A
cis
-Acting Diversification Activator Both Necessary and
Sufficient for AID-Mediated Hypermutation
Artem Blagodatski., Vera Batrak., Sabine Schmidl, Ulrike Schoetz, Randolph B. Caldwell, Hiroshi Arakawa, Jean-Marie Buerstedde¤*
Institute for Molecular Radiobiology, Helmholtz Center Munich, Neuherberg, Germany
Abstract
Hypermutation of theimmunoglobulin(Ig) genes requires Activation Induced cytidine Deaminase (AID) and transcription, but it remains unclear why other transcribed genes of B cells do not mutate. We describe a reporter transgene crippled by hypermutation when inserted into or near theIg light chain(IgL) locus of the DT40 B cell line yet stably expressed when inserted into other chromosomal positions. Step-wise deletions of theIgLlocus revealed that a sequence extending for 9.8 kilobases downstream of theIgLtranscription start site confers the hypermutation activity. This sequence, namedDIVACfor
diversification activator, efficiently activates hypermutation when inserted at non-Ig loci. The results significantly extend previously reported findings on AID-mediated gene diversification.They show by both deletion and insertion analyses that cis-acting sequences predispose neighboring transcription units to hypermutation.
Citation:Blagodatski A, Batrak V, Schmidl S, Schoetz U, Caldwell RB, et al. (2009) Acis-Acting Diversification Activator Both Necessary and Sufficient for AID-Mediated Hypermutation. PLoS Genet 5(1): e1000332. doi:10.1371/journal.pgen.1000332
Editor:Derry C. Roopenian, The Jackson Laboratory, United States of America
ReceivedApril 4, 2008;AcceptedDecember 9, 2008;PublishedJanuary 9, 2009
Copyright:ß2009 Blagodatski et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding:The work was supported by the European Framework VI grant ‘‘Geninteg’’, the Deutsche Forschungsgemeinschaft SFB ‘‘Networks in genome expression and maintenance’’, and a research grant to AB from the ‘‘Russian Foundation for Basic Research (project no. 07-04-01765a)’’.
Competing Interests:The authors have declared that no competing interests exist.
* E-mail: brstdd@googlemail.com
¤ Current address: Independent researcher, Gunta-Stoelzl Strasse 6, 80807 Munich, Germany
.These authors contributed equally to this work.
Introduction
Vertebrate B cells are able to diversify their rearranged immunoglobulin (Ig) genes by hypermutation, gene conversion and class switch recombination. All three phenomena require expres-sion of Activation Induced cytidine Deaminase (AID, NC_006088) [1–3] which most likely initiates Ig gene diversification by deaminating cytidines within the mutating and recombining sequences [4,5]. A further requisite for hypermutation and switch recombination is the transcription of theIggenes and the switch regions respectively [6,7].
Sequence analysis of transcribed non-Ig genes from AID expressing B cells revealed either no or only a low number of mutations compared to Ig genes [8]. A recent study of a large number of expressed genes in B cells found a significantly higher number of mutations in wild-type mice than in AID knock-out mice [9]. However, the mutation rates for thenon-Iggenes in AID expressing B cells were still orders of magnitude lower than for the Iggenes. To explain this difference betweenIgandnon-Iggenes it has been postulated thatcis-acting sequences in theIgloci activate hypermutation possibly by recruiting AID. However, intense efforts did not succeed to unambiguously define these sequences for the murine and human Ig loci [10]. Whereas studies using chimeric reporter genes in transgenic mice indicated that certainIg enhancers and their surrounding sequences conferred hypermuta-tion activity [11–14], delehypermuta-tion ofIgkenhancers in knock-out mice did not prevent hypermutation of the Igk gene (CAA36032) [15,16]. At least one murine B cell line [17] and AID expressing
fibroblasts [18] mutated transcribed transgenes in the absence of nearby Ig locus sequences, further confounding the issue of whethercis-acting regulatory sequences are needed for hypermu-tation.
The chicken B cell line DT40 diversifies its rearrangedIg light chain (IgL) gene by gene conversion in the presence of nearby pseudo V(yV) genes [2] and by hypermutation, if the yV genes are deleted [19]. Both activities strictly depend on the expression of AID. Consistent with the idea that the absence of homologous gene conversion donors leads to hypermutation, a Green Fluorescent Protein (GFP, AAB08058) transgene is rapidly diversified by mutations when inserted into the rearranged IgL locus [20]. The hypermutation activity of DT40 appeared however to be limited to the IgL locus, because no mutations were found in the highly transcribed Elongation Factor 1 alpha gene (NP_989488) [19]. This was confirmed by a recent study showing that neither the VpreB3 (NC_006102) nor the Carbonic Anhydrase (XP_415218) gene, immediate upstream and down-stream neighbors of theIgLlocus respectively, showed sequence heterogeneity in DT40 [21].
Results
A Hypermutation Reporter Based on GFP Expression
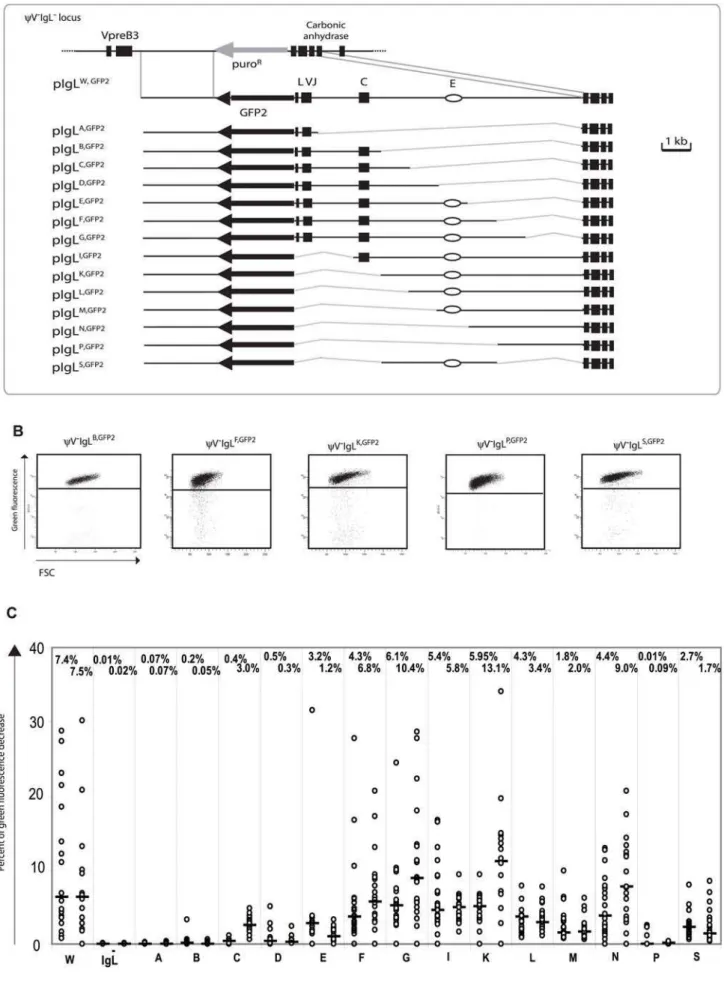
We have previously demonstrated that a GFP transgene in DT40 rapidly accumulates mutations, when integrated at the position of the promoter of the rearranged IgLlocus [20]. The hypermutation activity depended on AID expression and could be visualized by the appearance of cells displaying decreased green fluorescence due to detrimental GFP mutations. To exploit this phenomenon, we designed a new expression cassette namedGFP2 which consisted of the strongRSVpromoter followed by theGFP coding region, an internal ribosome entry site (IRES), the blasticidin resistancegene (P19997) and theSV40polyadenylation signal.GFP2 was incorporated into the targeting constructpIgLGFP2(Figure 1A) in the opposite transcriptional orientation of the IgL gene to minimize interference between transcriptional and post-transcrip-tional regulation of theGFP2transgene and theIgLgene.
Transfection ofpIgLGFP2
into the conditionally AID expressing clone AIDR2yielded a number of transfectants named IgLGFP2in which targeted integration had substituted theIgLpromoter by the GFP2 transgene. Fluorescence activated cell sorting (FACS) analysis of subclones from two independent primary transfectants revealed median values of 12.8% and 14.5% decreased green fluorescence two weeks after subcloning (Figure 1C and 1D). The result confirms our previous study indicating that the GFP2 transgene is mutated at high rate within the rearrangedIgLlocus and that cell populations with decreased green fluorescence can be used to quantify this hypermutation activity.
Hypermutation in the Vicinity of theIgLLocus
Targeted integration was used to insert theGFP2 reporter at various distances from the IgL locus into chromosome 15 [22] (Figure 1B and Figure S1A). FACS analysis of primary transfectants (Figure 1C and Table S1) and their subclones (Figure 1D) revealed that the medians of decreased green fluorescence fell to about 3% at the +26 kb and the 215 kb positions, to 0.5% at the+52 kb position and to about 0.05% at the 2135 kb position. The medians of decreased green fluorescence were only around 0.001% at the +52, +26 and 2135 kb positions in the absence of AID (Figure 1C and 1D and
Table S1), indicating that the decreased green fluorescence was dependent on AID expression.
Although we did not determine for theGFP2insertions outside theIgLlocus, whether the rearranged or the unrearranged allele was targeted, the results were representative for a large number of independent primary transfectants (Table S1). Thus, hypermuta-tion of theGFP2reporter was detectable at insertions up to 52 kb away from theIgLlocus, but mutations declined with increasing distance and were barely detectable at the2135 kb position.
Since surrounding sequences were unlikely to influence the post-transcriptional processing and translation of GFP2 transcripts, GFP2transcription should be reflected by the green fluorescence of the cells independent of the transgene insertion site. Even in the case of mutating transgenes, GFP2transcription levels could be deduced from the average green fluorescence of the major cell populations which most likely expressed the un-mutated GFP sequence. As seen by FACS analysis, the average green fluorescence of the major cell populations varied slightly among the primary transfectants (Figure 1C) most likely reflecting chromosomal position effects. However, the transfectants
+52IgLGFP2 and IgLGFP2 differed more than 20 fold in their
median fluorescence decreases despite similar green fluorescence of their major cell populations. This strongly suggested that the hypermutation differences among the transfectants reflected the distance of the GFP2 insertion sites to the IgL locus and not variation inGFP2transcription.
Identification of a Diversification Activator
The results could be explained by the presence of a cis-acting sequence which activated hypermutation in a distance dependent manner. We have named this putative regulatory sequence Diversification Activator (DIVAC) and attempted to map it by combining insertions of theGFP2reporter with deletions of theIgL locus.
To address the role of the yVpart of the IgLlocus, aGFP2 construct (Figure 2A, upper part) was transfected into the clone
yV2AIDR1 [20] in which the entire 20 kb containing the yV genes had been deleted. The transfectantsyV2IgLGFP2expressed the GFP2 reporter at the position of the IgL promoter in the absence of theyVlocus.
FACS analysis of yV2IgLGFP2 primary transfectants showed
sizable populations of cells showing decreased green fluorescence (Figure 2B) indicating that the GFP gene is diversified by hypermutation. To rule out that the decrease of green fluorescence is caused by gene silencing, the populations of high (GFP-high) and low (GFP-low) green fluorescence were sorted from one of the
yV2IgLGFP2 primary transfectants (Figure 2C, left), and GFP mRNA levels was analyzed by semi-quantitative reverse transcrip-tion polymerase chain reactranscrip-tion (PCR), (Figure 2C, right). RT-PCR of theEF1amRNA served as a control. Although GFP-low cells showed on average more than 100-fold lower green fluorescence than GFP-high cells, the levels ofGFPmRNA were comparable between sorted GFP-low, GFP-high and non-sorted cells, confirming that the decrease of green fluorescence is not due to silencing of GFP gene expression.
FACS analysis ofyV2IgLGFP2subclones revealed medians of 5.2% and 7.5% decreased green fluorescence (Figure 2D), only one fold lower than the medians of the yV positive IgLGFP2 subclones. As the difference between IgLGFP2 and yV2IgLGFP2 subclones could be due to fluctuation effects or different AID expression levels in the AIDR2andyV2AIDR1precursors, theyV locus seems to exert little, if any stimulation on the hypermutation activity of theGFP2reporter.
Author Summary
It remains an open question how AID-mediated gene diversification is targeted to the immunoglobulin loci. Here we define a cis-acting sequence, named DIVAC for diversification activator, which is required for hypermuta-tion of theIg light chain gene and sufficient to activate hypermutation at variousnon-Igloci in the DT40 B cell line.
yV2IgLGFP2still contained a 9.8 kb fragment of the rearranged IgLlocus extending from theIgLtranscription start site until the 39 end of the carbonic anhydrase gene and referred to in the following as fragment ‘W’. To test the relevance of this fragment, a GFP2construct was transfected into the cloneyV2IgL2in which the entire rearrangedIgLlocus had been replaced by thepuromycin resistancegene (P42670). The resulting transfectantsyV2IgL2,GFP2 had thepuromycin resistancegene replaced by theGFP2reporter at the position of the deletedIgLlocus (Figure 2A, middle part, and Figure 2B). Subclones ofyV2IgL2,GFP2showed medians of only 0.01% and 0.02% decreased green fluorescence (Figure 2D), more than 100 fold lower than the medians ofyV2IgLGFP2subclones. This indicated that the ‘W’ fragment, absent inyV2IgL2,GFP2but present inyV2IgLGFP2, was required for hypermutation of the GFP2 transgene. yV2IgL2 cells were then transfected by a construct including the GFP2 transgene and the ‘W’ fragment (Figure 2A, lower part). Subclones of the transfectants
yV2IgLW,GFP2 showed median green fluorescence decreases similar to the medians of yV2IgLGFP2 subclones (Figure 2D). Thus, the ‘W’ fragment efficiently activates hypermutation after reinsertion into the IgL locus as expected for a true DIVAC sequence.
Controls confirmed that the appearance of cells with decreased green fluorescence reflected hypermutation in theGFP2gene. As expected, the decrease of green fluorescence in yV2IgLGFP2 cultures depended on AID, because subclones of the AID negative transfectantyV2IgLGFP2AID2/2showed only very low medians of 0.001% decreased green fluorescence (Figure 2D). Furthermore, 723 bp of the GFP open reading frame amplified from
yV2IgLGFP2cells six weeks after subcloning showed an average of 0.9 nucleotide substitutions per sequence (Figure 2E). As the doubling time of the DT40 cell line is about 10 hours, the mutation rate of theGFPgene ofyV2IgLGFP2was calculated to be 1.361025mutation/bp/generation, which was similar to the mutation rate of the human hypermutating RAMOS cell line (2.261025mutation/bp/generation) [23,24]. The most prevalent mutations were C to G and Gto C transversions as previously observed for hypermutation of the IgL VJ segments from yV deleted DT40 clones [19]. In contrast, only a very low number of nucleotide substitutions, most likely reflecting polymerase chain reaction (PCR) artifacts, were found in the GFP gene of
yV2IgL2,GFP2cells (Figure 2E).
Fine Mapping ofDIVAC
A new series of targeting constructs was transfected into
yV2IgL2to characterize the ‘W’ fragment by step-wise deletions (Figure 3A). FACS analysis of subclones from the different transfectants showed a variable but progressive loss of hypermuta-tion activity when the ‘W’ fragment was shortened from either end (Figure 3C). The 4 kb ‘S’ fragment in the middle of the ‘W’ fragment, which included the previously identifiedIgL enhancer [25], still produced median green fluorescence decreases of 2.7% and 1.7%. In contrast, the upstream ‘B’ and the downstream ‘P’ fragments on their own produced median green fluorescence decreases of 0.13% and 0.05% respectively, which are low in absolute terms, but clearly above the medians ofyV2IgL2,GFP2. If either one of these fragments was combined with the ‘S’ fragment
in the ‘F’ and ‘K’ fragments respectively, the median decreases of green fluorescence were elevated about 3 times. This suggested that theDIVACof the chickenIgLlocus consisted of a central core region and partially redundant flanking regions which contributed to the overall activity. Clearly, more detailed analysis is needed to define the location, the nature and the configuration of the active motifs within theIgL DIVAC.
The average green fluorescence in the main population of
yV2IgLW,GFP2 was increased compared to yV2IgL2,GFP2 (Figure 2B) perhaps due to the additional stimulation of theRSV promoter in GFP2 by the IgL enhancer of the ‘W’ fragment. However, the relatively small decrease ofGFP2transcription seen in
yV2IgL2,GFP2was unlikely to be responsible for the more than 300 fold reduction of hypermutation. Analysis of the ‘W’ fragment deletions also strongly argued against the possibility that differences in hypermutation were caused by alterations ofGFP2transcription since primary transfectants of all fragments shown in Figure 3B showed similarGFPtranscription levels. Similar levels of steady-stateGFP2transcripts were confirmed by RT-PCR (Figure S2).
Hypermutation atnon-Ig Loci
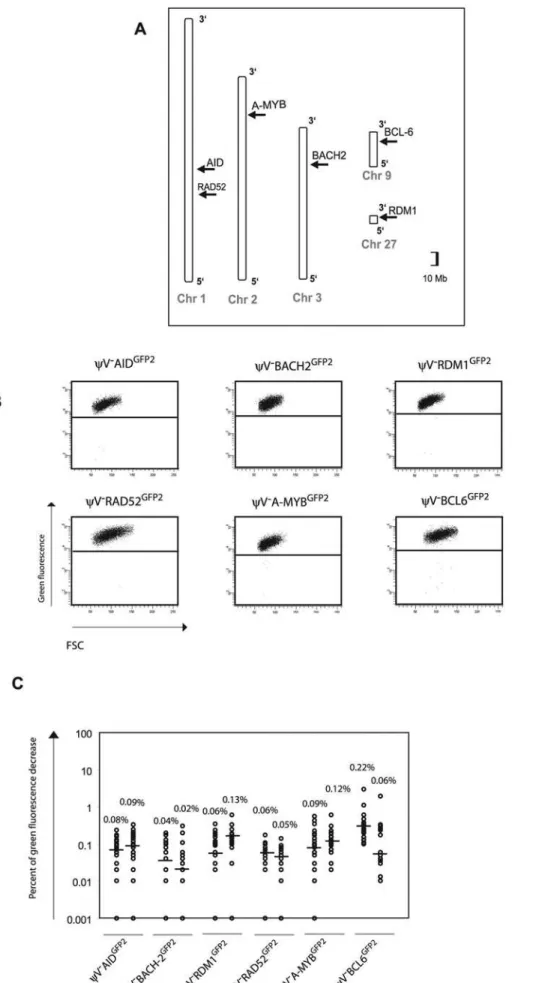
To confirm that theGFP2reporter on its own is stably expressed at non-Ig loci, six loci on five different chromosomes [22] were targeted by transfection of GFP2 constructs into yV2AIDR1 (Figure 4A and Figure S1B). Neither the primary transfectants (Figure 4B and Table S1B) nor their subclones (Figure 4C) showed high percentages of decreased green fluorescence. Depending on the experiment and the insertion site, the medians of the subclones ranged from 0.02% to 0.22% indicating that the mutation rates of theGFP2reporter at the chosen loci were 50 to 500 fold lower than at theIgLlocus. However, these medians were about 2–10 fold higher than the medians of various subclones from AID negative transfectants (Figures 2D and 5C) confirming a slight increase in the background mutation rates in AID expressing B cells [9].
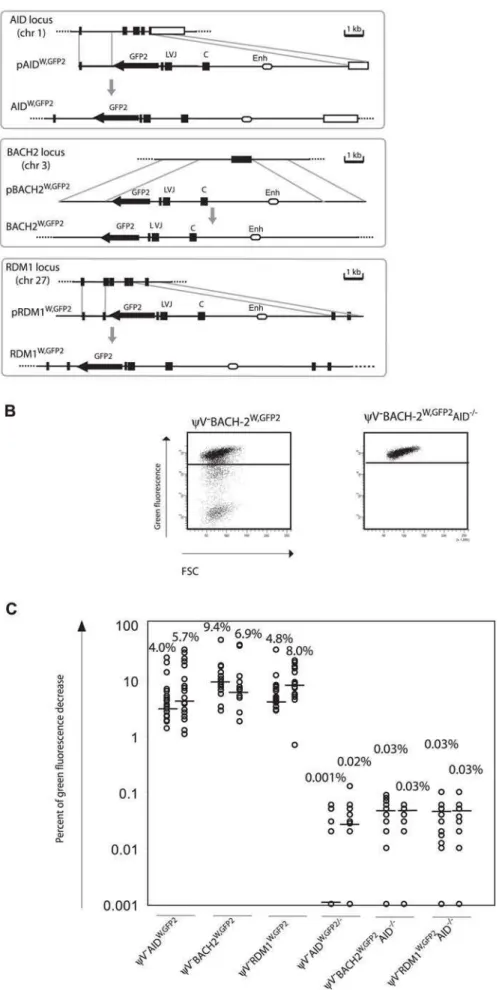
GFP2was then inserted together with the ‘W’ fragment into the respectiveAID,BACH2(NC_006090) andRDM1(BAC02561) loci ofyV2AIDR1(Figure 5A and 5B). Subclones of the transfectants
yV2AIDW,GFP2, yV2BACH2W,GFP2 and yV2RDM1W,GFP2 showed high median decreases of green fluorescence between 4.0% and 9.4% (Figure 5C) similar to the medians for
yV2IgLGFP2andyV2IgLW,GFP2subclones.GFP2hypermutation was AID dependent, since subclones of the AID negative transfectants yV2AIDW,GFP2/2, yV2BACH2W,GFP2/2AID2/2 and yV2RDM1W,GFP2/2AID2/2 showed very low medians of decreased green fluorescence in the range of 0.001% to 0.03%. These results demonstrated that the ‘W’ fragment was able to activateAIDmediated hypermutation at loci which otherwise did not support hypermutation.
Discussion
We have identified a cis-acting sequence that is needed for hypermutation at the chicken IgL locus and able to activate hypermutation at other loci upon insertion. The 9.8 kb sequence, namedDIVACfor diversification activator, extends from the IgL transcription start site towards the next downstream gene.DIVAC seems to be composed of multiple interacting regions. Whereas a 4
Figure 1. Hypermutation of theGFP2reporter at various distances from the rearrangedIgLlocus.(A) A physical map of the rearranged IgLlocus, a targeting construct including theGFP2reporter and theIgLlocus after targeted insertion of theGFP2reporter. (B) Locations ofGFP2 insertion relative to theIgLlocus on chromosome 15. The reference point is the insertion site ofGFP2in the IgLGFP2transfectant. (C) FACS analysis of primary transfectants having integrated the transfected construct targeted. (D) Fluctuation analysis of subclones. Each dot represents the analysis of a single subclone and the median of all subclones from the same primary transfectant is indicated by a bar.
kb core sequence which includes the knownIgLenhancer activates hypermutation more than 100 fold above background level, the flanking regions possess less activity on their own, but stimulate hypermutation when combined with the core. Surprisingly,DIVAC can act on either side of the hypermutation reporter and over long distances.
Given the conservation of AID mediatedIggene diversification during vertebrate evolution, the identification of the chickenIgL DIVACshould also be of relevance for mammals. Searches forcis -acting hypermutation regulatory sequences in transgenic mice showed that regions surrounding the Ig enhancers conferred hypermutation activity [11–14]. Intriguingly, the location and functional characteristics of these regions appear to be similar to the core of the chickenIgL DIVAC. In hindsight, the difficulty to unambiguously prove the existence of hypermutation activator sequences may relate to the large size of the murineIgloci and the fact that DIVACs seem to be composed of multiple interacting regions. As each of the murine Ig loci possesses at least two enhancers at different positions, murineDIVACs may be composed of multiple discontinuous sequences.
Bursal B cells and DT40 in the presence of nearbyyVgenes diversify their rearrangedIgLloci by gene conversion, suggesting that one of the physiological roles of theIgL DIVACis activation of gene conversion. This is supported by a recent publication showing that a deletion of the rearrangedIgLlocus downstream of the C region stopped IgLgene diversification in yV positive DT40 [26]. It seems also likely thatDIVACsplay a role for switch recombination which is accompanied by hypermutation of the recombining switch regions [27]. Possibly a dedicatedDIVACnear the switch regions activates switch recombination. As the chicken IgL DIVAC can activate hypermutation in both directions over large distances, it is also conceivable that a singleDIVACin the heavy chain loci regulates both hypermutation and switch recombination.
The mechanism of how acis-regulatory sequence can activate hypermutation in neighboring transcription units remains specu-lative. Intriguingly, the chicken IgL DIVACnot only includes the IgLenhancer, but also seems to act like an enhancer by activating hypermutation over long distances in upstream or downstream target genes. A plausible hypothesis may be thatDIVACpromotes the formation of protein complexes which first bind AID and then hand it over to the neighboring transcription initiation complex. Candidates for proteins involved in building such an AID docking station would be DNA binding factors which recognize sequence motifs within DIVAC. The described experimental system offers unique advantages to test this hypothesis.
Materials and Methods
Targeting Constructs
The GFP2 construct was made by combining the RSV promoter-GFP open reading frame of pHypermut2 [20] with a PCR amplicon including anIRES[19], theblasticidin resistancegene and the SV40 polyadenylation signal [28]. PCR was performed using the primers described in the Table S2.GFP2was flanked by
uniqueBamHIrestriction sites for easy cloning into the targeting vectors.
All targeting constructs except the ones belonging to the series of ‘W’ fragment deletions and reconstitutions were made by cloning the arms sequences intopBluescriptKS+ (Stratagene, CA) and then insertingGFP2either into uniqueBamHIorBglIIsites as shown in Figure 5A and Figure S1. Targeting ofAID[28] andRDM1[29] have been previously described. PCR amplifications of all target arms were performed using the Expand long template PCR System (Roche, Switzerland), DT40 genomic DNA as template and primers as described in the Table S2.
Since the ‘W’ fragment was difficult to amplify as a single sequence, it was sequentially cloned by combining upstream and downstream PCR amplicons with a 2.2 kbAvrII/SpeIrestriction fragment excised from the rearranged IgL targeting construct ‘Construct R’ [30]. The sequence of the AvrII/SpeI restriction fragment isA/Trich and localized between theJsegment and the Cregion. The assembled ‘W’ fragment of 9784 nucleotides was sequenced and deposited into Genbank under the accession number FJ482234. It starts at position27 relative to the first base of the IgL start codon and corresponds to the chicken genome coordinates chr15:8165070–8176699 but lacks theVJintervening sequence.
Constructs belonging to ‘W’ fragment deletion series were made by cloningGFP2between the target arms and then inserting the ‘W’ fragment or parts thereof into uniqueNheI/SpeIsites. ABamHI fragment containingGFP2and the ‘W’ fragment was incorporated into the AID, BACH2 and RDM1 targeting vectors to test the activity of the ‘W’ fragment innon-Igloci.
Cell Culture
Cells were cultured in chicken medium (RPMI-1640 or DMEM/F-12 with 10% fetal bovine serum, 1% chicken serum, 2 mM L-glutamine, 0.1mM b-mercaptoethanol and penicillin/ streptomycin) at 41uC with 5% CO2. Transfections were performed by electroporation, using 40mg of linearized plasmid DNA with a Gene Pulser Xcell (BIO-RAD) at 25mF and 700 V. Stable transfectants were selected by culturing in 15mg/ml of blasticidin. Transfectants having integrated the transgenic con-structs by targeted integration were identified by PCR using an inside primer from theSV40 polyadenylation signal sequence of GFP2together with a primer derived from the sequence outside the target arm (Table S2). In case of insertions into theIgLlocus, targeted integration into the rearranged allele was verified by amplifying theVJintervening sequence of the unrearranged locus. TheAIDreconstituted clone AIDR2was generated from theAID deleted clone AID2/2 [2] by transfection of a construct which targeted anAIDcDNA expression cassette into one of the deleted AID loci. The AID negative transfectants were produced by transfecting AID2/2andyV2AID2/2[19], respectively.
Flow Cytometry
The phenotype of each mutation was determined by FACS analysis of at least two independent targeted transfectants and
Figure 2.GFP2hypermutation after deletion and reconstitution of the rearrangedIgLlocus.(A) Design of deletions and insertions in the rearrangedIgLlocus usingGFP2targeting constructs. Upper part: Physical maps of the rearrangedIgLlocus after the deletion of theyVlocus, a targeting construct including theGFP2reporter and the locus after targeted integration. Middle part: Physical maps of the deleted rearrangedIgL locus, a targeting construct including theGFP2reporter and theGFP2insertion site after targeted integration. Lower part: Physical maps of the deleted rearrangedIgL locus, a targeting construct including theGFP2 reporter together with the ‘W’ fragment and the locus after targeted integration. (B) FACS analysis of primary transfectants. (C) Sorting of GFP-high and GFP-low cells from ayV2IgLGFP2primary transfectant, and semi-quantitative RT-PCR ofGFPandEF1amessages from sorted and non-sorted cells. (D) Fluctuation analysis of subclones. (E) The frequencies of particular nucleotide substitutions within theGFPopen reading frame ofGFP2.
Figure 3. Analysis of the ‘W’ fragment deletion series.(A) A physical map of the deletedIgLlocus and the aligned targeting constructs leading to the insertion of theGFP2reporter together with parts of the ‘W’ fragment. (B) FACS analysis of representative primary transfectants. (C) Fluctuation analysis of subclones. Only the letter of the reconstituted fragment and not the full name of the transfectants is indicated for clarity.
doi:10.1371/journal.pgen.1000332.g003
Figure 4. Insertions of theGFP2reporter intonon-Igloci.(A) Chromosomal locations of theGFP2insertions. (B) FACS analysis of primary transfectants. (C) Fluctuation analysis of subclones.
Figure 5. Hypermutation of theGFP2reporter atnon-Igloci in the presence of the ‘W’ fragment.(A) Physical maps of thenon-Igloci, the targeting constructs including theGFP2reporter together with the ‘W’ fragment and the loci after targeted insertion of theGFP2reporter. (B) FACS analysis of primaryAIDpositive andAIDnegative transfectants. (C) Fluctuation analysis of subclones.
doi:10.1371/journal.pgen.1000332.g005
twenty-four subclones of each. The primary transfectants were analyzed by FACS about three weeks after transfection and the subclones two weeks after subcloning. As the green fluorescence levels in the main populations varied slightly among the transfectants, the gates to separate the main population of green fluorescent cells from cells showing decreased or lost green fluorescence were adapted accordingly. At least 5000 events falling into the live cell gate were collected for each primary transfectant or subclone. Subclones in which more than 50% of the live cell events fell into the gates for decreased or lost green fluorescence were excluded from the analysis as they might represent the expansion of a precursor cell already expressing a mutatedGFP2 transgene at the time of subcloning.
GFPGene Sequencing
To minimize PCR-introduced mutations, Pfu Ultra hotstart polymerase (Stratagene) was used for the amplifications of theGFP open reading frames prior to sequencing. Sequencing and sequence analysis were performed as previously described [19].
RT-PCR
RT-PCR was performed as previously described [2]. Primer pairs used for amplification of the GFP and elongation factor 1a
transcripts are shown in Table S2.
Supporting Information
Figure S1 (A) Targeting strategy of theGFP2reporter into four different loci on chromosome 15. (B) Targeting strategy of the
GFP2reporter into theA-MYB,RAD52,BACH2, andBCL6loci. The targeting strategies used for the insertions into theAID and theRMD1loci were described previously [2,29].
Found at: doi:10.1371/journal.pgen.1000332.s001 (1.87 MB EPS)
Figure S2 Comparison of GFP gene expression levels analyzed by semi-quantitative RT-PCR of primary transfectants belonging to the stepwise deletions series of the ‘W’ fragment.
Found at: doi:10.1371/journal.pgen.1000332.s002 (2.27 MB EPS)
Table S1 Green fluorescence decrease in individual primary transfectants. (A) GFP2 reporter in the vicinity of theIgLlocus. (B) GFP2 reporter innon-Igloci.
Found at: doi:10.1371/journal.pgen.1000332.s003 (0.11 MB XLS)
Table S2 List of primers.
Found at: doi:10.1371/journal.pgen.1000332.s004 (0.12 MB DOC)
Acknowledgments
The authors would like to thank Claire Brellinger for excellent technical assistance.
Author Contributions
Conceived and designed the experiments: AB VB SS US RBC HA JMB. Performed the experiments: AB VB SS US RBC HA. Analyzed the data: AB VB SS US RBC HA JMB. Contributed reagents/materials/analysis tools: JMB. Wrote the paper: AB VB RBC JMB.
References
1. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, et al. (2000) Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102: 553– 563.
2. Arakawa H, Hauschild J, Buerstedde JM (2002) Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science 295: 1301–1306.
3. Harris RS, Sale JE, Petersen-Mahrt SK, Neuberger MS (2002) AID is essential for immunoglobulin V gene conversion in a cultured B cell line. Curr Biol 12: 435–438.
4. Di Noia J, Neuberger MS (2002) Altering the pathway of immunoglobulin hypermutation by inhibiting uracil-DNA glycosylase. Nature 419: 43–48. 5. Rada C, Di Noia JM, Neuberger MS (2004) Mismatch recognition and uracil
excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol Cell 16: 163–171.
6. Peters A, Storb U (1996) Somatic hypermutation of immunoglobulin genes is linked to transcription initiation. Immunity 4: 57–65.
7. Shinkura R, Tian M, Smith M, Chua K, Fujiwara Y, et al. (2003) The influence of transcriptional orientation on endogenous switch region function. Nat Immunol 4: 435–441.
8. Shen HM, Peters A, Baron B, Zhu X, Storb U (1998) Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science 280: 1750–1752.
9. Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, et al. (2008) Two levels of protection for the B cell genome during somatic hypermutation. Nature 451: 841–845.
10. Odegard VH, Schatz DG (2006) Targeting of somatic hypermutation. Nat Rev Immunol 6: 573–583.
11. Betz AG, Milstein C, Gonza´lez-Ferna´ndez A, Pannell R, Larson T, et al. (1994) Elements regulating somatic hypermutation of an immunoglobulin kappa gene: critical role for the intron enhancer/matrix attachment region. Cell 77: 239–248.
12. Klotz EL, Storb U (1996) Somatic hypermutation of a lambda 2 transgene under the control of the lambda enhancer or the heavy chain intron enhancer. J Immunol 157: 4458–4463.
13. Klix N, Jolly CJ, Davies SL, Bru¨ggemann M, Williams GT, et al. (1998) Multiple sequences from downstream of the Jkcluster can combine to recruit somatic
hypermutation to a heterologous, upstream mutation domain. Eur J Immunol 28: 317–326.
14. Kong Q, Zhao L, Subbaiah S, Maizels N (1998) Al39enhancer drives active and untemplated somatic hypermutation of al1transgene. J Immunol 161: 294–301.
15. van der Stoep N, Gorman JR, Alt FW (1998) Reevaluation of 39Ekfunction in stage- and lineage-specific rearrangement and somatic hypermutation. Immu-nity 8: 743–750.
16. Inlay MA, Gao HH, Odegard VH, Lin T, Schatz DG, et al. (2006) Roles of the Igk light chain intronic and 39 enhancers in Igk somatic hypermutation. J Immunol 177: 1146–1151.
17. Wang CL, Harper RA, Wabl M (2004) Genome-wide somatic hypermutation. Proc Natl Acad Sci U S A 101: 7352–7356.
18. Yoshikawa K, Okazaki IM, Eto T, Kinoshita K, Muramatsu M, et al. (2002) AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science 296: 2033–2036.
19. Arakawa H, Saribasak H, Buerstedde JM (2004) Activation-induced cytidine deaminase initiates immunoglobulin gene conversion and hypermutation by a common intermediate. PLoS Biol 2: e179. doi:10.1371/journal.pbio.0020179. 20. Arakawa H, Kudo H, Batrak V, Caldwell RB, Rieger MA, et al. (2008) Protein
evolution by hypermutation and selection in the B cell line DT40. Nucleic Acids Res 36: e1.
21. Gopal AR, Fugmann SD (2008) AID-mediated diversification within the IgL locus of chicken DT40 cells is restricted to the transcribed IgL gene. Mol Immunol 45: 2062–2068.
22. International Chicken Genome Sequencing Consortium (2004) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432: 695–716.
23. Sale J, Neuberger MS (1998) TdT-accessible breaks are scattered over the immunoglobulin V domain in a constitutively hypermutating B cell line. Immunity 9: 859–869.
24. Zhang W, Bardwell PD, Woo CJ, Poltoratsky V, Scharff MD, et al. (2001) Clonal instability of V region hypermutation in the Ramos Burkitt’s lymphoma cell line. Int Immunol 13: 1175–1184.
25. Bulfone-Paus S, Reiners-Schramm L, Lauster R (1995) The chicken immuno-globulin lambda light chain gene is transcriptionally controlled by a modularly organized enhancer and an octamer-dependent silencer. Nucleic Acids Res 23: 1997–2005.
26. Kothapalli N, Norton DD, Fugmann SD (2008) Cutting edge: a cis-acting DNA element targets AID-mediated sequence diversification to the chicken Ig light chain gene locus. J Immunol 180: 2019–2023.
27. Nagaoka H, Muramatsu M, Yamamura N, Kinoshita K, Honjo T (2002) Activation-induced deaminase (AID)-directed hypermutation in the immuno-globulin Smu region: implication of AID involvement in a common step of class switch recombination and somatic hypermutation. J Exp Med 195: 529–534. 28. Arakawa H, Lodygin D, Buerstedde JM (2001) Mutant loxP vectors for
29. Hamimes S, Arakawa H, Stasiak AZ, Kierzek AM, Hirano S, et al. (2004) RDM1, a novel RNA recognition motif (RRM)-containing protein involved in the cell response to cisplatin in vertebrates. J Biol Chem 280: 9225–9235.
30. Buerstedde JM, Takeda S (1991) Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67: 179– 188.