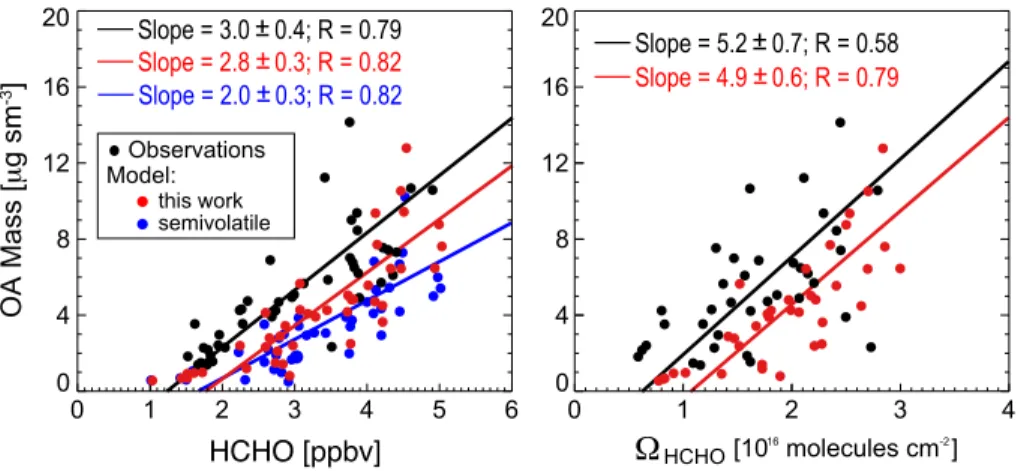
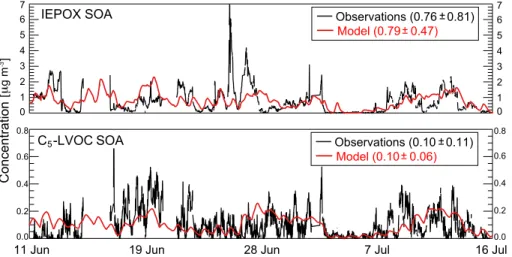
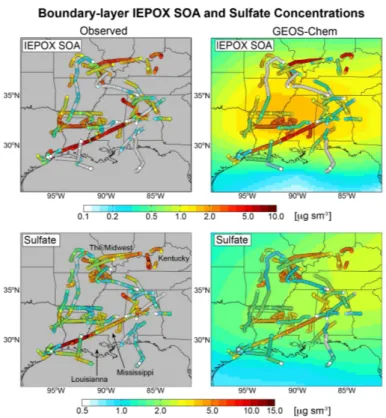
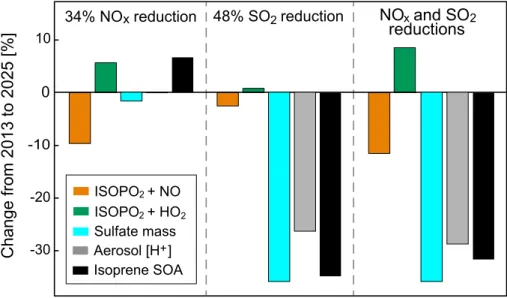
Aqueous-phase mechanism for secondary organic aerosol formation from isoprene: application to the Southeast United States and co-benefit of SO<sub>2</sub> emission controls
Texto
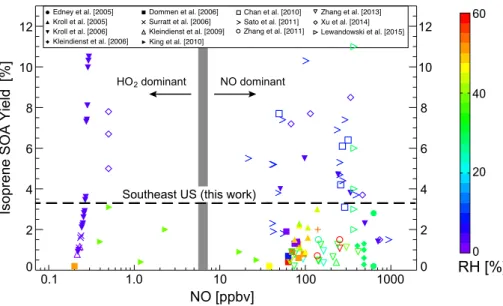
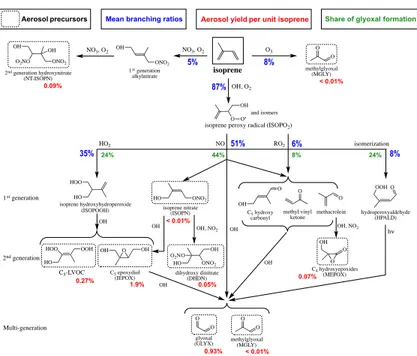
Imagem
![Table 1. Constants for reactive uptake of isoprene SOA precursors a . Species b H ∗ [M atm −1 ] k H + [M −1 s −1 ] k nuc [M −2 s −1 ] k HSO −](https://thumb-eu.123doks.com/thumbv2/123dok_br/18162458.328957/35.918.52.656.190.315/table-constants-reactive-uptake-isoprene-soa-precursors-species.webp)

Documentos relacionados
SOA formation from benzene, toluene, m -xylene, and their corresponding phenolic compounds were investi- gated using the UCR/CE-CERT Environmental Chamber to evaluate the importance
(A) SOA formation potentials of VOC emissions calculated from initial emission ratios of VOCs to CO in emission inventory of each province and from the results of field measure-
(2006) examined the dark forma- tion of the methyl tetrols in aerosol-phase from isoprene or specific photooxidation products in the presence of hydro- gen peroxide (H 2 O 2 )
They were either optical (LIDAR, Differential Op- tical Absorption Spectroscopy (DOAS), UV camera), com- bined with model-based estimates of fuel consumption, or based on the so
In that study their model was found to fit the field data the best with a 4% yield of alkyl nitrates from the reaction of isoprene peroxy radicals with NO.. By contrast, MCM was
For the US summer mean, the simulated ammonia surface concentrations increase by 8.8 % from 2008 to 2012 due to the anthropogenic emissions changes, compared to the 29 % decrease
(2014) – such vapours are shown to be essential for for- mation of secondary organic aerosol (Kulmala et al., 1998; Riipinen et al., 2011); secondly, the suggestion that stabi-
seems unlikely that SCI produced from isoprene ozonolysis alone are important for DMS oxidation, it is possible that (the sum of) SCI species produced from other alkene-