullania. his synonomy was adopted by Grolle and Meister (2004) who described a morphologically similar plant from Oligocene amber from Bitterfeld (Germany) as Frullania (subg. Dentatilobi) hamatosetacea Grolle. However, this fossil species appears morphologically closer to F. subg. Microfrullania than to
Neohattoria, and this issue will be explored in detail in a forthcoming monograph of the latter subgenus.
Lack of useable specimens has previously precluded inclusion of Neohattoria in molecular phylogenetic studies. As a result of recent collecting activities, fresh material became available that allowed for successful DNA extraction and ampliication. In the present study, we use molecular sequence data to investigate the phylogenetic position of Neohattoria. We investigate whether the genus should be placed in the Frullaniaceae or the Jubulaceae and evaluate whether molecular evidence supports the recognition of
Methods
Microscopy
For the production of microscopic images an Olympus BX51 microscope was used, equipped with both a QICAM Fast1394 camera from QIMAGING (Surrey, Canada), and a slide scanner (moving platform stage attached between the objectives and the condenser) from Objective Imaging Ltd. (Cambridge, UK). he software “Surveyor” from the latter company was used for the digitally rendered images.
DNA extraction, PCR amplification and sequencing
We worked with two independent datasets to address two diferent questions, (1) what is the position of Neohattoria relative to the Frullaniaceae, Jubulaceae and Lejeuneaceae, and once we obtained results from these analyses, we asked (2) what is the position of Neohat-toria within the Jubulaceae. For dataset 1 sequences were generated for two mitochondrial (nad1, rps3), and two chloroplast loci (psbA, rbcL), following DNA extraction, ampliica-tion and sequencing methods described by Shaw et al. (2003), and using primer sequences provided in Cooper et al. (2011). For dataset 2 we used the aforementioned plastid regions (psbA and rbcL) together with the nuclear ITS region following the methods described by Shaw et al. (2003), and the chloroplast trnL-trnF region, ampliied and sequenced as described in von Konrat et al. (2012). All sequences were edited and manually aligned in PhyDE v0.9971 (www.phyde.de) following the alignment rules and hotspot deinitions presented in Kelchner (2000), Olsson et al. (2009), and Borsch and Quandt (2009).
Taxon sampling and outgroup selection
For dataset 1 seven species of Radula were selected as outgroup taxa following the re-sults already published in recent liverwort phylogenies (Davis 2004, Forrest et al. 2006, Feldberg et al. 2014, Heinrichs et al. 2005, 2007). he same criteria were undertaken for dataset 2, including all taxa with sequences available in GenBank for Jubula and
Nipponolejeunea S.Hatt. (Ahonen 2006, Ahonen et al. 2003, Konstantinova and Vilnet 2011, Pätsch et al. 2010, Wilson et al. 2004, 2007), using selected taxa of the Lejeu-neaceae and species of Frullania as outgroup based on results from dataset 1. GenBank accession numbers for both newly generated sequences and for already published se-quences are provided in Appendices 1 and 2 for datasets 1 and 2 respectively.
Phylogenetic inferences
was partitioned setting one separate data block for each of the four genes used, each of them divided in three according to each codon position; introns and/or spacers were coded as extra partitions. Dataset 2 was partitioned in four parts, corresponding to the regions included only, without inner codon partition for the coding regions analysed. For dataset 1, phylogenetic reconstructions under maximum likelihood (ML) were performed in GARLI v2.01 (Zwickl 2006), setting up seven diferent models for the eleven partitions determined by PartitionFinder. Two independent searches each with 100 bootstrap replicates were made, and the 50% majority-rule consensus tree from all obtained trees was obtained with SumTrees v3.3.1 included in the package DendroPy v3.12.2 (Sukumaran and Holder 2010). Bayesian Posterior Probabilities analyses (PP) were executed in MrBayes v3.2.2 (Huelsenbeck and Ronquist 2001, Ronquist and Huelsenbeck 2003) also with the partitioned data set as given by PartitionFinder, and setting a diferent model for the individual partitions from the available options in MrBayes, with all characters given equal weight and gaps treated as missing data. he default settings of the program for a priori probabilities were used. Four runs, each with four MCMC chains (one million generations each) were run simultaneously, with the temperature of the heated chain set to 0.2 (default setting). Chains were sampled every 100 generations. Calculation of the consensus tree and posterior probabilities of clades was based on the set of trees sampled after the chains had converged, as observed graphically using Tracer v1.5 (Rambaut and Drummond 2007). For dataset 2, phylo-genetic reconstructions under ML were performed in GARLI v2.01 and Bayesian anal-yses were executed with MrBayes v3.2.2 following the protocols as described above. For this dataset only three diferent partitions were suggested by PartitionFinder, and the models given by this software for each partition were incorporated into the settings of both the ML and the Bayesian analysis. Trees were edited and support values added using TreeGraph v2.0.54-364 beta (Stöver and Müller 2010).
Results
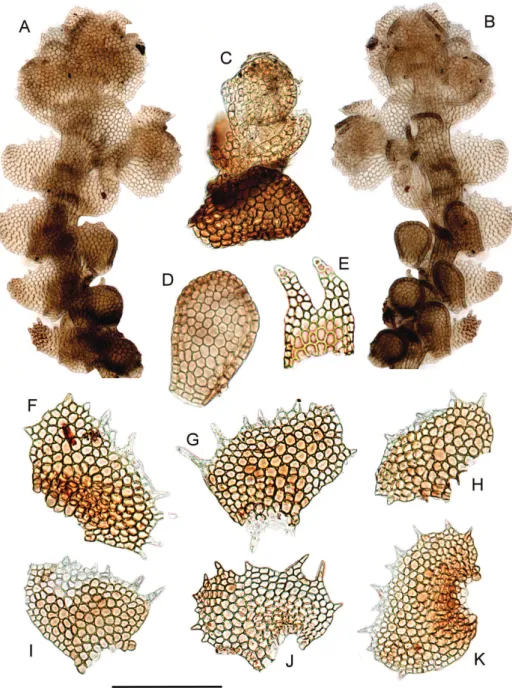
Figure 3.Neohattoria herzogii. A Habit, dorsal view B Habit, ventral view with distal lobules detached
C Regenerant shoot originating from a detached lobule D Lobule E Underleaf F–K Leaves. All from Furuki 22673 (F). Scale bar: 350 µm (A, B), 200 µm (C), 180 µm (D), 300 µm (E), 150 µm (F–K).
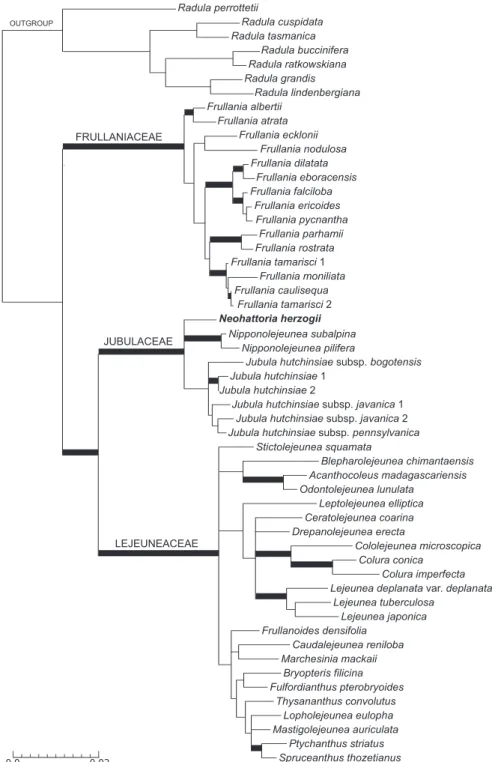
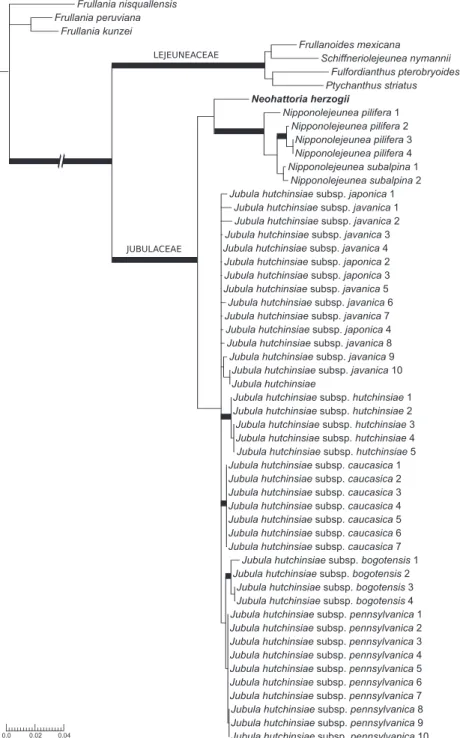
parsimony informative. he four diferent regions were not equally represented in the matrix, as shown in Appendix 2. he results of the analyses (Fig. 2) conirm with strong support the placement of Neohattoria within the Jubulaceae (ML = 100, PP = 1.0), and forming a sister clade to Nipponolejeunea, although recovered with strong support only by the Bayesian analysis (ML = 64, PP = 0.97). Jubula was resolved as the sister clade to the Neohattoria-Nipponolejeunea clade, although with low support (ML = 65, PP = 0.5).
he voucher of Neohattoria herzogii used for DNA extraction is illustrated in Figure 3.
Discussion
Our molecular analyses support recognition of the genus Neohattoria as distinct from the genus Frullania, as irst proposed by Kamimura (1961) almost 55 years ago. More-over, our molecular analysis strongly supports its inclusion within the Jubulaceae, to-gether with Jubula and Nipponolejeunea. A close relationship with Jubula, based on similarities in branch morphologies, was irst suggested by Kamimura (1961, p. 94), and also accepted by Hattori et al. (1972). Inoue et al. (1981) provided new karyologi-cal, chemical and ecological data on N. herzogii and concluded that the biosystematic evidence collected suggested distance between Jubula and Neohattoria, but, nonethe-less, retained Neohattoria in the Jubulaceae. While morphologically closer to Jubula
than Nipponolejeunea to which it is sister, it is clearly not nested in the Jubula clade. his combination of molecular and morphological evidence, in fact, supports its rec-ognition as a distinct genus in the Jubulaceae.
Circumscription and relationships of the Jubulaceae
Our results strongly support the position of the Jubulaceae (containing Jubula, Nip-ponolejeunea and Neohattoria) sister to the Lejeuneaceae, and the Frullaniaceae as sister of the latter clade, although without signiicant support (Fig. 1). hese results agree with several molecular phylogenies (e.g. Ahonen 2004, Forrest et al. 2006, Heinrichs et al. 2005, 2007). hus the traditional view of a widely circumscribed Jubulaceae including Frullania is further rejected in this study.
lobule ontogeny) Jubula belongs nearest to Frullania, which later lead Schuster (1992, p. 6) to describe Jubula as a “bona-ide genus of Jubulaceae [= Frullaniaceae]”. Mizutani (1961) was the irst to propose that, except for the lobule structure, Jubula had no align-ment with Frullania, and subsequently placed Jubula into the Lejeuneaceae. However, Asakawa et al. (1979) concluded that chemically, both Jubula and Frullania are quite diferent from Lejeunea Lib. species. Interestingly, the phylogenetic analysis by Crandall-Stotler and Crandall-Stotler (2000) of 40 gametophyte and 21 sporophyte characters distributed among 34 liverwort families, resolved F. asagrayana Mont. as sister to a clade contain-ing J. hutchinsiae (Hook.) Dumort. subsp. pennsylvanica (Steph.) Verd. and L. cavifolia
(Ehrh.) Lindb. However, in the systematic treatment of the same work (Crandall-Stotler and Stotler 2000) Jubulaceae is presented as including both Jubula and Frullania, where-as the Lejeuneaceae is presented where-as a separate family, following accepted clwhere-assiications of the time. he revised version of that classiication, incorporating some recent molecular data, presents the Frullaniaceae, Jubulaceae and Lejeuneaceae as three separate families within the suborder Jubulineae (Crandall-Stotler et al. 2008, 2009), which is accepted here but with the transfer of Neohattoria from the Frullaniaceae to the Jubulaceae.
Assessing the importance of diferent morphological characters in circumscribing Frullaniaceae, Jubulaceae and Lejeuneaceae has been a diicult problem, but there are several characters that are consistent with the molecular phylogenetic results presented here. In most Lejeuneaceae a true stylus does not develop, but instead a single, unstalked slime papilla is formed at the junction of the lobule base and the stem, while in Jubula
and Neohattoria there is a one- or two-celled ilament terminated by a slime papilla in this position (Crandall-Stotler and Guerke 1980, Stotler and Crandall-Stotler 1987). Both types of structures are clearly diferent from those of the Frullaniaceae, where the stylus is always formed by more than two cells and is usually very conspicuous. he Jubulac-eae and FrullaniacJubulac-eae can be clearly diferentiated from the LejeuneacJubulac-eae by the lobule, which is almost free from the larger dorsal lobe, and typically modiied into an inlated, balloon-like to helmet-shaped sac whose aperture is directed either toward the shoot base or toward the stem, with the exception of Nipponolejeunea which has Lejeuneaceae-like lobules. Guerke (1978) hypothesised that Jubula was more advanced than Frullania on the basis that Jubula has many specialized characteristics e.g., a highly reduced stylus, seta, and foot, and features associated with the sporeling. In contrast, Schuster (1992, p. 9) stated that taxa such as Amphijubula microcaulis (Gola) R.M.Schust. (≡F. microcaulis
Schuster (1980, 1992) questioned the division into two families and argued that only the single family Jubulaceae should be recognized, but commented that this area of classiication remains replete with ambiguities and contradictions. Interestingly, he also suggested that there was a possibility that Neohattoria might share a closer ainity to Jubulopsidaceae (= Lepidolaenaceae) than to Jubulaceae (Schuster 1996), a view irst expressed when Grolle (1966) transferred Jubula novae-zelandiae E.A.Hodgs. & S.W.Arnell, which is the generitype of Jubulopsis R.M.Schust., to Neohattoria. How-ever, recent molecular analyses (e.g., Heinrichs et al. 2005, Forrest et al. 2006) have demonstrated that Jubulopsis (= Lepidolaena) is far removed from the Jubulaceae.
Morphologically, the monogeneric Frullaniaceae can be diferentiated from the Jubulaceae by: (1) plants usually with conspicuous secondary pigmentation, often red-dish; (2) initial leaves of branches either triid or biid; and (3) spores with rosette-like protrusions. Conversely, in the Jubulaceae the plants are: (1) soft and without secondary pigmentation (thus usually dull green to pale brown); (2) the initial leaves of branches are small, subtriangular, and never tri- or biid; and (3) the spores without rosette-like protrusions. he irst two of these characters support the placement of Neohattoria
within Jubulaceae rather than Frullaniaceae (spores remain unknown in Neohattoria). Chemically, Frullania species in general, produce signiicant amounts of sesquiter-pene lactones, diterpenoids, and bibenzyl derivatives, which are considered important chemosystematic markers of the group (Asakawa et al. 1981, 1983, 1987, Kraut et al. 1994). On the other hand, cyclocolorenone and maalioxide have been isolated as major components of Jubula hutchinsiae (Hook.) Dumort. subsp. japonica (Steph.) Horik. & Ando (Asakawa et al. 1979); interestingly cyclocolorenone is also widely distributed in the Porellaceae. In contrast, no members of Jubula or Frullania produce parainic hydrocarbons which are characteristic for Neohattoria (Inoue et al. 1981).
Interestingly, Schuster (1996) suggested that there was a possibility that Neohattoria
might share a closer ainity to Jubulopsidaceae (= Lepidolaenaceae) than to Jubulac-eae. his view was irst expressed when Grolle (1966) transferred Jubula novae-zelandiae
E.A.Hodgs. & S.W.Arnell, which is the type species of Jubulopsis R.M.Schust., to Ne-ohattoria. However, preliminary unrooted trees made for this contribution including
Ascidiota C.Massal., Gackstroemia Trevis., Goebeliella Steph., Lepidogyna R.M.Schust.,
Lepidolaena Dumort. (= Jubulopsis) and Porella L. together with representatives outside the Porellales, showed Neohattoria far away from Lepidolaenaceae but within Jubulaceae (results not depicted). hese results are basically the same as the ones observed in recent molecular phylogenies (e.g. Heinrichs et al. 2005, Forrest et al. 2006), demonstrating that these groups are only distantly related to either the Jubulaceae or the Frullaniaceae.
Circumscription and relationships of Neohattoria
Our results place Neohattoria in the Jubulaceae with strong support, together with
Nipponolejeunea and Jubula. Within the Jubulaceae, Neohattoria is resolved as sister to
sen-sitive to taxon sampling (cf. Figs. 1 and 2), and not strongly supported in the analyses. When describing the genus Hattoria (later renamed Neohattoria), Kamimura (1961) conceived it as a monotypic genus containing only the Japanese endemic N. herzogii. he singularity of this taxon was well described and illustrated, highlighting its closer ainities to Jubula instead of Frullania, mostly because of its branching pattern and leaf insertion: “[…] the branch replaces the lobule of leaf in origin and the lobe is inserted partly to the stem and partly to the branch. he irst leaf and underleaf of branches are much deformed, being the “Vorblätter” of Verdoorn (1930).” (Kamimura 1961, p. 94). he characteristic combination of traits that led Kamimura to describe this new genus vanished when Schuster (1963, 1970) added more species in the circumscription of
Neohattoria as explained above. Schuster (1970) still recognized the taxonomic singu-larity of N. herzogii when placing it in its own subgenus within Neohattoria, but failed to see the relationships of this taxon with other Jubula species, precisely because of his wide concept of Neohattoria that includes members of F. subg. Microfrullania and F. subg. Diastaloba.
Oil-bodies in Neohattoria are homogenous, usually more than ten per cell, and similar in size to chloroplasts (Hattori et al. 1972, Inoue et al. 1981). Hattori et al. (1972) reported 10–20 oil-bodies per leaf lobe median cell for N. herzogii and later Inoue et al. (1981, p. 25) reported a similar number “usually 7–15 per leaf-lobe cell (rarely up to 22)”. Hattori et al. (1972) stated that oil-bodies of Neohattoria are hya-line and homogenous, and Inoue et al. (1981) recorded in their specimen of Neohat-toria that the oil-bodies were completely colourless and homogenous. However, they noted that sometimes they were faintly papillose with a few distinct granules; Inoue et al. (1981) were uncertain if this was due to degeneration of the oil-bodies. Reports of oil-body numbers for Jubula are ambiguous: although Guerke (1979) and Paton (1999) suggested they range between 3–7 in all Jubula taxa, Schuster (1992) stated that the oil-bodies are numerous in the North American material of J. pennsylvanica (≡
appear as almost homogeneous oil-droplets (von Konrat 2004). he oil-bodies of Neo-hattoria then appear closer to the other Jubulaceae genera in appearence (although smooth, homogeneous oil-bodies are also seen in Frullania subg. Microfrullania) and number, notwithstanding the number reported for Nipponolejeunea and some reports of Jubula taxa with fewer oil-body numbers.
Nomenclatural novelties
Neohattoria Kamim., Journal of Japanese Botany 37: 218. 1962.
≡Frullania subg. Dentatilobi Stotler & Crand.-Stotl., Memoirs of he New York Bo-tanical Garden 45: 542. 1987 (“Dentatiloba”). syn.nov. – Type: Frullania herzogii
S.Hatt.
Acknowledgements
We thank Anders Hagborg (he Field Museum) and Lars Söderström (Norwegian University of Science and Technology) and the Early Land Plants Today (ELPT) pro-ject for access to nomenclatural data. Support from the Biodiversity Synthesis Center of the Encyclopedia of Life provided important funding to help foster international initiatives. he Biodiversity Heritage Library is acknowledged for the facility they pro-vide that has greatly accelerated our efort. he generous support by the National Science Foundation (Awards No. 1145898, 1146168, and 0531730) is gratefully acknowledged. We also recognize the support of the Museum Collection Spending Fund, administered by he Field Museum, as well as curatorial support provided by Yarency Rodriguez, Lucia Kawasaki and Anna Balla (he Field Museum). Lauren Smith is acknowledged for providing the digital images used to compile the plates. Finally, we thank Matt Renner and an anonymous reviewer for their help in improv-ing the manuscript.
References
Ahonen I (2004) Molecular phylogeny of liverwort order Porellales (Marchantiophyta, Jun-germanniopsida). In: Goinet B, Hollowell VC, Magill R (Eds) Molecular Systematics of Bryophytes. Monographs in Systematic Botany from the Missouri Botanical Garden 98: 169–188.
Ahonen I (2006) he taxonomic position of the genus Nipponolejeunea Hatt. Journal of the
Hattori Botanical Laboratory 99: 319–342.
Asakawa Y, Tokunaga N, Toyota M, Takemoto T, Hattori S, Mizutani M, Suire C (1979) Chemosystematics of bryophytes II. he distribution of terpenoids in Hepaticae and An-thocerotae. Journal of the Hattori Botanical Laboratory 46: 67–76.
Asakawa Y, Matsuda R, Toyota M, Hattori S, Ourisson G (1981) Terpenoids and bibenzyls
of 25 liverwort Frullania species. Phytochemistry 20: 2187–2194. doi:
10.1016/0031-9422(81)80111-2
Asakawa Y, Matsuda R, Toyota M, Takemoto T, Connolly JD, Phillips WR (1983)
Sesquit-erpenoids from Chiloscyphus sp., Clasmatocolea sp., and Frullania sp. Phytochemistry 22:
961–964. doi: 10.1016/0031-9422(83)85030-4
Asakawa Y, Matsuda R, Cheminat A (1987) Bibenzyl derivatives from Frullania species.
Phyto-chemistry 26: 1117–1122. doi: 10.1016/S0031-9422(00)82361-4
Borsch T, Quandt D (2009) Mutational dynamics and phylogenetic utility of noncoding chloro-plast DNA. Plant Systematics and Evolution 282: 169–199. doi: 10.1007/s00606-009-0210-8 Casas C, Brugués M, Cros RM, Sérgio C, Infante M (2009) Handbook of liverworts and
hornworts of the Iberian Peninsula and the Balearic Islands. Ilustrated keys to genera and species. Institut d’Estudis Catalans, Secció de Ciències Biològiques, Barcelona, 177 pp. Cooper ED, Shaw AJ, Shaw B, Henwood MJ, Heslewood MM, Brown EA (2011) A
mul-ti-locus molecular phylogeny of the Lepidoziaceae: Laying the foundations for a stable classiication. Molecular Phylogenetics and Evolution 59: 489–509. doi: 10.1016/j. ympev.2011.02.006
Crandall-Stotler B, Guerke WR (1980) Developmental anatomy of Jubula Dum. (Hepaticae).
Bryologist 83: 179–201. doi: 10.2307/3242131
Crandall-Stotler B, Stotler RE (2000) Morphology and classiication of the Marchantiophyta. In: Shaw AJ, Goinet B (Eds) Bryophyte biology. Cambridge University Press, Cambridge, 21–70. doi: 10.1017/CBO9781139171304.003
Crandall-Stotler B, Stotler RE, Long DG (2008) Morphology and classiication of the March-antiophyta. In: Goinet B, Shaw AJ (Eds) Bryophyte biology, second edition. Cambridge University Press, Cambridge, 1–54.
Crandall-Stotler B, Stotler RE, Long DG (2009) Phylogeny and classiication of the Marchan-tiophyta. Edinburgh Journal of Botany 66: 155–198. doi: 10.1017/S0960428609005393 Damsholt K (2002) Illustrated lora of Nordic liverworts and hornworts. Nordic Bryological
Society, Lund, 837 pp.
Davis EC (2004) A molecular phylogeny of leafy liverworts (Jungermanniidae: Marchantiophyta). Monographs in Systematic Botany from the Missouri Botanical Garden 98: 87–118.
Engel JJ (1978) A taxonomic and phytogeographic study of Brunswick Peninsula (Strait of Magellan) Hepaticae and Anthocerotae. Fieldiana Botany 41: 1–319. doi: 10.5962/bhl. title.2426
Feldberg K, Schneider H, Stadler T, Schäfer-Verwimp A, Schmidt A, Heinrichs J (2014) Epi-phytic leafy liverworts diversiied in angiosperm-dominated forests. Scientiic Reports 4: 5974. doi: 10.1038/srep05974
and analyses. he Bryologist 109: 303–334. doi: 10.1639/0007-2745(2006)109[303:UT EHOT]2.0.CO;2
Frey W, Stech M (2009) Marchantiophyta. In: Frey W (Ed.) Syllabus of plant families – A. Engler’s Syllabus der Planzenfamilien. part 3: Bryophytes and seedless vascular plants. 13th edition, viii, Borntraeger, Berlin/Stuttgart, 13–115.
Gradstein SR, Churchill SP, Salazar-Allen N (2001) Guide to the bryophytes of tropical America. Memoirs of the New York Botanical Garden 86: 1–577.
Grolle R (1966) Miscellanea hepaticologica (51–60). Journal of Japanese Botany 41: 141–147. Grolle R, Meister K (2004) he liverworts in Baltic and Bitterfeld amber. Weissdorn, Jena, 1–91.
Guerke WR (1978) A monograph of the genus Jubula Dumortier. Bryophytorum Biblioteca
17: 1–118.
Hattori S (1955) A remarkable Frullania species from northern Japan. Feddes Repertorium
Specierum Novarum Regni Vegetabilis 58: 53–54.
Hattori S (1982) A synopsis of New Guinean Frullania, Hepaticae. Journal of the Hattori
Botanical Laboratory 51: 203–271.
Hattori S (1984) New Caledonian Frullaniaceae. Journal of the Hattori Botanical Laboratory 57: 405–426.
Hattori S (1986) A synopsis of New Caledonian Frullaniaceae. Journal of the Hattori Botanical Laboratory 60: 203–237.
Hattori S, Mizutani M (1982) A status of Amphijubula (Hepaticae) with special reference to
the seta anatomy. Journal of the Hattori Botanical Laboratory 52: 441–448.
Hattori S, Sharp AJ, Mizutani M (1972) Schusterella, a new genus of Jubulaceae (Hepaticae).
Journal of Japanese Botany 20: 329–338.
Heinrichs J, Gradstein SR, Wilson R, Schneider H (2005) Towards a natural classiication of
liverworts (Marchantiophyta) based on the chloroplast gene rbcL. Cryptogamie, Bryologie
26: 215–233.
Heinrichs J, Hentschel J, Wilson R, Feldberg K, Schneider H (2007) Evolution of leafy liverworts (Jungermanniidae, Marchantiophyta): estimating divergence times from chloroplast DNA sequences using penalized likelihood with integrated fossil evidence. Taxon 56: 31–44. Hentschel J, von Konrat MJ, Pócs T, Schäfer-Verwimp A, Shaw AJ, Schneider H, Heinrichs
J (2009) Molecular insights into the phylogeny and subgeneric classiication of Frullania
Raddi (Frullaniaceae, Porellales). Molecular Phylogenetics and Evolution 52: 142–156. doi: 10.1016/j.ympev.2008.12.021
Holmgren PK, Holmgren NH, Barnett LC (1990) Index herbariorum. Part I: he herbaria of the world (Regnum Veg. 120), eighth edition. New York Botanical Garden, New York, 693 pp.
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. doi: 10.1093/bioinformatics/17.8.754
Inoue H, Asakawa Y, Gradstein SR (1981) A biosystematic study of Neohattoria herzogii (Hatt.)
Kamim. Bulletin of the National Science Museum, Tokyo, Series B, 7: 23–30.
Kamimura M (1962) On the genus Neohattoria Kamim. nom. nov. (Hepaticae). Journal of Japanese Botany 37: 26.
Kelchner SA (2000) he evolution of non-coding chloroplast DNA and its application in plant systematics. Annals of the Missouri Botanical Garden 87: 482–498. doi: 10.2307/2666142
Konstantinova NA, Vilnet AA (2011) Jubula hutchinsiae subsp. caucasica subsp. nov.
(Jubu-laceae, Marchantiophyta) – a new taxon from the western Caucasus. Arctoa 20: 227–238. doi: 10.15298/arctoa.20.18
Kraut L, Mues R, Sim-Sim M (1994) Sesquiterpene lactones and 3-benzylphthalides from
Frul-lania muscicola. Phytochemistry 37: 1337–1346. doi: 10.1016/S0031-9422(00)90409-6 Lanfear R, Calcott B, Ho SYW, Guindon S (2012) PartitionFinder: combined selection of
partitioning schemes and substitution models for phylogenetic analyses. Molecular Biol-ogy and Evolution 2: 1695–1701. doi: 10.1093/molbev/mss020
Lanfear R, Calcott B, Kainer D, Mayer C, Stamatakis A (2014) Selecting optimal parti-tioning schemes for phylogenomic datasets. BMC Evolutionary Biology 14: 82. doi: 10.1186/1471-2148-14-82
Mizutani M (1961) A revision of Japanese Lejeuneaceae. Journal of the Hattori Botanical Laboratory 24: 115–302.
Müller K (1915) Die Lebermoose Deutschlands, Oesterreichs u. d. Schweiz mit Berücksichti-gung der übrigen Länder Europas, II. Abteilung. In: Dr. L. Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. 2. Aulage, 6. Band, 24. Lieferung. Eduard Kummer, Leipzig, 593–656.
Olsson S, Buchbender V, Enroth J, Hedenäs L, Huttunen S, Quandt D (2009) Phylogenetic analyses reveal high levels of polyphyly among pleurocarpous lineages as well as novel clades. he Bryologist 112: 447–466. doi: 10.1639/0007-2745-112.3.447
Paton JA (1999) he liverwort lora of the British Isles. Harley Books, Colchester, 626 pp. Pätsch R, Hentschel J, Linares-Palomino R, Zhu R-L, Heinrichs J (2010) Diversiication
and taxonomy of the liverwort Jubula Dumort. (Jungermanniopsida: Porellales)
in-ferred from nuclear and chloroplast DNA sequences. Systematic Botany 35: 6–12. doi: 10.1600/036364410790862515
Pedersen N, Holyoak DT, Newton AE (2007) Systematics and morphological evolution with-in the moss family Bryaceae: A comparison between parsimony and Bayesian methods for reconstruction of ancestral character states. Molecular Phylogenetics and Evolution 43: 891–907. doi: 10.1016/j.ympev.2006.10.018
Rambaut A, Drummond AJ (2007) Tracer, version 1.4. http://beast.bio.ed.ac.uk/Tracer Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under
mixed models. Bioinformatics 19: 1572–1574. doi: 10.1093/bioinformatics/btg180
Schuster RM (1961) Studies in Lophoziaceae. I. he genera Anastrophyllum and Sphenolobus
and their segregates. Revue Bryologique et Lichénologique 30: 55–73.
Schuster RM (1963) Studies on antipodal Hepaticae. I. Annotated key to the genera of antipodal Hepaticae with special reference to New Zealand and Tasmania. Journal of the Hattori Bo-tanical Laboratory 26: 185–309.
Schuster RM (1970) Studies on antipodal hepaticae, III. Jubulopsis Schuster, Neohattoria Kamimura
Schuster RM (1980) New combinations and taxa of Hepaticae, I. Phytologia 45: 415–437. Schuster RM (1992) he Hepaticae and Anthocerotae of North America east of the hundredth
meridan. Volume V. Field Museum of Natural History, Chicago, 854 pp.
Schuster RM (1996) On Jubulopsis Schust. (Jungermanniales: Jubulopsidaceae fam. nov.) and
its relationships. Journal of Bryology 19: 297–310.
Shaw AJ, Cox CJ, Boles SB (2003) Polarity of peatmoss evolution: who says mosses have no roots? American Journal of Botany 90: 1777–1787. doi: 10.3732/ajb.90.12.1777
Stotler RE, Crandall-Stotler B (1987) A re-evaluation of the genus Neohattoria (Jubulaceae).
Memoirs of the New York Botanical Garden 45: 535–543.
Stöver BC, Müller KF (2010) TreeGraph 2: Combining and visualizing evidence from dif-ferent phylogenetic analyses. BMC Bioinformatics 11: 7. doi: 10.1186/1471-2105-11-7 Sukumaran J, Holder MT (2010) DendroPy: A Python library for phylogenetic computing.
Bioinformatics 26: 1569–1571. doi: 10.1093/bioinformatics/btq228
Verdoorn F (1930) Die Frullaniaceae der Indomalesischen Inseln. (De Frullaniaceis VII). An-nales Bryologici Supplmentary Volume 1: 1–188. doi: 10.1007/978-94-015-5385-8
von Konrat M (2004) A systematic study of the liverwort genus Frullania Raddi: encompassing
a worldwide monograph of subg. Microfrullania (Schust.) Schust.; a revision of the New
Zealand species & study of subsidiary species. PhD dissertation, he University of Auck-land, NZ, xxviii + 408 pp.
von Konrat M, Hentschel J, Heinrichs J, Braggins JE, Pócs T (2010) Forty-one degrees below
and sixty years in the dark: Frullania sect. Inconditum, a new section of Australasian
Frul-lania species including F. colliculosa, sp. nov., F. hodgsoniae, nom. and stat. nov., F.
ater-rima, and F. hattorii (Frullaniaceae, Marchantiophyta). Nova Hedwigia 91: 471–500. doi:
10.1127/0029-5035/2010/0091-0471
von Konrat M, de Lange P, Greif M, Strozier L, Hentschel J, Heinrichs J (2012) Frullania
knightbridgei, a new liverwort (Frullaniaceae, Marchantiophyta) species from the deep south of Aotearoa-New Zealand based on an integrated evidence-based approach. PhytoKeys 8: 13–36. doi: 10.3897/phytokeys.8.2496
Wilson R, Gradstein SR, Heinrichs J, Groth H, Ilkiu-Borges AL, Hartmann FA (2004)
Phy-logeny of Lejeuneaceae: a cladistic analysis of chloroplast rbcL sequences and morphology
with preliminary comments on the mithocondrial nad4–2 spacer region. In: Goinet B,
Hollowell VC, Magill RE (Eds) Molecular Systematics of Bryophytes. Monographs in Systematic Botany from the Missouri Botanical Garden 98: 189–202.
Wilson R, Gradstein SR, Schneider H, Heinrichs J (2007) Unravelling the phylogeny of Lejeu-neaceae (Jungermanniopsida): Evidence for four main lineages. Molecular Phylogenetics and Evolution 43: 270–282. doi: 10.1016/j.ympev.2006.10.017
Appendix 1
Voucher information for data set 1. Information is presented in the following order: taxon name, collector followed by collection number (herbarium acronyms follow Holmgren et al. 1990), country: region (if known), GenBank accesion numbers (psbA/
rbcL/rps3/nad1). Lacking sequences are indicated by a dash (—). New sequences gen-erated for this study are marked by an asterisk (*).
Acanthocoleus madagascariensis (Steph.) Kruijt, Pócs 97145/AA (GOET), Uganda, EF011843/DQ983649/—/—; Blepharolejeunea chimantaensis van Slageren & Kruijt, Pócs & Rico 00234/A (F), Venezuela, KF851876/—/—/KF852465; Bryopteris i-licina (Sw.) Nees, Churchill, Magombo & Price 19855 (NY), Bolivia, AY607930/ DQ439681/KF851576/KF852481; Caudalejeunea reniloba (Gottsche) Steph., Pócs et al. 01090/AB (F), Australia, KF851845/KF852294/KF851541/KF852441; Cera-tolejeunea coarina (Gottsche) Schifn., Zartman 1235.1 (DUKE), Brazil, AY607934/ AY608026/—/KF852489; Cololejeunea microscopica (Taylor) Schifn., Long & Rothe-ro 37789 (E), Scotland: Wester Ross, KF851954/KF852386/KF851651/KF852552;
Colura conica (Sande Lac.) K.I.Goebel, Pócs & Streimann 9986/W (F), Australia: Queensland, KM817490*/KM817513*/KM817536*/KM817462*; Colura imperfec-ta Steph., Pócs & Pócs 07019/A (F), hailand, KF851881/KF852327/—/KF852469;
Drepanolejeunea erecta (Steph.) Mizut., Long 28691 (E), Bhutan, JF513393/ JF513452/KF851515/JF513342; Frullania albertii Steph., Davis 295 (DUKE), Ecua-dor, AY607942/DQ439685/KM817549*/KM817477*; Frullania atrata (Sw.) Nees ex Mont., Dauphin 3306 (F), Costa Rica, KM817491*/—/KM817540*/KM817466*;
Frullania caulisequa (Nees) Mont., Karst, Shaw & Gibbs 022 (DUKE), USA: North Carolina, KM817500*/KM817526*/KM817553*/KM817481*; Frullania dilatata
(L.) Dumort., Stotler 4666 (SIU), Portugal, KM817502*/KM817528*/KM817555*/ KM817482*; Frullania eboracensis Lehm., Stotler 80-4354 (ABSH), USA: Illinois, AY688827/AY688779/KM817547*/KM817475*; Frullania ecklonii (Spreng.) Spreng. ex Gottsche, Lindenb. & Nees, Pócs 02030/W (F), Kenya, KM817488*/ KM817510*/KM817533*/KM817459*; Frullania ericoides (Nees) Mont., Long 35167 (E), China: Yunnan, KM817486*/KM817507*/KM817531*/KM817456*;
Frullania falciloba Taylor ex Lehm., Engel, von Konrat & Braggins 26837 (F), New Zealand, KM817489*/KM817511*/KM817534*/KM817460*; Frullania moniliata
Stot-ler 4661 (SIU), Portugal: Sintra, KM817501*/KM817527*/KM817554*/—; Frul-lania tamarisci 2, Long 35371 (E), France, KM817487*/KM817508*/KM817532*/ KM817457*; Frullanoides densifolia Raddi, Gradstein 10171 (GOET), Ecuador, KF851930/KF852371/KF851634/KF852530; Fulfordianthus pterobryoides (Spruce) Gradst., Gradstein & Varon 11069 (GOET), Colombia, KF851931/KF852372/ KF851635/KF852531; Jubula hutchinsiae (Hook.) Dumort. 1, Long 29077 (E), UK: England, —/KM817509*/—/KM817458*; Jubula hutchinsiae 2, Drehwald 3007 (GOET), Portugal, EF011746/AY548101/—/—; Jubula hutchinsiae subsp. bogotensis
(Steph.) Verd., Gradstein s.n. (GOET), Mexico, EF011758/AY548100/—/—; Jubula hutchinsiae subsp. javanica (Steph.) Verd. 1, Konstantinova & Savchenko K479/1-07 (F), Russia, —/KM817506*/KM817542*/KM817468*; Jubulahutchinsiae subsp.
javanica 2, Kodama s.n. (ABSH), Japan: Wakayama Pref., AY507492/AY507408/ KF851585/JF513366; Jubula hutchinsiae subsp. pennsylvanica (Steph.) Verd., Risk 11005 (DUKE), USA, AY607954/KM817523*/KM817550*/—; Lejeunea deplana-ta Nees var. deplanata, Shaw F533 (DUKE), USA: North Carolina, KM817498*/ KM817524*/KM817552*/KM817479*; Lejeunea japonica Mitt., Bakalin s.n. (F), Russia, —/KM817518*/KM817543*/KM817469*; Lejeunea tuberculosa Steph., Long 28596 (E), Bhutan, JF513394/JF513453/KF851518/JF513344; Leptolejeu-nea elliptica (Lehm.) Besch., Yamaguchi s.n. (F), Japan, KM817485*/KM817515*/ KM817538*/KM817464*; Lopholejeunea eulopha (Taylor) Schifn., Pócs et al. 08036/U (F), Fiji, KF851868/KF852314/—/—; Marchesiniamackaii (Hook.) Gray, Buryova 2181 (DUKE), UK: Wales, —/KF852356/KF851619/KF852515; Mastigole-jeuneaauriculata (Wilson) Steph., Shaw 6222 (DUKE), USA: Alabama, KF851917/ KF852359/KF851622/KF852518; Neohattoria herzogii (S.Hatt.) Kamim., Furuki 22673 (F), Japan: Honshu, KM817504*/KM817530*/KM817557*/KM817484*;
Nipponolejeuneapilifera (Steph.) S.Hatt., Ohnishi 5975 (HIRO), Japan, AM396291/ AM392293/—/—; Nipponolejeunea subalpina (Horik.) S.Hatt., Ohnishi 5611 (GOET), Japan, AM396290/AM392292/—/—; Odontolejeunea lunulata (F.Weber) Schifn., Picon et al. 00227/CE (F), Venezuela, —/KM817514*/KM817537*/ KM817463*; Ptychanthus striatus (Lehm.) Nees, Pócs & Pócs 03288/O (F), Fiji, KF851872/KF852318/KF851558/KF852460; Radulabuccinifera (Hook.f. & Taylor) Taylor ex Gottsche, Lindenb. & Nees, Engel, von Konrat & Braggins 23569 (F), New Zealand, KM817495*/KM817521*/KM817545*/KM817472*; Radula cuspidata
Steph., Engel & von Konrat 23517 (F), New Zealand, KM817496*/—/KM817546*/ KM817473*; Radulagrandis Steph., Engel, von Konrat & Braggins 24847 (F), New Zealand, KM817494*/KM817520*/KM817544*/KM817471*; Radula lindenbergi-ana Gottsche ex C.Hartm., Stotler 4656 (SIU), Portugal, KM817503*/KM817529*/ KM817556*/KM817483*; Radulaperrottetii Gottsche ex Steph., Mizutani 15030 (F), Japan, —/DQ439700/KM817551*/KM817478*; Radula ratkowskiana K.Yamada, Engel, von Konrat & Braggins 24365 (F), New Zealand, KM817497*/KM817522*/—/ KM817474*; Radula tasmanica Steph., Engel, von Konrat & Braggins 24874 (F), New Zealand, KM817493*/KM817519*/—/KM817470*; Spruceanthus thozetianus
AM396273/AM384877/—/—; Stictolejeuneasquamata (Willd. ex F.Weber) Schifn., Dauphin & Gonzalez 2134 (GOET), Costa Rica: Alajeula, KF851951/—/—/ KF852549; hysananthusconvolutus Lindenb., Gradstein 10205 (GOET), Indonesia: Java, KF851953/DQ983737/KF851650/KF852551.
Appendix 2
Voucher information for data set 2. Information is presented in the following order: taxon name, collector followed by collection number (herbarium acronyms follow Holmgren et al. 1990), country: region (if known), GenBank accesion numbers (ITS region/rbcL/trnL-F/psbA). Lacking sequences are indicated by a dash (—). New se-quences generated for this study are marked by an asterisk (*).
Frullania kunzei (Lehm.) Lehm. & Lindenb., Costa & Gradstein 3769 (GOET), Brazil, FJ380536/FJ380863/FJ380387/FJ380697; Frullania nisquallensis Sull., Doyle 11001 (GOET), USA, FJ380503/FJ380826/FJ380349/FJ380661; Frullania pe-ruviana Gottsche, Schaefer-Verwimp & al. 24356 (GOET), Ecuador, FJ380543/ FJ380870/FJ380394/FJ380704; Frullanoides mexicana van Slageren, Burghardt 4421a, Mexico, DQ987366/DQ983682/DQ987464/EF011851; Fulfordianthus pterobryoides (Spruce) Gradst., Dauphin 2518, Costa Rica, AM237145/DQ983684/ AM237198/EF011832; Jubula hutchinsiae (Hook.) Dumort., Ahonen, Huttunen et Virtanen 3190 (H), Taiwan, AY125350/AY125946/AY144477/—; Jubula hutchin-siae subsp. bogotensis (Steph.) Verd. 1, Gradstein s.n. (GOET), Mexico: Veracruz, FN396818/—/FN398013/—; Jubula hutchinsiae subsp. bogotensis 2, Gradstein s.n. (GOET?), Mexico, DQ987273/AY548100/DQ987388/AM396281; Jubula hutch-insiae subsp. bogotensis 3, Gradstein 9449 (GOET), Costa Rica, FN396817/—/ FN398012/—; Jubula hutchinsiae subsp. bogotensis 4, Frahm et al. 1313 (GOET), Peru, FN396816/—/—/—; Jubula hutchinsiae subsp. caucasica Konstant. & Vil-net 1, Konstantinova K456-5-07 (KPABG), Russia: Caucasus, JN836964/—/ JN836974/—; Jubula hutchinsiae subsp. caucasica 2, Konstantinova K429-3-08 (KPABG), Russia: Caucasus, JN836961/—/JN836971/—; Jubulahutchinsiae subsp.
caucasica 3, Konstantinova K462-1-08 (KPABG), Russia: Caucasus, JN836960/—/ JN836970/—; Jubula hutchinsiae subsp. caucasica 4, Konstantinova K463-1-07 (KPABG), Russia: Caucasus, JN836962/—/JN836972/—; Jubula hutchinsiae subsp.
caucasica 5, Konstantinova K371-1-08 (KPABG), Russia: Caucasus, JN836958/—/ JN836968/—; Jubula hutchinsiae subsp. caucasica 6, Konstantinova K446-7-08 (KPABG), Russia: Caucasus, JN836959/—/JN836969/—; Jubula hutchinsiae subsp.
caucasica 7, Konstantinova K443-14-08 (KPABG), Russia: Caucasus, JN836963/—/ JN836973/—; Jubula hutchinsiae subsp. hutchinsiae 1, Long 29077 (GOET), UK: Devon, FN396813/—/FN398010/—; Jubula hutchinsiae subsp. hutchinsiae 2, Long 35296 (GOET), UK: Wales, FN396814/—/FN398011/—; Jubulahutchinsiae subsp.
Schaefer-Verwimp & Schaefer-Verwimp 25796 (GOET), Portugal: Boaventura, FN396812/—/ FN398009/—; Jubulahutchinsiae subsp. hutchinsiae 5, Drehwald & Reiner-Drehwald 3007 (GOET), Portugal, DQ987260/AY548101/DQ987380/AM396282; Jubula hutchinsiae subsp. japonica (Steph.) Horik. & Ando 1, Koponen et al. 54308 (H), China, AY125342/AY125938/AY144479/—; Jubulahutchinsiae subsp. japonica 2, In-oue BSE755 (GOET), Japan: Kochi, FN396809/—/—/—; Jubulahutchinsiae subsp.
japonica 3, Gradstein & Mizutani 2958 (GOET), Japan: Miyazaki, FN396810/—/ FN397098/—; Jubula hutchinsiae subsp. japonica 4, Bakalin P-68-10-08 (KPABG), Russia: Primorsky Kray, JN836967/—/JN836977/—; Jubula hutchinsiae subsp. ja-vanica (Steph.) Verd. 1, Zhu et al. 3361 (HSNU), China: Hainan, FN396800/—/— /—; Jubulahutchinsiae subsp. javanica 2, Zhu et al. 20050903-7a (HSNU), China: Hainan, FN396801/—/—/—; Jubula hutchinsiae subsp. javanica 3, Long 34765 (GOET), China: Yunnan, FN396805/—/FN397095/—; Jubula hutchinsiae subsp.
javanica 4, Pocs 98105/C (GOET), Viet Nam: Vin-Phuc, FN396807/—/—/—;
Jubula hutchinsiae subsp. javanica 5, Pocs & Tran Ninh 98103/A2 (GOET), Viet Nam: Vin-Phuc, FN396808/—/FN397097/—; Jubulahutchinsiae subsp. javanica 6, Schaefer-Verwimp & Verwimp 18870/A (GOET), Malaysia: Pahang, FN396802/—/ FN397094/—; Jubula hutchinsiae subsp. javanica 7, Zhu 555 (HSNU), China: Fu-jian, FN396806/—/FN397096/—; Jubulahutchinsiae subsp. javanica 8, Bakalin Kor-12-6-08 (KPABG), South Korea, JN836966/—/JN836976/—; Jubula hutchinsiae
subsp. javanica 9, Schaefer-Verwimp & Verwimp 18935 (GOET), Malaysia: Pahang, FN396803/—/—/—; Jubulahutchinsiae subsp. javanica 10, Wang 685B (HSNU), China: Yunnan, FN396804/—/—/—; Jubula hutchinsiae subsp. pennsylvanica
(Steph.) Verd. 1, Buck 39060 (H?), USA: West Virginia, AY776308/AY776303/ AY776309/—; Jubula hutchinsiae subsp. pennsylvanica 2, Davison 5045 (UNAF), USA: Alabama, FN396819/—/—/—; Jubulahutchinsiae subsp. pennsylvanica 3, Davi-son 5201 (UNAF), USA: West Virginia, FN396821/—/FN398015/—; Jubula hutch-insiae subsp. pennsylvanica 4, Davison 4707 (UNAF), USA: Alabama, FN396822/—/ FN398016/—; Jubula hutchinsiae subsp. pennsylvanica 5, Davison 3775a (UNAF), USA: Alabama, FN396823/—/FN398017/—; Jubulahutchinsiae subsp. pennsylvanica
6, Davison & Risk 2537 (UNAF), USA: Kentucky, FN396820/—/FN398014/—;
Jubulahutchinsiae subsp. pennsylvanica 7, Konstantinova ACH-3-92 (KPABG), USA, JN836965/—/JN836975/—; Jubula hutchinsiae subsp. pennsylvanica 8, Davison 4690 (UNAF), USA, Alabama, FN396824/—/FN398018/—; Jubula hutchinsiae
Nippon-olejeuneasubalpina (Horik.) S.Hatt. 1, Ohnishi 5611 (HIRO), Japan, DQ987289/ AM392292/FJ380227/AM396290; Nipponolejeunea subalpina 2, Higuchi 41358 (H?), Japan, AY776306/AY776305/AY776311/—; Ptychanthus striatus (Lehm.) Nees, Gradstein 10217, Indonesia: Java, DQ987297/DQ983723/DQ987403/EF011777;