Nuclear Medicine
Diagnostic
Reference Levels
(DRL’s) in a large
oncology hospital
José Miguel Ferreira Gonçalves
Mestrado em Física Médica
Departamento de Física e Astronomia 2019
Orientador
João António Miranda dos Santos, PhD
Assessor de Saúde (Física Médica) no Instituto Português de Oncologia Francisco Gentil, EPE;
Professor Afiliado da Universidade do Porto (ICBAS)
Coorientadora
Vera Catarina Marques Antunes, MSc Física Médica no Instituto Português de Oncologia Francisco Gentil, EPE;
O Presidente do Júri,
Acknowledgements
First and foremost I would like to express my gratitude to Professor Jo ˜ao Santos, who supervised this thesis, sharing his time and expertise while empowering me with oppor-tunities to improve not only this dissertation, but above all, myself.
I would also like to mention one of the major contributors to this study, in the person of my co-supervisor, Vera Antunes, for her knowledge and availability, always ensuring that I had the necessary tools to perform. Thank you!
To all the professionals at the IPO-Porto Nuclear Medicine department, especially to its Director, Dr. Hugo Duarte, I appreciate all the hours spent in teaching and assisting me, along with the input that everyone gave to this thesis.
Without my brother, friends and girlfriend, this dissertation would not be the same. As such, I thank them for their direct and indirect contributions.
Abstract
Diagnostic Reference Levels (DRL’s) have been part of the radiation protection landscape for more than thirty years and, according to Directive 2013/59/Euratom, its development, regular review and use should be ensured by every member state. However, for Nuclear Medicine procedures, only 64% of the European countries have established DRL’s, with Portugal being one of the countries that has yet to develop them.
At the IPO-Porto Nuclear Medicine department, thousands of patients are received every year for radiodiagnostic purposes. Each day, almost thirty PET scans are
per-formed, using 18F-FDG, 68Ga-DOTANOC and 68Ga-PSMA as the selected
radiotrac-ers, according to the procedure. Furthermore, this centre has a new state of the art PET/CT equipment, since in 2017, the old Siemens Biograph 6 HighRez was replaced by a Siemens Biograph mCT Flow, equiped with time-of-flight technology.
For18F-FDG, this upgrade forced an adjustment of the procedures, especially in the way the Administered Activities (AA) were selected. As such, the AA values for the old equipment were compared with the ones acquired with the new PET, in both adult and paediatric patients, proving that the equipment heavily influences the activity necessary to acquire a good diagnostic image. Regarding the Gallium-68 based radiotracers, there were no modifications in the procedures with the arrival of the new PET, and this thesis proves that the generator is the main responsible for the variation of AA. The evolution of each radiotracer values was internally analysed, using various graphical and numerical tools, being then compared with international guidelines and publications.
Dispersion of the AA values was the main problem for every radiotracer. In an attempt to reduce it, some preliminary results were presented in the department, with the goal of comparing the AA recorded in 2019, before and after the intervention. With the excep-tion of paediatric exams, the dispersion decreased after the intervenexcep-tion, but there is no conclusive evidence to credit this result to the effectiveness of the intervention.
An image quality survey was performed, with the assistance of local physicians, in order to quantify a sample of 12018F-FDG images, which would be associated to several parameters in an attempt to discover which ones influenced the quality of a diagnostic im-age. No significant correlation was found, but the results proved that when the dispersion decreased, good images could still be acquired, while reducing under and overexposures. It became clear that the IPO-Porto Nuclear Medicine department operates within the reference values, performing similarly to other centres. This study was one of the first attempts of developing local DRL’s for PET radiotracers in Portugal and, for each one of them, a comprehensive analysis on the AA values throughout the years and across different equipment and circumstances was performed. Nonetheless, DRL’s were not established, as the information gathered was not sufficient to effectively determine them.
Keywords: Diagnostic Reference Levels (DRL’s); Nuclear Medicine; Administered
Resumo
Os N´ıveis de Refer ˆencia de Diagn ´ostico s ˜ao, h ´a mais de trinta anos, uma das ferramen-tas usadas em protec¸ ˜ao radiol ´ogica e, de acordo com a Diretiva 2013/59/Euratom, o seu desenvolvimento, uso e supervisionamento ´e da responsabilidade de cada estado membro. No entanto, em Medicina Nuclear, apenas 64% dos pa´ıses Europeus j ´a estab-eleceram os NRD, com Portugal a ser um dos que ainda n ˜ao os desenvolveu.
No departamento de Medicina Nuclear do IPO-Porto, milhares de pacientes s ˜ao re-cebidos todos os anos para realizar exames de radiodiagn ´ostico. Todos os dias, quase trinta imagens PET s ˜ao adquiridas, utilizando os seguintes radiof ´armacos: 18F-FDG, 68Ga-DOTANOC e68Ga-PSMA. Para al ´em disso, o departamento adquiriu, em 2017, um novo equipamento PET/CT, passando de um Siemens Biograph 6 HighRez para o novo Siemens Biograph mCT Flow, equipado com a tecnologia de tempo de voo.
Com a chegada do novo PET, para exames de18F-FDG, os procedimentos de selec¸ ˜ao das Atividades Administradas (AA) foram alterados. Como tal, os valores das AA uti-lizadas em cada equipamento foram comparadas, provando assim a influ ˆencia dos mes-mos nas atividades necess ´arias para adquirir uma boa imagem de diagn ´ostico. Nos restantes radiof ´armacos, baseados em G ´alio-68, n ˜ao existiram alterac¸ ˜oes no procedi-mento, tendo sido provado neste estudo que o gerador de G ´alio ´e o principal respons ´avel pela variac¸ ˜ao das AA. A evoluc¸ ˜ao dos valores administrados para cada radiof ´armaco foi analisada, recorrendo a diversas ferramentas gr ´aficas e num ´ericas que permitiram a sua comparac¸ ˜ao com as normas e publicac¸ ˜oes internacionais.
A dispers ˜ao das AA foi sempre o maior problema do servic¸o. Com o objetivo de a reduzir, alguns dos resultados preliminares foram apresentados no departamento, permitindo assim a comparac¸ ˜ao dos exames efetuados em 2019, antes e depois da intervenc¸ ˜ao. `A excec¸ ˜ao dos exames pedi ´atricos, a dispers ˜ao diminuiu ap ´os a intervenc¸ ˜ao, apesar de n ˜ao existirem provas contundentes que permitam relacionar os dois eventos.
No ˆambito deste trabalho foi ainda desenvolvido um estudo sobre a qualidade de im-agem, dirigido aos m ´edicos do servic¸o, com o objetivo de quantificar a qualidade de 120 imagens PET, obtidas com FDG, cujo resultado seria associado a diversos par ˆametros para tentar descobrir a influ ˆencia de cada um na qualidade de imagem. N ˜ao foi en-contrada nenhuma correlac¸ ˜ao assinal ´avel, apesar de os resultados terem provado que quando a dispers ˜ao diminui, as imagens mant ˆem uma qualidade acima da m ´edia, evi-tando assim exposic¸ ˜oes `a radiac¸ ˜ao que sejam insuficientes ou excessivas.
O presente estudo prova que o departamento de Medicina Nuclear do IPO-Porto apresenta uma performance semelhante `a de outros centros, operando de acordo com os valores de refer ˆencia. Esta dissertac¸ ˜ao foi uma das primeiras tentativas nacionais para estabelecer NRD em exames PET, e, para cada radiof ´armaco, uma extensa an ´alise das AA foi efetuada. Apesar de tudo, n ˜ao foram estabelecidos NRD no servic¸o.
Palavras-chave: N´ıveis de Refer ˆencia de Diagn ´ostico (NRD); Medicina Nuclear;
List of presentations in scientific meetings
• ”Single centre evaluation of18F-FDG,68Ga-DOTANOC and68Ga-PSMA
admin-istered activities (AA) and comparison with international guidelines” - J.M. Gonc¸alves, V. Antunes, J.A.M. Santos
Accepted as an oral presentation in the 2nd International Conference on Radiations and Applications (ICRA) , 28-30 October, 2019, Algiers - Algeria.
• ”Single centre evaluation of18F-FDG,68Ga-DOTANOC and68Ga-PSMA
admin-istered activities (AA) in a large oncology hospital and comparison with inter-national guidelines” - J.M. Gonc¸alves, V. Antunes, J.A.M. Santos
Accepted as a poster and as a ”Speed talk” in the XXVII Congresso Nacional de Medicina Nuclear, 28-30 November, 2019, Porto - Portugal.
Contents
1 Introduction 1
1.1 Motivation . . . 1
1.2 Nuclear Medicine & Diagnostic Reference Levels’s (DRL’s). . . 2
1.3 Thesis Goals . . . 4
1.4 Thesis Structure . . . 5
2 State of art 6 2.1 Diagnostic Reference Levels (DRL’s) . . . 6
2.2 DRL’s in Nuclear Medicine . . . 9
3 Equipment and parameters 13 3.1 Theoretical framework . . . 13
3.1.1 Radioactivity . . . 13
3.1.2 Radionuclide production . . . 14
3.2 Dose definitions . . . 20
3.3 Positron Emission Tomography . . . 23
3.4 IPO-Porto Nuclear Medicine Department. . . 27
3.4.1 Overview . . . 27
3.4.2 Practical effects on DRL’s . . . 30
4 Methodology 32 4.1 Overview . . . 32
4.2 Data acquisition and statistical analysis . . . 33
4.2.1 Global considerations . . . 33
4.2.2 18F-FDG . . . 38
4.2.3 68Ga-DOTANOC . . . 40
4.2.4 68Ga-PSMA. . . 41
4.3 Intervention . . . 41
4.4 Image quality survey . . . 42
5 Assessment of administered activities 45 5.1 Data acquisition and statistical analysis . . . 45
5.1.1 18F-FDG . . . 45 5.1.1.1 Paediatric. . . 45 5.1.1.2 Adults . . . 50 5.1.2 68Ga-DOTANOC . . . 54 5.1.2.1 Paediatric. . . 54 5.1.2.2 Adults . . . 55 5.1.3 68Ga-PSMA. . . 58 5.2 Intervention . . . 61
5.2.1 18F-FDG . . . 61
5.2.1.1 Paediatric. . . 61
5.2.1.2 Adults . . . 63
5.2.2 68Ga-DOTANOC . . . 65
5.2.3 68Ga-PSMA. . . 67
5.3 Image quality survey . . . 69
6 Discussion of results 71 6.1 Data acquisition and statistical analysis . . . 71
6.1.1 18F-FDG . . . 71 6.1.1.1 Paediatric. . . 71 6.1.1.2 Adults . . . 73 6.1.2 68Ga-DOTANOC . . . 77 6.1.2.1 Paediatric. . . 77 6.1.2.2 Adults . . . 78 6.1.3 68Ga-PSMA. . . 82 6.2 Intervention . . . 85 6.2.1 18F-FDG . . . 85 6.2.1.1 Paediatric. . . 85 6.2.1.2 Adults . . . 87 6.2.2 68Ga-DOTANOC . . . 89 6.2.3 68Ga-PSMA. . . 91
6.3 Image quality survey . . . 93
6.4 Establishment of DRL’s. . . 98 7 Future work 100 Appendix A Questionnaires 101 A.1 18F-FDG. . . 101 A.2 68Ga-DOTANOC . . . 102 A.3 68Ga-PSMA . . . 103
List of Figures
2.1 Diagnostic Reference Levels for Nuclear Medicine [2] . . . 11
3.1 Schematic representation of a nuclear reactor [39] . . . 15
3.2 Schematic representation of a cyclotron: top (left) and side (right) views [39] 16 3.3 Schematic representation of a radionuclide generator [38] . . . 17
3.4 Representation of a periodic elution in a99Mo (orange) /99mTc (blue) gen-erator [39] . . . 18
3.5 Representation of secular equilibrium [39] . . . 18
3.6 Radiation weighting factor (ωR), for neutrons versus neutron energy [21] . . 21
3.7 Schematic representation of a coincidence detector [39] . . . 24
3.8 Differences in LOR probability for equipment with and without TOF tech-nology [39] . . . 25
3.9 Differences between 2D and 3D PET acquisition [48] . . . 26
3.10 IPO-Porto Nuclear Medicine Department. . . 30
4.1 Limiting distributions for high-precision and low-precision measurements. [51] . . . 34
4.2 The ”Error function” [51] . . . 35
4.3 Density values of each interval (black) and the resulting KDE function (red) [52] . . . 36
4.4 KDE function example . . . 37
4.5 Activity per weight plot example . . . 37
4.6 EANM Dosage Card 2016 for18F-FDG - Activity per Weight . . . 39
4.7 EANM Dosage Card 2016 for18F-FDG - MBq/kg per Weight. . . 39
4.8 EANM Dosage Card 2016 for68Ga-DOTANOC - Activity per Weight . . . . 40
4.9 EANM Dosage Card 2016 for68Ga-DOTANOC - MBq/kg per Weight . . . . 40
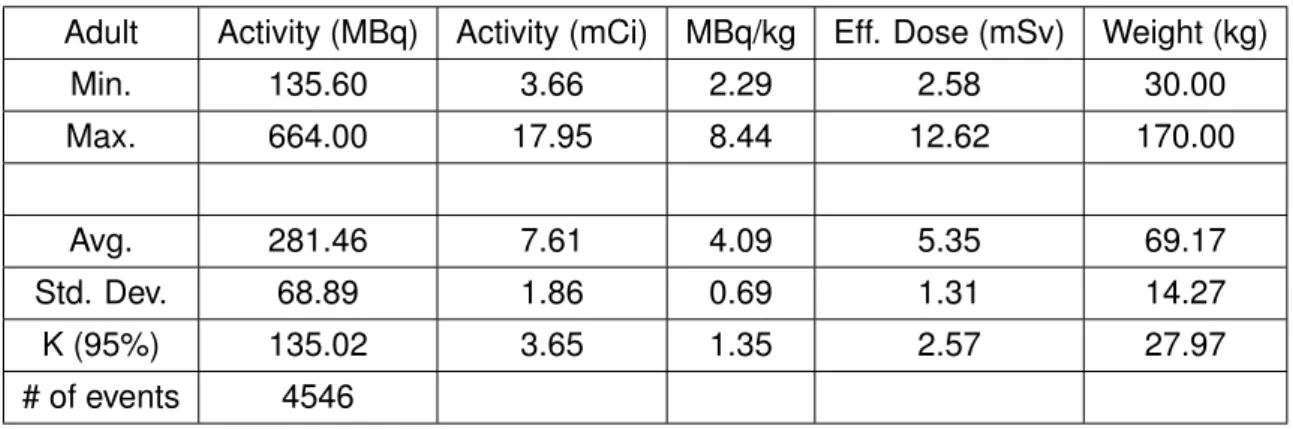
5.1 Administered activity of 18F-FDG per Weight in paediatric patients (2005-2017) . . . 46
5.2 MBq/kg of18F-FDG per Weight in paediatric patients (2005-2017) . . . 46
5.3 Effective dose of18F-FDG per Weight in paediatric patients (2005-2017) . 46 5.4 AA of18F-FDG per Weight in paediatric patients (2016) . . . 47
5.5 MBq/kg of18F-FDG per Weight in paediatric patients (2016) . . . 47
5.6 Effective dose of18F-FDG per Weight in paediatric patients (2016) . . . 48
5.7 AA of18F-FDG per Weight in paediatric patients (2018) . . . 49
5.8 MBq/kg of18F-FDG per Weight in paediatric patients (2018) . . . 49
5.9 Effective dose of18F-FDG per Weight in paediatric patients (2018) . . . 49
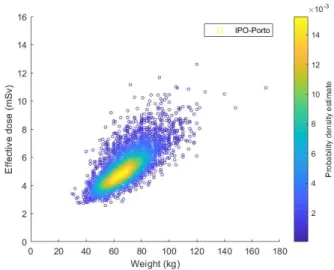
5.10 AA of18F-FDG per Weight in adult patients (2005-2017) . . . 50
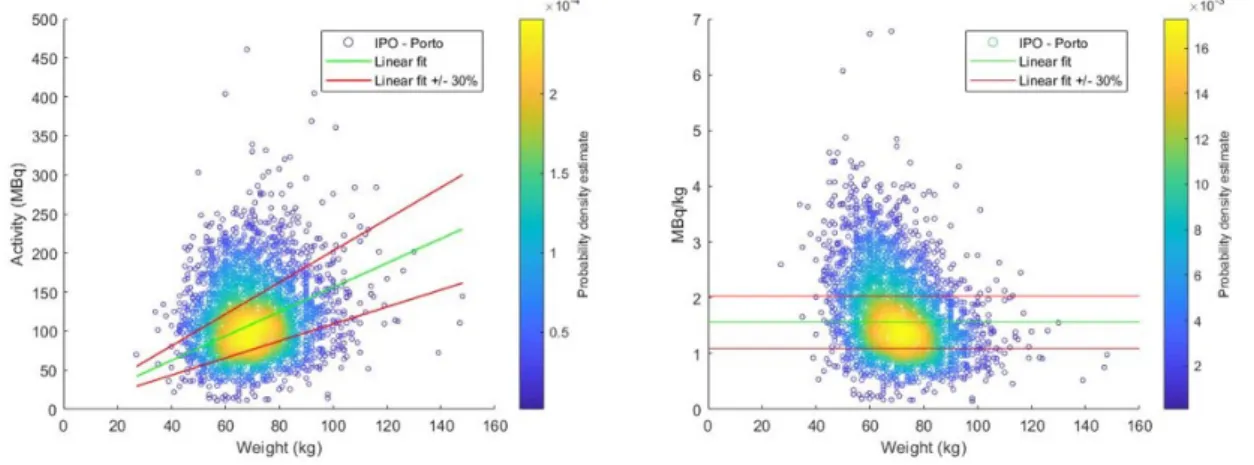
5.11 MBq/kg of18F-FDG per Weight in adult patients (2005-2017) . . . 50
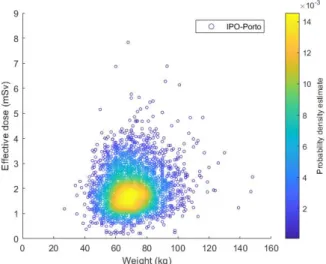
5.12 Effective dose of18F-FDG per Weight in adult patients (2005-2017) . . . . 51
5.13 AA of18F-FDG per Weight in adult patients (2016) . . . 52
5.15 AA of18F-FDG per Weight in adult patients (2018) . . . 53
5.16 MBq/kg of18F-FDG per Weight in adult patients (2018) . . . 53
5.17 Effective dose of18F-FDG per Weight in paediatric patients (2018) . . . 53
5.18 AA of68Ga-DOTANOC per Weight in paediatric patients (2010-2018) . . . . 54
5.19 MBq/kg of68Ga-DOTANOC per Weight in paediatric patients (2010-2018) . 54 5.20 AA of68Ga-DOTANOC per Weight in adult patients (2010-2018) . . . 55
5.21 MBq/kg of68Ga-DOTANOC per Weight in adult patients (2010-2018) . . . . 55
5.22 Effective dose of68Ga-DOTANOC per Weight in adult patients (2010-2018) 56 5.23 AA of68Ga-DOTANOC per Weight in adult patients (Generator A). . . 57
5.24 MBq/kg of68Ga-DOTANOC per Weight in adult patients (Generator A) . . . 57
5.25 AA of68Ga-DOTANOC per Weight in adult patients (Generator B). . . 58
5.26 MBq/kg of68Ga-DOTANOC per Weight in adult patients (Generator B) . . . 58
5.27 AA of68Ga-PSMA per Weight in adult patients (2015-2018) . . . 59
5.28 MBq/kg of68Ga-PSMA per Weight in adult patients (2015-2018) . . . 59
5.29 Effective dose of68Ga-PSMA per Weight in adult patients (2015-2018) . . . 59
5.30 AA of68Ga-PSMA per Weight in adult patients (Generator A) . . . 60
5.31 MBq/kg of68Ga-PSMA per Weight in adult patients (Generator A) . . . 60
5.32 AA of68Ga-PSMA per Weight in adult patients (Generator B) . . . 61
5.33 MBq/kg of68Ga-PSMA per Weight in adult patients (Generator B) . . . 61
5.34 AA of18F-FDG per Weight in paediatric patients before the intervention . . 62
5.35 MBq/kg of18F-FDG per Weight in paediatric patients before the intervention 62 5.36 AA of18F-FDG per Weight in paediatric patients after the intervention . . . 63
5.37 MBq/kg of18F-FDG per Weight in paediatric patients after the intervention . 63 5.38 AA of18F-FDG per Weight in adult patients before the intervention . . . 64
5.39 MBq/kg of18F-FDG per Weight in adult patients before the intervention . . 64
5.40 AA of18F-FDG per Weight in adult patients after the intervention . . . 65
5.41 MBq/kg of18F-FDG per Weight in adult patients after the intervention . . . 65
5.42 AA of68Ga-DOTANOC per Weight in adult patients before the intervention . 66 5.43 MBq/kg of 68Ga-DOTANOC per Weight in adult patients before the inter-vention. . . 66
5.44 AA of68Ga-DOTANOC per Weight in adult patients after the intervention . . 67
5.45 MBq/kg of68Ga-DOTANOC per Weight in adult patients after the intervention 67 5.46 AA of68Ga-PSMA per Weight in adult patients before the intervention . . . 68
5.47 MBq/kg of68Ga-PSMA per Weight in adult patients before the intervention. 68 5.48 AA of68Ga-PSMA per Weight in adult patients after the intervention . . . . 69
5.49 MBq/kg of68Ga-PSMA per Weight in adult patients after the intervention. . 69
5.50 AA of18F-FDG per Weight in adult patients (Questionnaires) . . . 70
5.51 MBq/kg of18F-FDG per Weight in adult patients (Questionnaires) . . . 70
6.1 Comparison of the number of paediatric18F-FDG exams per MBq/kg . . . . 72
6.2 Probability density function of paediatric18F-FDG exams per MBq/kg. . . . 72
6.4 Evolution of MBq/kg in18F-FDG paediatric exams . . . 72
6.5 Comparison of the mean AA of18F-FDG for a patient with (30 ± 5) kg, in different regions [57] . . . 73
6.6 Comparison of the number of adult18F-FDG exams per MBq/kg . . . 74
6.7 Probability density function of adult18F-FDG exams per MBq/kg . . . 74
6.8 Evolution of AA in18F-FDG adult exams . . . 75
6.9 Evolution of MBq/kg in18F-FDG adult exams . . . 75
6.10 Comparison of the mean AA of18F-FDG adult exams[58][59][60][28][61][62]. 76 6.11 Mean DRL (75th percentile) value per region [2][59][28][62][24][63][27] . . . 77
6.12 Evolution of AA in paediatric68Ga-DOTANOC exams. . . 78
6.13 Evolution of MBq/kg in paediatric68Ga-DOTANOC exams . . . 78
6.14 AA of68Ga-DOTANOC per date of exams in adult patients (Generator A) . 79 6.15 AA of68Ga-DOTANOC per date of exams in adult patients (Generator B . . 79
6.16 MBq/kg of68Ga-DOTANOC per date of exams in adult patients (Generator A) . . . 79
6.17 MBq/kg of68Ga-DOTANOC per date of exams in adult patients (Generator B) . . . 79
6.18 Comparison of the number of adult68Ga-DOTANOC exams per MBq/kg . . 80
6.19 Probability density function of adult68Ga-DOTANOC exams per MBq/kg . . 80
6.20 Evolution of AA in68Ga-DOTANOC adult exams . . . 81
6.21 Evolution of MBq/kg in68Ga-DOTANOC adult exams . . . 81
6.22 Comparison of the mean AA values in Ga-68 DOTANOC adult exams [64] [65] [66] . . . 82
6.23 AA of68Ga-PSMA per date of exams in adult patients (Generator A) . . . . 83
6.24 AA of68Ga-PSMA per date of exams in adult patients (Generator B) . . . . 83
6.25 MBq/kg of68Ga-PSMA per date of exams in adult patients (Generator A) . 83 6.26 MBq/kg of68Ga-PSMA per date of exams in adult patients (Generator B) . 83 6.27 Comparison of the number of adult68Ga-PSMA exams per MBq/kg. . . 84
6.28 Probability density function of adult68Ga-PSMA exams per MBq/kg . . . . 84
6.29 Evolution of AA in68Ga-PSMA adult exams . . . 85
6.30 Evolution of MBq/kg in68Ga-PSMA adult exams . . . 85
6.31 Comparison of the number of paediatric 18FFDG exams per MBq/kg -Intervention . . . 86
6.32 Probability density function of paediatric18F-FDG exams per MBq/kg - In-tervention . . . 86
6.33 Probability density function of paediatric 18FFDG exams per MBq/kg -Global comparison . . . 86
6.34 Comparison of the number of adult18F-FDG exams per MBq/kg - Intervention 87 6.35 Probability density function of adult18F-FDG exams per MBq/kg - Interven-tion. . . 87
6.36 Probability density function of adult18F-FDG exams per MBq/kg - Global comparison . . . 88
6.37 Comparison of the number of adult68GaDOTANOC exams per MBq/kg -Intervention . . . 89
6.38 Probability density function of adult68GaDOTANOC exams per MBq/kg -Intervention . . . 89
6.39 Probability density function of adult68GaDOTANOC exams per MBq/kg
-Global comparison . . . 90
6.40 Comparison of the number of adult68Ga-PSMA exams per MBq/kg - Inter-vention. . . 91
6.41 Probability density function of adult68Ga-PSMA exams per MBq/kg - Inter-vention. . . 91
6.42 Probability density function of adult68Ga-PSMA exams per MBq/kg - Global comparison . . . 92
6.43 Questionnaire results . . . 94
6.44 Specific comparisons between 18F-FDG images and the corresponding Overall Scores . . . 96
List of Tables
3.1 ICRP Publication 103 recommended radiation weighting factors [21] . . . . 20
3.2 ICRP Publication 103 recommended tissue weighting factors [21] . . . 22
3.3 Effective doses per age for18F-FDG (F = Female; M = Male) . . . 23
4.1 Interpretation on the values of the Pearson Correlation Coefficient [54] . . . 38
4.2 Weighting factors, ωV, of each questionnaire question . . . 42
5.1 Paediatric exams using18F-FDG (2005-2017). . . 45
5.2 Paediatric exams using18F-FDG (2016) . . . 47
5.3 Paediatric exams using18F-FDG (2018) . . . 48
5.4 Adult exams using18F-FDG (2005-2017) . . . 50
5.5 Adult exams using18F-FDG (2016) . . . 51
5.6 Adult exams using18F-FDG (2018) . . . 52
5.7 Paediatric exams using68Ga-DOTANOC (2010-2018) . . . 54
5.8 Adult exams using68Ga-DOTANOC (2010-2018) . . . 55
5.9 Adult exams using68Ga-DOTANOC (Generator A) . . . 56
5.10 Adult exams using68Ga-DOTANOC (Generator B) . . . 57
5.11 Adult exams using68Ga-PSMA (2015-2018). . . 58
5.12 Adult exams using68Ga-PSMA (Generator A) . . . 60
5.13 Adult exams using68Ga-PSMA (Generator B) . . . 61
5.14 Paediatric exams using18F-FDG before the intervention . . . 62
5.15 Paediatric exams using18F-FDG after the intervention . . . 63
5.16 Adult exams using18F-FDG before the intervention. . . 64
5.17 Adult exams using18F-FDG after the intervention . . . 65
5.18 Adult exams using68Ga-DOTANOC before the intervention . . . 66
5.19 Adult exams using68Ga-DOTANOC after the intervention . . . 67
5.20 Adult exams using68Ga-PSMA before the intervention . . . 68
5.21 Adult exams using68Ga-PSMA after the intervention . . . 69
5.22 Results of the18F-FDG questionnaires for adult patients . . . 70
6.1 Correlation coefficients of paediatric exams using18F-FDG . . . 71
6.2 Correlation coefficients of adult exams using18F-FDG . . . 73
6.3 Percentage of exams outside the ± 30% margin in adult exams using18 F-FDG . . . 75
6.4 Correlation coefficients of adult exams using68Ga-DOTANOC . . . 78
6.5 Percentage of exams outside the ± 30% margin in adult exams using68 Ga-DOTANOC . . . 81
6.6 Correlation coefficients of adult exams using68Ga-PSMA . . . 82
6.7 Percentage of exams outside the ± 30% margin in adult exams using68 Ga-PSMA . . . 84
6.8 Correlation coefficients of paediatric exams using18F-FDG - Intervention . 85 6.9 Correlation coefficients of adult exams using18F-FDG - Intervention . . . . 87
6.10 Percentage of exams outside the ± 30% margin in adult exams using18
F-FDG - Intervention . . . 88
6.11 Correlation coefficients of adult exams using68Ga-DOTANOC - Intervention 89 6.12 Percentage of exams outside the ± 30% margin in adult exams using68 Ga-DOTANOC - Intervention. . . 90
6.13 Correlation coefficients of adult exams using68Ga-PSMA - Intervention . . 91
6.14 Percentage of exams outside the ± 30% margin in adult exams using68 Ga-PSMA - Intervention . . . 92
6.15 Summary of paediatric exams using18F-FDG . . . 99
6.16 Summary of adult exams using18F-FDG. . . 99
6.17 Summary of adult exams using68Ga-DOTANOC . . . 99
List of Abbreviations and Acronyms
131I Iodine-131 32P Phosphorous-32 89Sr Strontium-89 99mTc Technetium-99m 90Y Yttrium-90 A Activity AA Administered Activities AEC Automatic exposure controlALARA As low as reasonably achievable Bq Becquerel
BMI Body Mass Index BSA Body Surface Area Ci Curie
CT Computed Tomography dps disintegrations per second DRL’s Diagnostic Reference Levels
EANM European Association of Nuclear Medicine Euratom European Atomic Energy Community eV Electron-volt
FDG Fluorodeoxyglucose Gy Gray
ICRP International Commission for Radiation Protection
ICRU International Commission on Radiation Units and Measurements IPO-Porto Instituto Portugu ˆes de Oncologia do Porto
MED Medical Exposure Directive MRI Magnetic Ressonance Imaging
Nal(TI) Thallium-activated Sodium Iodide NM Nuclear Medicine
PDF Probability Density Function PET Positron Emission Tomography
PSMA Prostate-specific Membrane Antigen ROI Region-of-interest
SI International System of Units
SPECT Single Photon Emission Computed Tomography UK United Kingdom
UNSCEAR United Nations Scientific Committee on the Effects of Atomic Radiation U.S. United States
1
Introduction
1.1
Motivation
All around the world, ionizing radiation is used in medical procedures, either in diagnosis or treatment itself, exposing patients to radiation in order to achieve its purpose. Since ionizing radiation can induce harmful effects on human tissues and cells, it is mandatory that limits and recommendations on how to do it as safely as possible are established, which correlates to attain the desired result while causing the least amount of damage possible. One of the ways to achieve that is by creating Diagnostic Reference Levels (DRL’s), adjusted to the reality of each country, centre, or even equipment. To enforce that, Directive 2013/59/Euratom declares: ”Member States shall ensure the establish-ment, regular review and use of diagnostic reference levels for radiodiagnostic examina-tions, having regard to the recommended European diagnostic reference” [1], reinforcing the importance of developing and reviewing DRL’s for all exams. With a vast majority of centres using radiodiagnostic imaging techniques everyday, the necessity of implement-ing DRL’s increases, in order to monitor and establish reference points for administered activities. However, in a survey performed by the European Commission [2], it was found that, for Nuclear Medicine (NM) alone, only 64% of European countries have established DRL’s, with Portugal not being one of them.
Regarding Nuclear Medicine centres in Portugal, IPO-Porto has one of the busiest de-partments in the country, where a multidisciplinary team works both in diagnostic imaging and therapy. Every month, hundreds of patients perform diagnostic exams there, resulting in a huge amount of acquired images and data per year. Despite this fact, the aforemen-tioned DRL’s are yet to be implemented in this centre.
Combining the necessity of establishing reference levels for the Nuclear Medicine department at IPO-Porto with the sheer amount of data to analyse, there is a great op-portunity to implement DRL’s adjusted to the centre reality and equipment, improving both the safety and quality of procedures. Furthermore, the recorded data of PET scans goes back to 2004, with two different equipment being used during that period, which, upon review, can provide useful information regarding the centre evolution, tendencies and differences in administered activities and its correlation to the change of equipment.
In short, a profound analysis to the centre history and its evolution, combined with the necessity of acquiring diagnostic images while giving hundreds of patients per month the least amount of dose possible, can be enough to seize the opportunity of establishing local DRL’s, fulfilling the directive and improving the service quality of one of the biggest Nuclear Medicine departments in the country.
1.2
Nuclear Medicine & Diagnostic Reference Levels’s (DRL’s)
As with most of today’s research and clinical fields, Nuclear Medicine does not fall under one specific area of knowledge. Instead, it combines discoveries in natural sciences, such as Physics, Chemistry and Biology, with the evolution of technology and engineering, to develop medical applications in both imaging and therapy. Nuclear Medicine specializes in using unsealed sources of radiation to obtain images capable of providing diagnos-tic information on a wide array of diseases and their stage, as well as treating some of them. Between 1997 and 2007, it is estimated that more than 32 million diagnostic Nu-clear Medicine examinations were conducted every year, reaching 0.5 % of the world population [3].
In this field, radiation comes from radiotracers (also referred as radiopharmaceuti-cals), which are molecules or compounds labelled with small amounts of radioactivity (coming from radionuclides or radioisotopes), allowing the compound to be traceable and detected by imaging systems. While other modalities of diagnosis, such as Computed To-mography (CT) or Magnetic Resonance Imaging (MRI), can retrieve anatomic structures with a higher detail, they cannot provide much information on biological mechanisms. On the other hand, due to the nature of radiotracers, Nuclear Medicine examinations can retrieve sensitive data on the physiological processes, since after being adminis-tered, the radiotracer disseminates through the body, allowing the imaging techniques to record its emissions at different moments. Technetium-99m (99mTc) is the most used radionuclide, but there are many others being administered for different examinations and treatments, such as the Fluorine and Gallium based radiotracers (e.g.18F-FDG and
68Ga-DOTANOC).
Two of the ways a radionuclide can decay are by emitting gamma-rays or through the emission of positrons, which, in Nuclear Medicine, results in two distinct forms of imaging. Single photon imaging takes advantage of radionuclides that decay by gamma-ray emission, and, by collecting the data of radiotracer distributions in the target’s body from one angle, a planar image can be obtained. Most of the times this is achieved using a Gamma Camera, which has a gamma-ray detecting system, (e.g. Sodium Iodide (Nal(TI)) scintillation detector), paired with a collimator and electronic devices that register, amplify and process the signals, allowing the computer to create an image. Other modality of this type of imaging is the Single Photon Emission Computed Tomography (SPECT), in which the photons are detected from different angles around the source, combining them to reconstruct the radiotracer distribution in the patients anatomy, giving another layer of information to the resulting image. Finally, Positron imaging techniques are based on radionuclides that decay by emitting positrons, which have a very short lifetime, since they annihilate when colliding with electrons, negating its detection. Instead, the camera collects data of two high-energy photons originated by the mentioned collision, that go in opposite directions, reaching the detector at almost the same time and allowing a precise localization of the annihilation. Images are formed from the detection of photons
in various angles and are called Positron Emission Tomography (PET) images, which can be combined with CT scans to give a better spatial resolution, forming PET-CT images. The potential of both techniques arise from the fact that the energy of these photons is high enough to allow most of them to escape from the human body without being too affected by scattering or attenuation events. Therefore, radionuclides are essential for diagnostic imaging, providing the basis for several types of examinations.
Nonetheless, that is not the only way they can be used since Nuclear Medicine is also useful for treatment purposes. As such, therapy in Nuclear Medicine takes advantage of the knowledge about physical, chemical and biological properties of radiotracers, using them to target specific areas of the body, while sparing the surrounding tissues. The amount and type of radiopharmaceuticals employed in each treatment varies, with its ad-ministration being mostly by injection or ingestion. Iodine therapy is a common treatment and uses Iodine-131 (131I) to target the thyroid, either to treat benign conditions (e.g. hyperthyroidism) or malign diseases (e.g. thyroid cancer). Other unsealed sources can also be used for therapy, such as Phosphorous-32 (32P) for cystic craniopharyngioma, Yttrium-90 (90Y) to treat hepatic metastasis and even for palliative purposes, like in the case of Strontium-89 (89Sr) for painful bone metastasis [4], which is a form of palliative care. Due to the high administered activities, patients cannot leave the clinical facilities until the activity decreases to a certain level, with the rooms where they stay requiring special conditions, such as a specific sewage system that goes into a decay tank. This increases the treatment time per patient and is one of the main drawbacks of therapy in Nuclear Medicine.
The aforementioned precautions regarding the activity of radiotracers are examples of radiation protection measures that aim to protect the population, being it clinical workers, patients or public in general, against high and unnecessary exposure to ionizing radia-tion. According to the International Commission for Radiation Protection (ICRP), efficient radiation protection is based on three key principles: justification, optimization and limi-tation. The first principle aims to ensure that every time someone is exposed to ionizing radiation, the benefits of such exposure should outweigh the detriment it causes, justify-ing the procedure. Optimization has the goal of keepjustify-ing the dose “as low as reasonably achievable, economic and social factors being taken into account” [5], which means that when someone has to be exposed to ionizing radiation, the given dose should be the minimum required to provide the desired result (e.g. obtaining a diagnostic image). This is also known as the ALARA (as low as reasonably achievable) principle and is one of the key aspects of radiation protection. Lastly, limitation establishes dose limits to individuals from planned exposures. However, those limits can be surpassed in medical exposure, as long as there is reasonable justification.
Expanding on the optimization principle, the ICRP 73 [6] introduces the term Diagnos-tic Reference Levels (DRL’s). DRL’s purpose is advisory, aiming to avoid unnecessarily high doses to the patients by setting dose levels (Radiology) or activity levels (Nuclear Medicine) in typical procedures for standard-sized groups of patients or standard
phan-toms. They are derived from relevant national, regional or local data, and defined by a numerical value that can be compared with one observed value for a reference group of patients (e.g. mean value) for a typical exam. The reference group only includes patients within a range of parameters, such as height, weight or age. Typically, if good practices are applied, these levels are not exceeded, but they are not a dose constraint or limit, nor do they define good or bad medicine and can be surpassed if there is reasonable justification. If DRL’s are constantly exceeded they should be reviewed by the competent body, who has the responsibility of taking corrective measures.
These reference levels can be applied on both Radiological and Nuclear Medicine procedures, but their definitions vary. In Diagnostic Radiology, DRL’s are based on values of patient doses, while for Nuclear Medicine, they are expressed in administered activities (MBq), either absolute or weight adjusted. According to the European Commission, the reference administered activity is based ”on the administered activity necessary for a good image during a standard procedure” [7]. However, qualifying a ”good image” is dependent on the evaluator, introducing a subjectivity factor as different physicians can have different opinions. Besides that, DRL’s are also affected by the equipment (e.g a poor Gamma Camera needs a higher administered activity or increased exposure time to produce a better image) and the quality of the dose calibration. As such, Nuclear Medicine DRL’s should not be surpassed, but are expected to be closely approached, resulting in an optimum value and not on a limit based one. For paediatric patients, DRL’s should also be enforced, but the administered activity will be a fraction of the one given to an adult, adjusted to the age and weight of the children. When setting and revising DRL’s, all the mentioned parameters should be closely inspected and reviewed, ensuring they are up to date with both the equipment and requirements of the responsible entities. Furthermore, the importance of DRL’s in Nuclear Medicine should not be understated and its implementation needs to be enforced.
1.3
Thesis Goals
The present dissertation has the following goals:
• Analyse the Administered Activities (AA) of all PET procedures;
• Devise graphical and numerical methods to compare the results, adjusted to the different radiotracers;
• Investigate the differences in AA between the two PET scans used by the centre; • Conduct an experiment to assess the importance of DRL’s in radiation protection; • Perform a survey on image quality and the parameters associated to it;
1.4
Thesis Structure
This dissertation is divided into seven chapters, including this one where the theme, mo-tivation, goals and structure are laid out. On the second one, the history and concepts of DRL’s are presented, with a special focus on its applications in Nuclear Medicine, both nationally and internationally. Following it, the theoretical foundations are reviewed in chapter 3, explaining radiotracer production, dose definitions, PET equipment and show-casing the department on which this thesis was performed. In the fourth chapter, methods and approaches are discussed, explaining how the data will be treated and the results will be acquired, dividing the work into three phases: Data acquisition and statistical analysis, Intervention and Image quality survey. The fifth chapter presents the attained results for each phase, that will serve as the basis for the subsequent analysis that is performed in chapter 6, where they are discussed and compared, enabling some conclusions and cul-minating in a DRL’s proposal for all PET exams performed in this centre. Finally, the last chapter discusses the future work that could and should follow this study, summarising the importance of DRL’s and its development.
2
State of art
2.1
Diagnostic Reference Levels (DRL’s)
More than a century ago, Wilhelm Conrad R ¨ontgen discovery of x-rays revolutionized the landscape of science and medicine, paving the way for a world of possible applications that to this day are a cornerstone on both medical diagnosis and therapeutics. When it was publicly announced in 1895, presenting a hand radiograph of R ¨ontgen’s wife, the discovery sparked a huge interest on this form of energy, resulting in numerous medical applications, which, soon enough, induced x-ray related injuries. The first symptoms were eye irritation, dermatitis, blistering and other skin related problems, as well as pain and other severe effects [8], alarming the community and generating some safety recommen-dations, mainly the ones proposed in 1896 by Wolfram Fuchs, of minimizing the time of exposure, rubbing the skin with a layer of Vaseline and be at least 30 cm away from the x-ray tube, marking the beginning of radiation protection [9]. In the following year, with Henri Becquerel discovering Radioactivity, the ability of some materials to naturally or artificially emit ionizing radiation, and two years later with the discovery of Radium by Pierre and Marie Curie, more radiation related symptoms (e.g. abdominal erythema [8]) surfaced, product of both scientists constantly being in contact with radioactive materials. Despite those unfortunate events, the huge potential of ionizing radiation to treat diseases like cancer continued to fuel the use of radioactive materials and x-rays in medicine, inducing the advance of radiation protection.
Through the next decades, protection against deterministic effects was a primary con-cern, with organizations being created to address it. In London (1925), the International Commission on Radiation Units and Measurements (ICRU) was created with the goal of defining concepts (such as dose), units and procedures, as well as having a protec-tion system based on duraprotec-tion of exposure, barriers and distance to the source, which were the basic principles behind the first recommendations made almost 30 years be-fore. Three years later, the International Commission on Radiological Protection (ICRP) was founded in Stockholm, aiming to provide guidance and recommendations on protec-tion against ionizing radiaprotec-tion, doing so until this day in the form of publicaprotec-tions on various topics related to the subject.
However, despite the concern for deterministic effects, there was not much control on the doses patients received over the course of a treatment and its consequences. Nowadays it is well known that ionizing radiation can cause stochastic effects such as cancer and genetic mutations, but it was not until after the second World War that ICRP Publications started to include the induction of malignant tumours and genetic effects as part of the health consequences provoked by ionizing radiation that should be kept under review [9]. In 1955, the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) was established by the United Nations, aiming to assess risks and effects of ionising radiation on human health and report levels of exposure to
ion-izing radiation. Their first publications focused on biological effects caused by ionion-izing radiation, including somatic and hereditary effects [10]. At the same time, national sur-veys on quantities related to patient dose were taken in the U.S. and UK [11], aiming to review and optimize radiation protection, with following studies in the 70’s and 80’s, such as the U.S Nationwide Evaluation of X-Ray Trends (NEXT) and surveys performed by the National Radiation Protection Board in the UK [12], laying the foundations to the first recommendations on quantities and levels of the surveyed parameters, resulting in dose limits, procedures and instructions.
Based on the knowledge gathered until that point by multiple studies and surveys, ICRP issued Publication 26 in 1977 [13], introducing three principles that became a cor-nerstone of radiation protection:
‘(a) no practice shall be adopted unless its introduction produces a positive net benefit; (b) all exposures shall be kept as low as reasonably achievable, economic and social factors being taken into account;
(c) the doses to individuals shall not exceed the limits recommended for the appropriate circumstances by the Commission’.
Those principles evolved into three keywords: Justification, Optimization and Limita-tion, respectively. The first one, justificaLimita-tion, specifies that every time a planned exposure occurs, the positive results have to outweigh the negative consequences, ”justifying” the procedure. Optimization aims to ensure that the every exposure is performed with the least amount of ionizing radiation possible to execute the procedure, following the ”As low as reasonably achievable (ALARA)” principle, aiming to reduce the exposure without com-promising the results, optimizing the process. Lastly, limitation intends to impose levels that are not to be surpassed, protecting individuals against excessive exposures, satis-fying deontological perspectives of ethics. However, assessing the optimization principle is far from being trivial, since it is difficult to assure that a procedure is being performed keeping the exposure as low as it could be without considering economic and social fac-tors. With that in mind, the common take on the rule is that every exam and treatment should be optimized to a point where the potential benefits of such improvement would not justify the costs necessary to attain it.
Publication 26 influence in radiation protection cannot be overstated, with those prin-ciples being implemented in both European and U.S. legislation. Shortly after the pub-lication, European Atomic Energy Community (EAEC or Euratom) emitted a directive [14] regarding radiation protection for general public and workers, establishing dose lim-its and guidelines, mentioning the principles aforementioned and effectively inducing lim-its members to include them in their own legislation. Four years later, in 1984, two more directives were issued, adding information and amending the first one [15] [16], being effective for more than a decade. In the U.S., Federal agencies responsible for radia-tion protecradia-tion were restructured and adopted the same principles, with the publicaradia-tion serving as the foundation for almost all Federal and State regulations in the country [17]. Building on that, ICRP revised those principles in 1991 with Publication 60[5],
rein-forcing them and introducing the concept of ”constraints”. While limits of dose protect the individual, intending that no one is exposed to certain levels of radiation, the mentioned constraints were a reference value applied to a source, not limiting, but aiming that the most exposed individuals were not in excessive risk when subjected to the mentioned source. Dose constraints could be perceived as investigation levels implemented by the responsible entities, that would help planning common diagnostic procedures, without limiting, since higher doses could be necessary, if properly justified. While the U.S. kept on using Publication 26 as the base for their regulations, Euratom issued directive 96/29 in 1996 [18] based on Publication 60, introducing the term ”dose constraint” and stating that ”Dose constraints should be used, where appropriate, within the context of optimiza-tion of radiological protecoptimiza-tion.” [18], clarifying its purpose.
Expanding on the idea of investigation levels, Publication 73 (1996) [6] separated the concept of a reference level from dose constraints, creating the Diagnostic Reference Level (DRL). Being defined as advisory levels, DRL’s are not in any way dose limits or constraints, nor do they serve regulatory purposes. However, they aim to identify if some common diagnostic exams (both in Radiology and Nuclear Medicine), present unusually high values, alerting the responsible bodies to act accordingly, by reviewing procedures and/or equipment. Despite focusing on levels being surpassed, in theory, DRL’s can also have a lower value, implying that below a certain dose, the resulting image could be insufficient to provide an useful diagnostic image.
DRL’s are created for standard procedures or groups of standard-sized patients or phantoms, but never for individual exposures, so the quantities on which those levels are based should be easily measured, and could be set locally, regionally, or nationally, providing information adjusted to the reality of where they were implemented. When analysing those measurements for a specific imaging task, unjustified values in the dis-tribution, high or low, can indicate that there are some issues that need to be addressed, ultimately improving the procedure and, ideally, promoting an optimum range of values for said procedure. As such, DRL’s were deemed an effective tool for improving and optimiz-ing radiation protection in diagnostic imagoptimiz-ing, actoptimiz-ing as a supplement to the professionals involved, providing information on the way exams are performed, without representing a distinction between good and bad medicine.
One year later, Euratom issued directive 97/43 [19], replacing directive 84/466 [15] on health protection of individuals against the dangers on ionizing radiation related to med-ical exposure. This directive is known as the ”Medmed-ical Exposure Directive” (MED) and included the aforementioned DRL’s, by stating that ”Member States shall promote the es-tablishment and the use of diagnostic reference levels for radiodiagnostic examinations” while also ensuring ”that appropriate local reviews are undertaken whenever diagnostic reference levels are consistently exceeded and that corrective actions are taken where appropriate.”
In 1999, aiming to help with the establishment of reference levels, the European Com-mission emitted a guide on DRL’s for medical exposures, ”Radiation Protection 109” [7],
that to this day serves as an important tool to understand the mentioned levels, provid-ing explanations and guidelines on legal and practical applications, as well as levels and examples that were already set in some European countries. As a result, more centres began to implement DRL’s, improving its effectiveness.
The latest update by Euratom on the subject was directive 2013/59 [20], based on ICRP Publication 103 [21], responsible for ”laying down basic safety standards for pro-tection against the dangers arising from exposure to ionising radiation” and revoking previous directives ([18] & [19]).The importance of DRL’s was reinforced, with its imple-mentation and supervision becoming a responsibility of the resident Medical Physics Ex-pert, a decision based on the fact that it is a meticulous process with a lot of variables, such as the diagnostic procedure itself, the equipment associated, context of the centre, measurements and analysis required, in order to properly apply them.
Despite its evolution, some problems subsisted and demanded clarification, mainly regarding definitions and determination of values, time intervals for revaluating and updat-ing DRL’s, how to use them in clinical practice and how to adjust them for new equipment and technology. As such, in 2017, ICRP issued Publication 135 [12], focusing entirely on DRL’s in medical imaging, explaining how to conduct the necessary surveys and de-termining the values for adults and paediatric patients, be it in Radiography, Diagnostic Fluoroscopy, Interventional Procedures or Nuclear Medicine.
2.2
DRL’s in Nuclear Medicine
Identifying examinations on which DRL’s are to be implemented is the first step. Although it would be ideal to have them for every procedure, it is advised to prioritize high fre-quency exams, since an extensive survey to acquire the desired measurable quantities is necessary. This involves collecting data from as many patients as possible in a single centre, or in case of a region, from a considerable number of centres, in order to acquire enough data to set the mentioned levels.
In Nuclear Medicine, the recommended measurable quantity is Administered Activity (AA) and is normally expressed in MBq. However, since it is related to the activity of the radiopharmaceutical given to each patient, it is typically adjusted for the weight of the patients and expressed in MBq/kg. With AA relying heavily on the size and weight of patients, mostly due to the way radiotracers disseminate in the human body and the tissues that gamma rays have to go through before reaching the detectors, DRL’s are typically set for a group of standard sized patients, with similar weights, enhancing the importance of setting these levels on high frequency exams in order to collect sufficient data. On top of that, Nuclear Medicine exams do not use the same radiotracers for every procedure, with this diversity being one of the main difficulties in establishing DRL’s.
Adding on that, the equipment plays a key role in the quality of the images and, consequentially, the development of DRL’s. In fact, a poorly-functioning detector will, in theory, require a higher AA to achieve the desired result, increasing dose to patients,
corroborating the notion that equipment quality and characteristics can interfere with the time it takes to obtain a useful image and compromise the results. Lower acquisition times demand higher AA, which is far from being ideal, whereas a longer period means the equipment is occupied and the centre has to reduce the number of patients it can receive, as well as potentially increasing image artefacts and defects induced by patient motions, who struggle to stay still for prolonged periods of time.
Besides the aforementioned difficulties, paediatric imaging brings additional problems since children are more vulnerable to the harmful effects of ionizing radiation and have, in theory, a longer life expectancy on which those effects can manifest. As such, most considerations regarding DRL’s setting are similar to the ones employed for adult patients, excluding the premise that AA per weight can be the same for all ages and bodies. Pae-diatrics range from newborn babies, weighting a few kilograms, to adult-sized teenagers that can weigh more than adults, which justifies the necessity of adjusting the AA for dif-ferent ages and/or weights. Those adjustments evolved through time, starting from being fractions of the activities given to adults, to include BSA measurements and other formu-las [22], ultimately culminating in the European Association of Nuclear Medicine (EANM) Dosage Card [23]. For a list of radiotracers, a baseline activity (minimum AA necessary to acquire an image for a children weighing 3 kilograms) was determined, as well as hav-ing them divided into three classes, that, for various weights, have a value associated. By multiplying the baseline activity by the weight-class corresponding value, the final AA is determined, ensuring a method that considers not only the radiotracer used and the weight of patients, but also the minimum necessary to obtain a good image, contributing to the main goal of DRL’s.
When developed, DRL’s serve as a guidance level for administered activities in a spe-cific procedure, that should result in a good diagnostic image. This approach differs from the one employed in Radiology where reference levels are not expected to be surpassed, while in Nuclear Medicine, beyond not being exceeded, they should be approached as closely as possible to obtain the best result. Since it is difficult to objectively quantify the quality of a diagnostic image, a local review of the images obtained should be regu-larly performed, guaranteeing that the AA are high enough to provide the desired image, effectively establishing a range of values for each examination. Even between similar equipment, gamma cameras or PET scans can have different sensitivities and dose cali-brations, introducing more variables that have to be considered when examining patients and, as a consequence, when setting DRL’s. Regarding PET scans, new equipment are normally equipped with time-of-flight (TOF) technology that improves image contrast and has higher sensitivity, effectively requiring less AA to acquire a useful image, with reports claiming a 20% reduction in the average administered activity [24].
Gradually, some countries released their surveys, establishing values for many proce-dures and radiopharmaceuticals. In 2012, the National Council on Radiation Protection and Measurements (NCRP) published a report with recommendations and guidelines, that, for Nuclear Medicine were based on mean, minimum and maximum administered
radiotracer doses for standard procedures [25]. Recently, the UK [26], Australia [27], Brazil [28] and Japan [29] also published their respective national DRL’s, allowing com-parisons and a better understanding on the practical differences between each region. As for Europe, in 2014, the European Commission issued Radiation Protection No 180 [2], an analysis on thirty-six European countries regarding DRL’s in both Radiology and Nuclear Medicine. For the later, it was found that only 64% of the countries have devel-oped them, with the majority being acquired through national surveys, while the rest were based on published local studies.
Figure 2.1: Diagnostic Reference Levels for Nuclear Medicine [2]
As seen in figure2.1, Portugal is one of the countries that has yet to develop DRL’s. Historically, Portugal followed the Euratom directives, with decree 9/90 [30] implement-ing the basic principles of radiation protection devised by ICRP Publication 26. The next directive to be transposed was the Euratom 97/43, when law decree 180/2002 [31] ef-fectively induced DRL’s into Portuguese legislation, stating that every diagnostic centre using ionizing radiation has to ensure that all medical exposures are in line with the Eu-ropean recommendations and guidelines, having to act accordingly if they are regularly surpassed. Through the years, more decrees were issued, attributing responsibilities and setting dose limits for specific groups of people [32], with the latest being law decree 108/2018 [33], which follows Euratom directive 2013/59, reinforcing the order to develop and review DRL’s, empowering Medical Physics Experts to do so. The aforementioned decree also states that the Portuguese Agency of the Environment (APA) is the respon-sible entity for ensuring an excellent level of radiation protection, assuring the directives
established by the decree are closely fulfilled by every centre or institution. Despite all decrees, only a few surveys tried to establish single centre DRL’s, mostly focusing on Ra-diology procedures [34]. There were also studies in NM, mainly focused on the frequency and average AA of certain exams [35] [36], yielding insight on the collective ionizing dose due to NM procedures. Nonetheless, as of this moment, national DRL’s are yet to be developed.
3
Equipment and parameters
3.1
Theoretical framework
3.1.1 Radioactivity
In Nuclear Medicine, when setting DRL’s, the recommended quantity to measure and assess is the Administered Activity (AA). The term traces back to the discovery of natural Radioactivity, by Henry Becquerel in the final years of the 19th century, a phenomenon where an unstable nucleus (parent) spontaneously transforms into one that is more stable (daughter), releasing energy in the process. Almost 40 years later, in 1934, F. Joliot and I. Curie found that a naturally stable nuclide could decay if properly activated, discovering artificial radioactivity [37]. For a radioactive material, the number of such atoms decaying (dN) in a period of time (dt) is called Activity (A).
A = −dN/dt (1)
Activity decreases with time, hence the negative signal, and was traditionally ex-pressed in units of curies (Ci), named after the famous physicist and defined as 3.7 × 10−10 disintegrations per second (dps). Nowadays, the scientific community follows the Interna-tional System of Units (SI), that for this quantity utilizes becquerel (Bq), a unit named after the discoverer of radioactivity, defined as 1 dps. A famous and handy relation in Nuclear Medicine is that one millicurie (mCi) corresponds to thirty-seven megabecquerels (MBq), (1 mCi = 37 MBq).
In itself, radioactivity is a stochastic process, making it impossible to predict when a singular atom will decay. Nonetheless, for a radioactive material that contains more of those atoms, determining the probability of a nucleus to decay, per unit of time, is feasible, being defined as the decay constant (λ), an intrinsic property of each radionuclide. Since the number of unstable atoms (N) is proportional to the number of atoms decaying per unit of time, activity can be expressed as:
A = λN (2)
Another useful parameter associated to the decay constant is the physical half-life (t1
2), defined as the time required for the number of radioactive atoms in a material to
decrease by half of its initial value. Both quantities are related by the following equation:
λ = ln2
t1 2
(3) As a consequence of its relation with the decay constant, the physical half-life time is also a characteristic of each radionuclide, ranging from fractions of seconds to billions of years.
Knowing the initial activity of a sample (A0) it is possible to calculate its activity at any given point in time (t), predicting its decay, as expressed in the following equation:
At= A0 e−λt (4)
Besides the number of disintegrations and its relations, one of the most important aspects of radioactive decay is the energy released, both in quantity and quality. Each disintegration process can be defined by a general equation, demonstrating the changes from a parent radionuclide (X) to its daughter (Y), either on the number of nucleons (mass number , A) and/or on the number of protons in a nuclei (atomic number, Z).
A
ZX → A 0
Z0Y + x + Q (5)
Each disintegration emits a type of radiation (x), with a total energy (Q), according to the type of radioactive decay, with the main processes being alpha decay (α), beta decay (β−and β+), electron capture (EC) and isomeric transitions.
Particularly, β+ decay is known as positron decay, being the fundamental basis of the Positron Emission Tomography (PET). A nucleus with an excess of protons is un-stable, usually decaying by positron (β+) emission, converting one proton into a neutron and becoming a different element with atomic number Z-1. Being the electron antiparti-cle, a positron has the same rest mass energy of his counterpart, of 511 keV, effectively inducing a transition energy threshold of 1.02 MeV, between parent and daughter ra-dionuclides, for a positron decay to occur. This process also emits a neutrino and can be described by the following equation:
A ZX
β+
→AZ−1Y + β++ ν (6)
After the emission, positrons quickly interact with a nearby electron, in a process known as pair annihilation, instantly emitting two 511 keV photons in opposite directions. Since positrons have a short range in matter, the photons point of emission is near the point where the radioactive decay occurred and, by detecting those photons, it is pos-sible to have a relatively precise indication of where the radionuclide was, allowing for numerous applications in Nuclear Medicine imaging (e.g. PET).
3.1.2 Radionuclide production
Building on the previous idea, radioactive markers that could be administered to patients were developed, being known as radiopharmaceuticals or radiotracers. Those markers are molecules or compounds that combine a radionuclide, which can be detected due to the aforementioned processes, with a biological marker that disseminates though the human body. The selected biological molecules have preferential targets, either organs or tissues, on which they accumulate after some time. Since they are marked with a radionuclide, their location can be determined and used in diagnostic imaging, with plenty
of radiopharmaceuticals available today, specially designed to target specific tissues and diseases, yielding insight on the metabolism and biological processes that occur within the human body.
Radiotracers production starts with obtaining the desired radionuclide. There are roughly 2700 radioactive nuclides [38], with most of them being artificially produced in nuclear reactors and cyclotrons, while others with a shorter half-life time can be attained using smaller radionuclide generators.
Nuclear reactors are devices capable of establishing a controlled and self-sustaining nuclear reaction. Inside its core there is a fissionable material, usually Uranium-235 (235U), that spontaneously splits into two fragments and emits two or three neutrons of about 1.5 MeV, while also releasing around 200 MeV in the form of heat, that is usually di-rected to energy production. With each fission, the emitted neutrons can cause additional fissions on the fuel materials (rods), initiating a chain reaction that, if properly contained by using control rods to moderate the reaction, can be used to produce various radionu-clides. Those rods limit the number of available neutrons, by absorbing them, and can be manipulated to control the core reactivity. Figure3.1shows a schematic representation of a nuclear reactor.
Figure 3.1: Schematic representation of a nuclear reactor [39]
In a nuclear reactor, radionuclides can be produced by Fission or Neutron Activation. The first method occurs when the emitted neutrons bombard the235U, creating (236U*), which is a highly unstable nucleus that promptly splits into two fragments. Among those fission product, more than 100 nuclides can be found, always neutron rich, which results in radionuclides that decay by β− emission. The resultant products have a high spe-cific activity, which is the activity per quantity of a radioisotope (Bq/g), and can be sep-arated through various chemical procedures such as precipitation, solvent extraction or chromatography. The most common examples of clinically useful radionuclides acquired through this process are99Mo,131I, and137Cs.
After the fission of Uranium, the resulting neutrons can be used to interact with a stable sample, in a process called Neutron Activation. The target nucleus can capture the mentioned neutron, producing an isotope of the same element while emitting a γ-ray, or it can release a proton, in this case producing a nuclide that is not of the same element. In both cases, the resultant products tend to decay by β−emission and usually have low specific activities.
Another popular method of radionuclide production is by bombarding stable nucleus with high-energy charged particles, using cyclotrons and other particle accelerators to do so. With this technique, positively charged particles such as protons, deuterons and α particles, as well as negative ones (hydrogen ions) are accelerated to high kinetic energies (10-20 MeV) and directed to a nucleus, overcoming the repulsive barrier created by Coulomb forces and striking its target. When struck, the nucleus goes to an excited state, promptly emitting nucleons and γ-rays, depending on the energy deposited and particles used, leading to the production of different radioisotopes.
Cyclotrons are commonly used devices to achieve this, producing many clinically rel-evant radionuclides. As shown in figure3.2, they have a vacuum chamber between the poles of an electromagnet, with a pair of semicircular electrodes called ”dees” (D) inside, separated by a small gap. An alternating high voltage is applied between the ”dees”, and when positive ions are injected in the centre of the cyclotron, they are accelerated towards the ”negative dee”, maintaining a circular path due to the existent magnetic field. As the particles travel, they gain kinetic energy and increase the radius of its movement, with the polarity of the electrical field being reversed right before they reach the gap, being again accelerated towards the ”new negative dee”. This cycle is repeated (hence the name cyclotron) until the final kinetic energy is achieved, which depends on the diameter of the ”dees”, magnetic field strength and on the particle itself. Finally, ions are removed from this cycle by a charged deflector and strike the target nuclei, causing the aforementioned emission of nucleons. By choosing the particle to be accelerated, its final kinetic energy and the targets composition, several radionuclides can be produced, typically decaying by β+emission or EC.
Cyclotron produced radionuclides have numerous applications in Nuclear Medicine, mostly due to the fact that some of the short-lived positron emitters attained are key constituents of the human body (e.g. 11C, 13N, and 15O). However, industrial cyclotron facilities are expensive to build and sustain, a fact that led to the invention of smaller and specialized cyclotrons that could be installed in hospitals and other centres, especially producing positron emitting radioisotopes for PET scans.
Lastly, short-lived radioisotopes can also be produced at a smaller scale by specially developed radionuclide generators. A generator basic principle is that it has to contain a parent radionuclide that has a longer half-life than that of its daughter nuclide, so the par-ent can decay while its short-lived daughter is chemically separated by simple processes, allowing repeated extractions during the generator lifetime.
Regarding its structure, which can be seen in figure3.3, a generator has a column containing an absorbent material that will retain the parent nuclide, on which it will decay, increasing the activity of daughter nuclide until an equilibrium is established. At that point, it appears that both nuclides decay with the same half-life. Afterwards, and taking advantage of the fact that the parent and daughter nuclides are not the same elements, having different chemical properties, the daughter is eluted with a selected solvent, being extracted from the generator to fulfil his purpose.
Figure 3.3: Schematic representation of a radionuclide generator [38]
There are two common concepts for this kind of generators, known as transient equi-librium generator and secular equiequi-librium generator. On the first one, the half-life of the parent nuclide is not much greater than that of its daughter, with the primary example being the99Mo/99mTc generator. It is the most known and used radionuclide generator in the world, not only due to its half-life times relation (99Mo → 66h and 99mTc → 6h), but also for its applications with exams performed in gamma cameras. As the time passes, the activity of99mTc grows until it reaches a maximum ( 23h), on which the production rate and decay rate are the same, with the daughter nuclide having an apparent half-life equal
to the one of its parent, achieving the aforementioned transient equilibrium. If the daugh-ter nuclide is eluted, the process starts over, but since the parent has also been decaying, the total activity that the daughter radionuclide will reach at the transient equilibrium point will be lower than before, as seen in figure3.4.
Figure 3.4: Representation of a periodic elution in a99Mo (orange) /99mTc (blue) generator [39]
As for the secular equilibrium, the main difference is that the parent half-life is much longer than that of his daughter, meaning its decay can be neglected during several half-lives of its daughter. Similarly to the transient equilibrium, the production rate is, in the beginning, higher than the decay rate, increasing the daughters activity, until it reaches a point where they are the same, with the daughter apparently decaying with the same half-life of his parent, reaching the secular equilibrium point shown in figure3.5.
Figure 3.5: Representation of secular equilibrium [39]
There are several examples of parent/daughter pairs with this behaviour (e.g. 81Rb → 4.58h and81mKr → 13s) with many clinical applications. Despite its differences, both are used to locally produce radiopharmaceuticals, promptly used in various examinations.
In most cases, radionuclides are attached as markers to compounds of biomedical interest, whose behaviour inside the human body is predictable, enabling them to seek
![Figure 3.8: Differences in LOR probability for equipment with and without TOF technology [39]](https://thumb-eu.123doks.com/thumbv2/123dok_br/15734253.1071816/41.892.239.676.592.800/figure-differences-lor-probability-equipment-tof-technology.webp)
![Figure 4.1: Limiting distributions for high-precision and low-precision measurements. [51]](https://thumb-eu.123doks.com/thumbv2/123dok_br/15734253.1071816/50.892.251.646.418.657/figure-limiting-distributions-high-precision-low-precision-measurements.webp)