Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
Regression of cardiac hypertrophy in the SHR by
combined renin-angiotensin system blockade and
dietary sodium restriction
Emad Abro, Cory D Griffiths, Trefor O Morgan, Lea MD Delbridge Keywords:
angiotensin receptor blocker, angiotensin-converting enzyme inhibitor, cardiac
hypertrophy, myocardial fibrosis, cardiomyocyte
Department of Physiology, University of Melbourne, Parkville, Victoria, 3010, Australia Correspondence to: Dr Lea Delbridge Department of Physiology,
University of Melbourne, Parkville,
Victoria, 3010, Australia
Tel: +61 3 8344 5853 Fax: +61 3 8344 5818 E-mail: l.delbridge@ physiology.unimelb. edu.au
JRAAS2001;2 (suppl 1):S148-S153
Abstract
Altered operation of the renin-angiotensin-aldosterone system (RAAS) and dietary sodium intake have been identified as independent risk factors for cardiac hypertrophy. The way in which sodium intake and the operation of the renin-angiotensin-aldosterone system interact in the pathogenesis of cardiac hypertrophy is poorly understood. The aims of this study were to investigate the cardiac effects of the renin-angiotensin system (RAS) blockade in the spontaneously hypertensive rat (SHR), using co-treatment with an angiotensin II receptor blocker (ARB) and an angiotensin-converting enzyme (ACE) inhibitor with different sodium intakes. Our experiments with SHR show that, at high levels of sodium intake (4.0%), aggressive RAS blockade treatment with candesartan (3 mg/kg) and perindopril (6 mg/kg) does not result in regression of cardiac hypertrophy. In contrast, RAS blockade coupled with reduced sodium diet (0.2%) significantly regresses cardiac hypertrophy, impairs animal growth and is associated with elevated plasma renin and dramatically suppressed plasma
angiotensinogen levels. Histological analyses indicate that the differential effect of reduced sodium on heart growth during RAS blockade is not associated with any change in myocardial interstitial collagen, but reflects modification of cellular geometry. Dimensional measurements of enzymatically-isolated ventricular myocytes show that, in the RAS blocked, reduced sodium group, myocyte length and width were decreased by about 16–19% compared with myocytes from the high sodium treatment group. Our findings highlight the importance of ‘titrating’ sodium intake with combined RAS blockade in the clinical setting to optimise therapeutic benefit.
Introduction
The occurrence of cardiac hypertrophy is inde-pendently associated with increased cardiovascu-lar morbidity and mortality.1The development of
cardiac hypertrophy is influenced by a variety of interacting genetic, haemodynamic, neurohumoral trophic and dietary factors.2 Myocardial
remodel-ling during the hypertrophic growth response involves both cellular hypertrophy and interstitial fibrosis. There is an increase in the size of individ-ual cardiomyocytes, the nature of which depends on the specific hypertrophic stimuli, and an increase in the collagen type 1 component of the extracellular matrix.3,4
Altered operation of the
renin-angiotensin-aldosterone system and angiotensin II (Ang II) overproduction is implicated in the occurrence of cardiac hypertrophy. Experimentally and clinically, antihypertensive treatment with an angiotensin-converting enzyme inhibitor (ACE-I) is more effec-tive at regressing hypertrophy than other treat-ments with equivalent blood pressure lowering effects.5Thus, with renin-angiotensin system (RAS)
suppression treatment, the haemodynamic unloading effect is combined with an antitrophic effect on the myocardium. This antitrophic effect may be due to suppression of both systemic and intra-cardiac Ang II production. In humans, an association has been demonstrated between cardiac ACE levels, hypertrophy and the occur-rence of a particular ACE genotype termed the ‘DD’ type.6 Experimentally, in
transgenically-manipulated mice in which there is a specific cardiac upregulation of Ang II production, hyper-trophy is induced without alteration of blood pressure.7Thus, upregulated RAS function and Ang
II production, both systemically and locally, are important determinants of cardiac hypertrophy.
In humans, dietary sodium intake has also been identified as an independent risk factor for left ventricular hypertrophy.8,9 Experimental studies
have similarly demonstrated that elevated sodium intake is associated with the induction of cardiac hypertrophy, with or without an increase in blood pressure.10,11
The way in which sodium intake and the RAS interact in the pathogenesis of cardiac hypertro-phy is poorly understood. In relation to antihyper-trophic treatments, the impact of dietary sodium intake on the efficacy of RAS suppression therapy has not been extensively investigated. This issue is of increased relevance with the development of new combination therapies involving co-treat-ment with an ACE-I and an Ang II receptor blocker (ARB).12,13With a co-treatment regimen, more
pro-nounced suppression, approaching total blockade of the RAS, is achievable than with either agent in monotherapy. Using the spontaneously hyperten-sive rat (SHR), Menard et al.14 demonstrated
exper-imentally that the combination of a competitive inhibitor of the Ang II AT1-receptor with an ACE-I
can produce more effective regression of cardiac hypertrophy than treatment with higher doses of either agent alone. The use of the more recently developed type of ‘insurmountable’ AT1-receptor
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
this combination therapy RAS blockade effect. With the expanding role for combination therapy in the management of cardiovascular disease, there is a requirement to better under-stand how sodium intake influences the treatment outcomes, particularly in relation to the regression of cardiac hypertrophy. Thus, the aims of this study were to investigate the cardiac effects of RAS blockade in the SHR, using co-treatment with an insurmountable AT1-receptor blocker
(can-desartan cilexetil) and an ACE-I (perindopril), under conditions of high (4.0%) and reduced (0.2%) dietary sodium intake. The effect of the combination treatment on the status of the sys-temic RAS in the two sodium loading states was evaluated by measuring plasma renin activity and angiotensinogen levels. The effects of differing sodium levels on the antihypertrophic effects of RAS blockade were evaluated by assessing car-diomyocyte size and extracellular collagen matrix density. The results of these investigations indi-cate that optimisation of combination RAS block-ade therapy requires consideration of dietary sodium intake.
Materials and methods
Animals and treatments
Age-matched SHR (16–20 weeks) were obtained from the Animal Resources Centre (Perth, Australia). Experiments were conducted in com-pliance with the principles outlined in the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and with the approval of the University of Melbourne Animal Experimentation Ethics Sub-Committee. Animals were fed either a high (4.0% w:w) or reduced (0.2% w:w) sodium chloride-containing chow, prepared as previously described,16 for 14 days.
Animals were weighed daily at 0900–1000 hours. On days 7–14, a subset of rats in each dietary group were injected (i.p.) with perindopril (6 mg/kg, Servier, France) and candesartan cilex-etil (3 mg/kg, AstraZeneca, Sweden). Diet-only treated animals received vehicle injections. The completeness of conversion of candesartan cilex-etil to the active metabolite candesartan when administered via this route has not been quanti-fied. However, the blood pressure-lowering effects of i.p. treatment observed in a small number of animals, monitored using telemetry, confirmed the
in vivoeffectiveness of this treatment. At day 14, systolic blood pressure was measured using tail-cuff plethysmography. Hearts were excised under anaesthesia (pentobarbitone sodium, 60 mg/kg, i.p.), weighed and used either for histological analysis or cardiomyocyte isolation.
Plasma renin and angiotensinogen measurements
Immediately after heart excision, up to 5 ml of blood was collected from the thoracic cavity into chilled, heparinised vials. Samples were centrifuged at 800G for 10 minutes and the plasma supernatant was collected and stored frozen.Assays were performed as previously described.17,18
Plasma active renin assay
Plasma active renin levels were approximated using an enzyme kinetic method, with hog renin as the reference standard and 24-hour nephrectomised rat plasma as the substrate angiotensinogen. Plasma active renin was measured by the generation of 125
I-angiotensin I (Ang I), using a radio-immuno assay with Ang I (10–1000 fmol) standards.
Plasma angiotensinogen assay
Plasma angiotensinogen concentrations were measured using excess renin (mouse submaxillary gland) to fully catalyse Ang I production from the angiotensinogen in the samples. Plasma angiotensinogen levels were indirectly measured by the generation of 125I-Ang I using a
radio-immuno assay.
Radio-immuno assay
Radio-immuno assay was the common final step for both renin and angiotensinogen assays. Ang I antibody (100 µl of rabbit IgG) and 125I-Ang I tracer
were added to the incubated samples and allowed to equilibrate by refrigeration for 24 hours at 4°C. Cold charcoal solution was rapidly added to the samples, allowing the separation of antibody-bound fractions from the free tracer.The samples were centrifuged at 2000G for 15 minutes at 4°C. The supernatant was separated and counted against the charcoal pellets on a Gamma counter.
Cardiomyocyte isolation and dimension measurement
Following excision, hearts were immediately mounted onto the cannula of a Langendorff column and retrogradely perfused with bicarbon-ate-buffered Krebs solution at 36oC, to wash blood
from the coronary vessels. Enzymatic dissociation of ventricular myocytes was commenced by addi-tion of collagenase type II (0.45 mg/ml) to the per-fusate, as previously described.19 After
dissocia-tion, myocytes were filtered, washed and resus-pended in Hepes buffered medium containing trypsin inhibitor. An aliquot of left ventricular myocyte suspension was transferred to a chamber mounted on the stage of an inverted light micro-scope and cells were viewed at 400 x magnifica-tion. Fifty viable left ventricular myocytes per heart, which were rod shaped with regular stria-tions, were randomly selected for length and width measurement using a calibrated eye piece graticule. Mean myocyte maximum width and length was calculated for each heart.
Histological analysis
ven-Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2 Supplement 1
tricular wall) and digital images acquired by a SPOT®
camera and software. To exclude perivascular fibrosis from the analysis, all major blood vessels were excluded from the imaging field. Images were analysed semi-quantitatively using ImagePro-Plus (version 3.0). Colour images were threshold-ed, converted to gray scale binary masks and den-sitometrically analysed. The sectional area occu-pied by stained collagen was computed for each image and expressed as a percentage of total cross-sectional area (% CSA). All counts were pooled for each group and the mean % CSA stained for collagen calculated.
Statistical analysis
All results are expressed as mean (± standard error of mean) and were analysed using 1-way or 2-way ANOVA where applicable. For each ANOVA, post-hoc analysis was performed using the Student-Newman Keuls test. A probability value less than 0.05 was designated as significant (p<0.05).
Results
Animal growth and the systemic RAS status
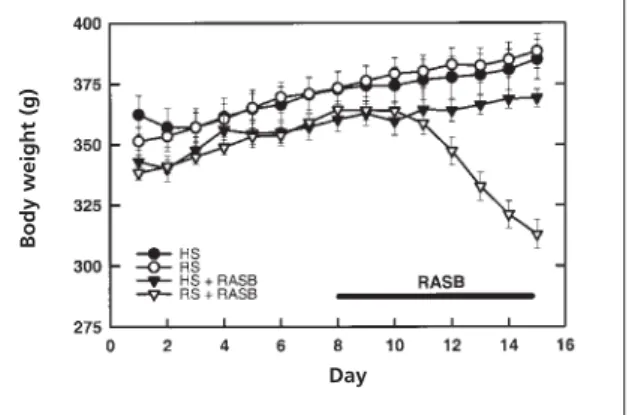
The SHRs fed on high- or reduced-sodium diet gained weight in a comparable and consistent fashion (22.0±5.1 g vs. 34.2±3.1 g net weight gain, Figure 1).The SHRs on a high-sodium intake with the RAS blockade had a similar behavioural pattern and weight gain (26.6±3.2 g net gain) compared with the diet-only control groups. However, although the SHRs fed a reduced-sodium diet with the RAS blockade initially gained body weight in parallel with the other groups, after three to four days of RAS blockade these animals appeared lethargic and exhibited substantial body weight loss (–21.6±6.4 g net loss).The final body weight of the SHRs in the reduced-sodium and RAS blockade group was below the initial mean level, and was significantly different from all other groups. This failure to thrive was associated with a significant decrease in blood pressure in the reduced-sodium group with RAS blockade, com-pared with SHRs on a high-sodium intake with RAS blockade (98.2±4.4 vs. 156.8±3.8 mmHg,
p<0.05, n=5/group, data not shown).
In the SHRs receiving diet-only treatment, the plasma active renin was suppressed in animals on a high-sodium intake (1.35±0.13 pmol Ang I/ml plasma vs. 11.36±2.78 pmol Ang I/ml plasma, Figure 2 ).The combination of high-sodium intake with the RAS blockade resulted in plasma active renin levels significantly higher (22.45±3.74 pmol Ang I/ml plasma) than in both diet-only treatment groups (p<0.05). In the SHRs on a reduced-sodium intake with RAS blockade, plasma active renin was markedly elevated (67.53±4.14 pmol Ang I/ml plasma) compared with all other groups.
Plasma angiotensinogen levels were similar for both the high-sodium and the reduced-sodium diet-only groups (424.20±77.18 pmol Ang I/ml plasma vs. 530.48±36.49 pmol Ang I/ml plasma).A high-sodium intake combined with the RAS block-ade had a blunting effect on plasma angiotensino-gen compared with the diet-only control groups, but the most dramatic effect was seen in the reduced-sodium group with the RAS blockade, where plasma angiotensinogen was profoundly reduced compared with all other groups (9.27±0.28 pmol Ang I/ml plasma).
Combined RAS blockade with
reduced sodium regresses cardiac hypertrophy
In Figure 3, the effects of the various treatments on heart weight normalised for body weight are presented. These data show that the cardiac indices for the diet-only treated animals were
Figure 2 Plasma active renin [upper panel] and angiotensinogen [lower panel] measured in SHRs receiving high (HS) or reduced (RS) sodium diets with RASB and without combined perindopril and candesartan treatment (HS, n=7; RS, n=8; HS+RASB, n=7; RS+RASB, n=8 animals) * p<0.05 vs.all groups, 2-way ANOVA.
Figure 1 Body weights recorded daily for SHRs receiving high (HS) or reduced (RS) sodium diets, with and without combined perindopril and candesartan treatment (designated as RAS blockade, RASB). (HS, n=9; RS, n=9; HS+RASB, n=9; RS+RASB, n=9 animals).
Body weight (g)
Plasma active r
enin
(pmol Ang I / ml plasma)
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
not significantly different (3.88±0.028 mg/g vs. 3.74±0.079 mg/g). In fact, these indices were similar to the index obtained for the high-sodium with RAS blockade group. However, the SHRs on a reduced-sodium intake with RAS blockade had a markedly diminished mean cardiac weight index (3.22±0.039 mg/g) compared with all other groups. In this group, despite the reduction in body weight, which would tend to counteract heart weight-dependent changes in the cardiac weight index, there is clearly a major effect of hypertrophic regression associated with the low-sodium status and RAS blockade.
Myocardial structural changes
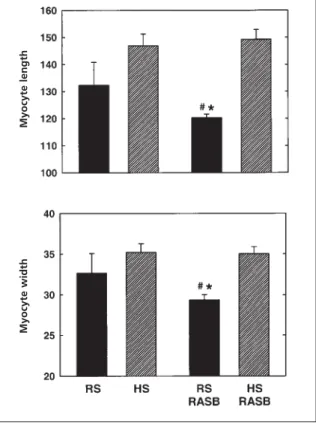
The myocyte dimension studies and the histologi-cal analysis, summarised in Figures 4 and 5 respec-tively, provide some insight into the nature of the structural changes occurring in the myocardium in association with hypertrophic regression. Left ventricular myocytes isolated from the SHRs receiving reduced-sodium diet with RAS blockade were significantly smaller than myocytes from the other treatment groups. In this group, both mean myocyte width and length were significantly reduced, and the data show that these dimension-al changes were prevented by a high-sodium intake. The length-to-width ratios of myocytes were similar in all groups (data not shown), indi-cating that myocyte dimensional changes were proportional in both aspects.
In Figure 5, the results of the histological sis of collagen content is presented. In this analy-sis, the two RAS blockade groups were selected for comparison, as they showed the greatest contrast in myocyte dimensional analysis. At all three myocardial regions examined (left and right ven-tricular walls and septum) there was no evidence of a differential extent of fibrosis. The cross-sec-tional area occupied by collagen ranged from 1.55 to 1.90%, with no significant differences detectable. Combining the values obtained for dif-ferent regions to calculate a value for average total
collagen yields the values of 1.74±0.24% CSA vs. 1.66±0.14% CSA for the high- and reduced-sodium groups respectively. Thus, the effect of dietary sodium reduction in decreasing cardiomyocyte dimensions when administered with RAS blockade was not associated with a differential effect on the collagen deposition in the myocardial interstitium.
Figure 4 Left ventricular cardiomyocyte dimensions of SHRs receiving high (HS) or reduced (RS) sodium diets with RASB and without combined perindopril and candesartan treatment. 50 myocytes/animal (HS, n=4; RS, n=4; HS + RASB, n=4; RS + RASB, n=4 animals). Myocyte length [upper panel] and myocyte width [lower panel]. * p<0.05 vs.HS, # p<0.05 vs.HS + RASB; 1-way ANOVA.
Figure 5 Percentage myocardial cross-sectional area stained for collagen (% CSA, mid-region transverse sections) using Van Gieson’s technique, in SHR receiving high or reduced sodium (Na) diets with combined perindopril and candesartan treatment (RASB).Tissue regions analysed: left ventricle (LV), right ventricle (RV) and septum (Sep). 1-way ANOVA, ns.
Figure 3 Cardiac weight indices (heart weight/body weight) of SHRs receiving high (HS) or reduced (RS) sodium diets with RASB and without combined perindopril and candesartan treatment (HS, n=7; RS, n=8; HS + RASB, n=7; RS+RASB, n=8 animals). * p<0.05 vs.all groups, 2-way ANOVA.
Car
diac weight index (mg/g)
Myocyte length
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2 Supplement 1
Discussion
Cardiac hypertrophy and somatic growth maintenance depend on dietary sodium intake
In this study, we demonstrate that the regressive effects of combination treatment with an insur-mountable ARB and an ACE-I on cardiac hypertro-phy and somatic growth are dependent on the dietary sodium intake. Our experiments with SHRs show that, at high levels of sodium intake (4.0%), aggressive RAS blockade with candesartan (3 mg/kg) and perindopril (6 mg/kg) does not result in regression of cardiac hypertrophy. We also determined that dietary intervention alone, either reduced- (0.2%) or high- (4.0%) sodium intake, did not affect the cardiac weight index over a 14-day intervention period. However, when the combination RAS blockade treatment was coupled with reduced-sodium intake for the final seven days of the diet intervention period, a sig-nificant regression in cardiac hypertrophy was evident, even over this relatively short period. Presumably, this negative growth effect reflects both the direct antitrophic effects of Ang II sup-pression and the indirect antigrowth effects of the hypotensive unloading of the heart.
Previous studies have reported the pro-hyper-trophic effects of elevated dietary sodium in the SHR20and in the control Wistar-Kyoto rat, where an
increase in cardiac weight index was observed with no increase in blood pressure.21 The
antihyper-trophic effect of RAS suppression with a competitive ARB and/or ACE-I has also been reported in the SHR.22In our investigation, we extend these findings
by showing that sodium dietary status is an impor-tant determinant of the efficacy of RAS-targeted pharmacological interventions in the SHR hyper-trophic and hypertensive experimental model.
Animal growth, sodium intake and RAS status
Our observation, that with RAS blockade and reduced-dietary sodium intake animals are lethar-gic and fail to thrive, is consistent with an earlier report by Menard et al.14 In their study, involving
a 14-day treatment regimen with enalapril (10 mg/kg) and losartan (10 mg/kg), Menard et al.
found that the body weight loss and activity deficit was sufficiently severe to merit euthanisa-tion of the animals prior to the conclusion of the treatment period.14 We have found that animals
which experience this degree of deterioration can be ‘rescued’ by modest dietary sodium supple-mentation (up to 0.45%).23Menard et al.14did not
provide details of the sodium content of the chow used in their study, although a ‘standard’ chow would be expected to have a sodium content ranging up to 0.6% (w:w).
The relative shifts in plasma active renin and angiotensinogen levels provide an explanation for the profound effect of reduced-sodium diet together with the RAS blockade treatment on somatic growth and development. The failure to thrive observed in the reduced-sodium RAS block-ade group was associated with the highest active
renin and the lowest angiotensinogen levels in plasma. As would be expected, in response to the RAS blockade combined treatment, there is a large increase in plasma renin. This increase is caused by the interruption of the negative feedback nor-mally in operation where Ang II levels and AT1 -receptor occupancy serve to suppress renin pro-duction.24 The renin increase is blunted in the
high-sodium situation, as elevated sodium exerts a negative feedback on renin production. This blunted renin increase appears to have a ‘sparing’ effect on plasma angiotensinogen. An alternative (but less straightforward) explanation for the depleted angiotensinogen levels could be downregulated pro-duction of this substrate, possibly mediated by nega-tive feedback in response to elevated Ang I levels.On the basis of the present data, it is not possible to dif-ferentiate between these possibilities.
In the reduced dietary sodium animals, it appears from the growth data that within 3–4 days of commencement of RAS blockade treatment, the angiotensinogen supply is depleted and marked hypotension and impairment of somatic growth is observed from this point. The profile of renin and angiotensinogen changes observed in the plasma would be expected to occur in parallel with alter-ations in various tissue ‘local’ RAS. In particular, angiotensinogen depletion in the heart would effectively represent withdrawal of local trophic and inotropic support.25 Central effects of local
RAS downregulation would also impact on blood pressure regulation26and on appetite.27
Cellular basis of regression of cardiac hypertrophy
It has been well established that Ang II is an impor-tant mediator of the fibrotic response evident in the hypertrophic myocardium.28 Thus, it would be
expected that RAS blockade, amplified by the low sodium dietary conditions, would have an antifi-brotic effect on the SHR myocardium. Our data are not consistent with this expectation – indeed there was no differential antifibrotic effect of low-sodium diet when administered with pharmaco-logical RAS blockade. On the basis of our data, it is not possible to assess the extent of interstitial col-lagen reduction induced by RAS blockade per se,
as the diet-only treatment groups were not evalu-ated histologically. Extensive investigations have been reported confirming that RAS blockade reduces myocardial fibrosis.29
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
The finding that combined RAS blockade treat-ment can exert a profound effect on myocardial cell growth under certain dietary sodium condi-tions is an important finding.The high-sodium diet used in this study represents a significant and arti-ficial elevation of sodium used for experimental contrast. However, the level of dietary restriction utilised here is not extreme, and is equivalent to an intermediate level of human sodium intake.30Our
findings highlight the importance of ‘titrating’ sodium intake with combined RAS blockade in the clinical setting to optimise therapeutic benefit. It is not yet clear whether chronic Ang II depletion during long-term combined ACE-I and ARB treat-ment in a relatively normal sodium state causes undesirable myocardial effects at a cellular level.
Acknowledgements
This study was supported by the National Health and Medical Research Council of Australia. Candesartan cilexetil was kindly supplied by AstraZeneca, Sweden. Perindopril was kindly sup-plied by Servier Laboratories, France.
References
1. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study.N Eng J Med1990;322:1561-6.
2. Morgan TO, Griffiths CD, Delbridge LMD. Wall stress, angiotensin II and left ventricular hypertrophy. In: M Epstein and H Brunner Eds. Angiotensin-II Receptor antagonists. Philadelphia: Hanley & Belfus, 2001; Chapter 15:223-34. 3. Hunter J, Chien K. Signalling pathways for cardiac hyper-trophy and failure.N Eng J Med1999;341:1276-83.
4. Farhadian F, Contard F, Corbier A, Barriex A, Rappaport L, Samuel JL. Fibronectin expression during physiological and pathological cardiac growth.Journal of Molecular & Cellular Cardiology1995;27: 981-90.
5. Dahlof B, Pennert K, Hansson L. Reversal of left ventricu-lar hypertrophy in hypertensive patients.A metaanalysis of 109 treatment studies.Am J Hypertens1992;5:95-110.
6. Danser AHJ, Schalekamp ADH, Bax WA,et al. Angiotensin-converting enzyme in the human heart.Circulation 1995; 92:1387-8.
7. Mazzolai L, Pedrazinni T, Nicoud F, Gabbiani G, Brunner H, Nussberger J. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensive mice.
Hypertension2000;35:985-91.
8. Schmeider RE, Messerli FH, Garavaglia GE, Nunez BD. Dietary salt intake: a determinant of cardiac involvement in essential hypertension.Circulation1988;78: 951-6.
9. du Cailar G, Ribstein J, Grolleau R, Mimran A. Influence of sodium intake on left ventricular structure in untreated essen-tial hypertensives.J Hypertens1989;7:S258-9.
10. Wilson RB, Smith DM, Newberne PM. Excess sodium chlo-ride intake in neonatal rats.Arch Pathol1973;96:372-6. 11. McGormick CP, Rauch AL, Buckalew Jr WM. Differential effect of dietary salt on renal growth in Dahl salt-sensitive and salt-resistant rats.Hypertension1989;13:122-7.
12. Azizi M, Guyene TT, Chatellier G, Wargon M, Menard J. Additive effects of losartan and enalapril on blood pressure and plasma active renin.Hypertension1997;29:634-40.
13. Sever P. Intra-individual responses to blockade of the renin-angiotensin system by an ACE inhibitor (lisinopril) and an angiotensin receptor antagonist (candesartan).JRAAS 2000; 1:104.
14. Menard J, Campbell DJ, Azizi M, Gonzales MF. Synergistic effects of ACE inhibition and Ang II antagonism on blood pres-sure, cardiac weight, and renin in spontaneously hypertensive rats.Circulation1997;96:3072-8.
15. Morsing P, Adler G, Brandt-Eliasson U et al.Mechanistic differences of various AT1-receptor blockers in isolated vessels of different origin.Hypertension1999;33:1406-13.
16. Di Nicolantonio R, Silvapulle MJ, Spargo S, Morgan TO. High salt diet decreases longevity in the spontaneously hyper-tensive rat. Clin Exp Pharmacol Physiol1988;15:357-9. 17. Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat.Hypertension1995;25:1014-20.
18. Kelly DJ, Wilkinson-Berka JL, Allen TJ, Cooper ME, Skinner SL. A new model of diabetic nephropathy with progressive renal impairment in the transgenic (mRen-2)27 rat (TGR).
Kidney Int1998;54:343-52.
19. Delbridge LMD, Connell PJ, Harris PJ, Morgan TO. Acute effects of low ethanol concentrations on isolated rat cardiac myocyte contractility. Clin Science2000;4:401-7.
20. Mervaala E, Laakso J, Vapaatalo H, Karppanen H. Effects of enalapril and hydrochlorothiazide on salt-induced cardiac and renal hypertrophy in normotensive rats. Naunyn-Schmiedeberg’s Archiv Pharmacol1994;350:416-25. 21. Yuan B, Leenen FHH. Dietary sodium and left ventricular hypertrophy in normotensive rats. Am J Physiol 1991; 261:H1397-H1401.
22. Mervaala E, Malmberg L, Teravainen T, Laakso J, Vapaatalo H, Karppanen H. Influence of dietary salts on the cardiovascu-lar effects of low-dose combination of ramipril and felodipine in spontaneously hypertensive rats.Br J Pharmacol 1998; 123:195-204.
23. Griffiths CD, Delbridge LMD, Morgan TO. High salt intake reverses the cardiovascular effects of high dose perindopril and losartan treatment in normotensive rats.Proc Aust Physiol Pharmacol Soc1999;30(2):76P.
24. Barret G, Morgan TO, Smith M,Alcorn D,Aldred P. Effect of converting enzyme inhibition on renin synthesis and secretion in mice.Hypertension1989;14:385-95.
25. Delbridge LM, Morgan TO, Harris PJ. Effects of endothelin-1 on the contractility of cardiomyocytes from the spontaneously hypertensive rat.Clin Exp Pharmacol Physiol1995;22:755-62. 26. Song K,Allen AM, Paxinos G, Mendelsohn FA. Mapping of angiotensin II receptor subtype heterogeniety in rat brain.
J Comp Neurol1992;316:467-84.
27. Blair-West JR, Carey KD, Denton DA, Weisinger RS, Shade RE. Evidence that brain angiotensin II is involved in both thirst and sodium appetite in baboons. Am J Physiol 1998; 275:R1639-46.
28. Brown L, Duce B, Miric G, Sernia C. Reversal of cardiac fibrosis in deoxycortisone acetate-salt rats by inhibition of the renin-angiotensin system.J Am Soc Nephrol1999;10:S143-S148. 29. Kagoshima T, Masuda J, Sutani T et al.Angiotensin II recep-tor antagonist, TCV-116, prevents myocardial hypertrophy in spontaneously hypertensive rats.Blood Pressure(suppl) 1994; 5:89-93.