InternationalJournalofAntimicrobialAgents45(2015)420–423
ContentslistsavailableatScienceDirect
International
Journal
of
Antimicrobial
Agents
j ou rn a l h om ep a ge :ht t p : / / w w w . e l s e v i e r . c o m / l o c a t e / i j a n t i m i c a g
Short
communication

-Lactam
antibiotics
and
vancomycin
inhibit
the
growth
of
planktonic
and
biofilm
Candida
spp.:
An
additional
benefit
of
antibiotic-lock
therapy?
José
J.C.
Sidrim
a,
Carlos
E.C.
Teixeira
a,
Rossana
A.
Cordeiro
a,
Raimunda
S.N.
Brilhante
a,∗,
Débora
S.C.M.
Castelo-Branco
a,
Silviane
P.
Bandeira
a,
Lucas
P.
Alencar
a,
Jonathas
S.
Oliveira
a,
André
J.
Monteiro
b,
José
L.B.
Moreira
c,
Tereza
J.P.G.
Bandeira
a,
Marcos
F.G.
Rocha
a,daDepartmentofPathologyandLegalMedicine,CollegeofMedicine,PostGraduatePrograminMedicalMicrobiology,SpecializedMedicalMycologyCenter, FederalUniversityofCeará,Fortaleza,CE,Brazil
bDepartmentofStatisticsandAppliedMathematics,FederalUniversityofCeará,Fortaleza,CE,Brazil
cDepartmentofPathologyandLegalMedicine,CollegeofMedicine,FederalUniversityofCeará,Fortaleza,CE,Brazil dCollegeofVeterinaryMedicine,PostGraduatePrograminVeterinarySciences,StateUniversityofCeará,Fortaleza,CE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received26May2014 Accepted1December2014
Keywords: Candidaspp. Biofilms Catheterinfection Antibiotic-locktherapy
a
b
s
t
r
a
c
t
Theaimofthisstudywastoevaluatetheeffectsofcefepime,meropenem,piperacillin/tazobactam(TZP) andvancomycinonstrainsofCandidaalbicansandCandidatropicalisinplanktonicandbiofilmforms. Twentyazole-derivative-resistantstrainsofC.albicans(n=10)andC.tropicalis(n=10)weretested.The susceptibilityofplanktonicCandidaspp.totheantibacterialagentswasinvestigatedbybroth microdi-lution.TheXTTreductionassaywasperformedtoevaluatetheviabilityofgrowingandmaturebiofilms followingexposuretothesedrugs.Minimuminhibitoryconcentrations(MICs)rangedfrom0.5mg/mL to2mg/mLforcefepime,TZPandvancomycinandfrom0.5mg/mLto1mg/mLformeropenemand thedrugsalsocausedstatisticallysignificantreductionsinbiofilmcellularactivitybothingrowingand maturebiofilm.Sinceallofthetesteddrugsarecommonlyusedinpatientswithhospital-acquired infec-tionsandinthosewithcatheter-relatedinfectionsunderantibiotic-locktherapy,itmaybepossibleto obtainanadditionalbenefitfromantibiotic-locktherapywiththesedrugs,namelythecontrolofCandida biofilmformation.
©2015ElsevierB.V.andtheInternationalSocietyofChemotherapy.Allrightsreserved.
1. Introduction
Candidaspp.arethefourthandthirdleadingcausesof hospital-acquiredbloodstream and urinary tractinfections, respectively,
and most of these infections are associated with implanted
medical devices such as central venous and bladder catheters
owingtobiofilmformationwithinthesematerials[1].Arelevant characteristicofCandidabiofilmsisresistancetoantifungalagents, whichcanbeintrinsicoracquiredbytransferofgeneticmaterial betweenbiofilm cells [2]. Biofilm-associated Candida infections are usually difficult to diagnose, causing delayed therapy and highlethalityrates in hospitalised patients worldwide [3], and
∗Correspondingauthor.Tel.:+558533668319;fax:+558532951736.
E-mailaddress:brilhante@ufc.br(R.S.N.Brilhante).
theabilityof Candidaspp.toformdrug-resistantbiofilmsisan importantcontributingfactortohumandiseases[1].
In systemic infections, biofilms can also be polymicrobial,
formed by Candida spp. and bacteria [1]. Patients with
con-firmedorstronglysuspectedhospital-acquiredinfectionsprimarily
receive antibacterial therapy, including cefepime, meropenem,
piperacillin/tazobactam(TZP)andvancomycin[4],which,onthe otherhand,predisposesthemtotheoccurrenceofCandida infec-tionsbecauseitdecreasesmicrobialcompetitionwithinthehost’s
microbiome [1,3]. It has already been shown that
antibacte-rialdrugs can affectCandida biofilmformation. Tigecycline, for instance,ishighlyactiveagainstgrowingandmaturebiofilmsof Candidaalbicans[5],whilstrifampicincaninducebiofilm forma-tionbythisCandidaspecies[6].Thus,thisstudyaimedtoevaluate theeffectsof-lactams(cefepime,meropenemandTZP)and van-comycinonstrainsofC.albicansandCandidatropicalisinplanktonic andbiofilmforms.
http://dx.doi.org/10.1016/j.ijantimicag.2014.12.012
J.J.C.Sidrimetal./InternationalJournalofAntimicrobialAgents45(2015)420–423 421
2. Materialsandmethods
2.1. Fungalstrains
Azole-derivative-resistantstrainsofCandidaspp.fromhuman casesof candidaemia (four C.albicans and sixC. tropicalis)and healthyanimals(sixC.albicansandfourC.tropicalis)wereincluded inthisstudy.Thestrainscamefromtheculturecollectionofthe SpecializedMedicalMycologyCenterofFederalUniversityofCeará (Fortaleza,Brazil)andwereselectedbasedontheirantifungal resis-tance[7].Theidentityofthestrainswasconfirmedaspreviously described[7].
2.2. AntimicrobialagentsandantifungalsusceptibilityofCandida planktoniccells
A stock solution of amphotericin B (AmB) (Sigma-Aldrich,
St Louis, MO) at 1mg/mL was prepared according to
Clini-caland LaboratoryStandards Institute(CLSI)guidelines[8] and wasusedas acontrol. Theantibacterial drugscefepime (Nova-farma,Anápolis,GO,Brazil),meropenem(AstraZeneca,Cotia,SP,
Brazil), TZP (Novafarma) and vancomycin (AstraZeneca) were
diluted with sterile distilled water as recommended by the
manufacturers. A stock solution of vancomycin was prepared
with distilled water at 50mg/mL as previously described [5]
and, based on this research [5], the other antibacterial drugs
were diluted to the same concentration. Serial two-fold
dilu-tionsof each drugwere prepared in RPMI 1640 medium with
l-glutamine and without sodium bicarbonate (Sigma-Aldrich,
St Louis, MO), buffered to pH 7.0 with 0.165M MOPS
(3-[N-morpholino]propane sulfonic acid) (Sigma-Aldrich, St Louis, MO).
Thesusceptibilityof Candidaspp.totheantibacterial agents wasinvestigatedbybrothmicrodilutionaccordingtoCLSI guide-lines[8].Thefinal inoculum wasdiluted withRPMI toreacha concentrationof0.5–2.5×103cells/mL.AmBwasalsotestedasa
controldrug.Todeterminethesusceptibilityofplanktoniccells,the
testedconcentrationrangeswere0.0039–4mg/mLforcefepime,
meropenem,TZPandvancomycinand0.03125–16g/mLforAmB.
Allisolatesweretestedinduplicate.Fortheantibacterialdrugs,
theminimuminhibitoryconcentration(MIC)wasdefinedasthe
lowest drug concentration capable of inhibiting 50% of fungal
growth[5]comparedwiththecontrolwell,whilstforAmBtheMIC wasdefinedasthelowestdrugconcentrationcapableof inhibi-ting100%ofplanktonicfungalgrowth[8].EscherichiacoliATCC 25922,StaphylococcusaureusATCC25923andCandida parapsilo-sisATCC22019wereincludedasqualitycontrolstrainsforeach test[8].
2.3. Biofilmformation
For biofilm testing, inocula were prepared as previously
described [9] with some modifications. Strains of C. albicans
(n=10)andC.tropicalis(n=10)weregrowninSabourauddextrose
broth (Himedia, Mumbai, India) at 30◦C for 24h in a rotary
shaker at 150rpm. After this period, cells were collected by
centrifugation (3000rpm, 10min) and the pellet was washed
twice with phosphate-buffered saline (PBS). Suspensions were
adjusted to 1×106cells/mL in RPMI medium and then 100L
aliquots of inoculum were transferred to flat wells of 96-well polystyrene plates (TPP, Trasadingen, Switzerland). The plates wereincubatedat37◦Cfor48handthewellswerewashedthree
timeswith0.05%Tween20(Sigma-Aldrich,SãoPaulo,SP,Brazil) in Tris-buffered solution (Sigma-Aldrich, São Paulo, SP, Brazil)
to remove non-adherent cells. Biofilm viability was monitored
through the use of
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT)
(Sigma-Aldrich,StLouis,MO)asdescribedpreviously[9].
2.4. EffectofantibacterialdrugsongrowingandmatureCandida biofilms
TheabilityofthetesteddrugstoinhibitformationofC.albicans andC.tropicalisbiofilmswasevaluatedasdescribedpreviously[9]. Duringtheplateinoculationstep,100Laliquotsofthe antibac-terialsolutionswereaddedtoeachwell.Eachdrugwastestedat fivedifferentconcentrations(MIC/50,MIC/10,MIC,10×MICand
50× MIC). Biofilm formationwasthen performedaspreviously
described.Followingincubationat37◦Cfor48h,theeffectofthe
antimicrobialdrugsongrowingbiofilmswasevaluatedbasedon biofilmcellactivityusingtheXTTreductionassaywith75Lof XTTsalt solution(1mg/mLin PBS),6L ofmenadione solution (1mMinacetone)(Sigma-Aldrich,StLouis,MO)and50Lof ster-ilePBS,whichwereaddedtoeach well,followedbyincubation at36◦Cfor5h.Themetabolicactivityofbiofilmcells was
mea-suredwithamicroplatereader(Epoch;Bio-Tek,Winooski,VT)at
492nm.
The inhibitory activity of the tested drugs against mature
biofilmsofC.albicansandC.tropicaliswasevaluatedaspreviously described[9].Theantibacterialdrugsweretestedatfivedifferent concentrations(MIC/50,MIC/10,MIC,10×MICand50×MIC).For
thispurpose,aliquotsof200Lofeachdrugwereaddedtoviable 48-h-oldbiofilmsgrowninflatwellsof96-wellpolystyreneplates, followedbyincubationat35◦Cfor48h.Afterthisperiod,inhibition
ofbiofilmmetabolicactivitywasmonitoredbyXTTreduction[9]
asdescribedabove.
Allbiofilmexperimentswereperformedinduplicateandwere
repeatedatthreeindependentmoments.Controlsweregrownin
mediumwithoutantimicrobials,andAmBwasusedasthecontrol drugforbiofilminhibition.
2.5. Statisticalanalysis
Inordertoverifydifferencesinabsorbancevaluestoevaluate theeffectsofantibacterialdrugsonbiofilmcellactivity,Student’s t-testforpairedsampleswasused.Foralloftheanalyses,a signifi-cancelevellowerthan5%indicatedstatisticallysignificantfindings (P<0.05).
3. Results
The antibacterialMICs against the20 tested Candida strains rangedfrom0.5mg/mLto2mg/mLforcefepimeandTZPandfrom
0.5mg/mLto1mg/mLformeropenem.TheMICsforvancomycin
rangedfrom0.5mg/mLto1mg/mLagainstC.albicansand from 0.5mg/mL to 2mg/mL against C. tropicalis. For AmB, the MICs rangedfrom0.5g/mLto2g/mLagainstC.albicansand from 0.5g/mLto4g/mLforC.tropicalis.
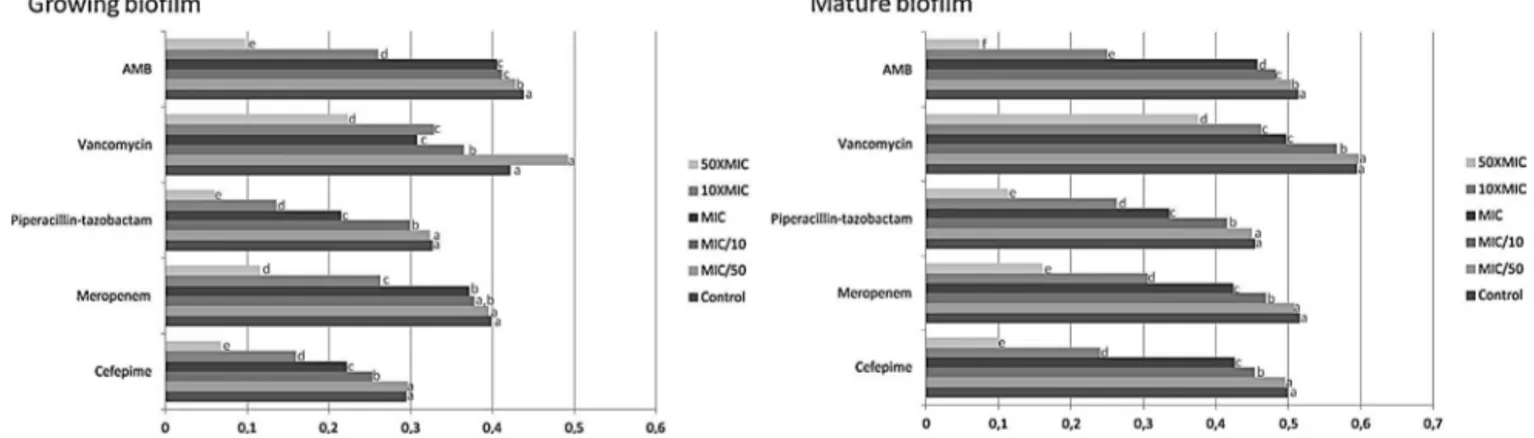
RegardingtheeffectsofantibacterialdrugsongrowingCandida
spp. biofilms, cefepime, TZP and vancomycin caused
statisti-callysignificantreductionsinbiofilmcellularactivityatMIC/10 (P<0.001), MIC(P<0.0001),10× MIC(P<0.0001) and 50×MIC
(P<0.0001),whilstmeropenemonlycausedsignificantreductions attheMICandhigherconcentrations(P<0.05).AmBsignificantly inhibitedgrowingbiofilmcellularactivityatalltested concentra-tions(P<0.01)(Fig.1).
Regarding mature Candida biofilms, cefepime, meropenem,
TZPandvancomycincausedstatisticallysignificantreductionsin biofilmcellular activityatMIC/10(P<0.05), MIC(P<0.01), 10×
MIC (P<0.0001) and 50× MIC (P<0.0001), but not at MIC/50
422 J.J.C.Sidrimetal./InternationalJournalofAntimicrobialAgents45(2015)420–423
Fig.1.Effectofdifferentconcentrationsofcefepime,meropenem,piperacillin/tazobactam,vancomycinandamphotericinB(AMB)onthemetabolicactivityofgrowingand maturebiofilmsofCandidaspp.analysedbytheXTTreductionassay.Theminimuminhibitoryconcentration(MIC)isthatobtainedagainstplanktonicgrowthofCandidaspp. Lettersindicatestatisticallysignificantdifferencescomparedwiththecontrolandwithgrowthatdifferentantibacterialconcentrations(P<0.05).Thetestedantimicrobial concentrationsarepresentedinthefollowingordertop-down:50×MIC,10×MIC,MIC,MIC/10,MIC/50andcontrol.
inhibitedmaturebiofilmcellularactivityatalltested concentra-tions(P<0.05)(Fig.1).
4. Discussion
ThecapacityofCandidatoformbiofilmsonabioticandbiotic surfacesisanimportantvirulencefactorfortheestablishmentof recurringcandidiasis[10].Itiswellknownthattheuseofsystemic antibioticspredisposestheproliferationofCandidaspp.,hencethe occurrenceofcandidiasis[1,3].However,littleisknownaboutthe effectsofantibioticsongrowingandmaturebiofilmsofCandida spp.withinmedical devices,whicharecontinuouslyexposedto antibacterialdrugswhenpatientsareundertreatment.Thislack ofknowledgewasamotivationtoinvestigatetheinvitroeffects
ofcefepime,meropenem,TZPandvancomycin, whichare
com-monlyused againsthospital-acquiredinfections [4],onCandida biofilmsand,consideringthatthesedrugspredisposethe occur-renceofCandidainfections,itwasinitiallyhypothesisedthatthey
could induce biofilm formation or the maintenance of mature
biofilms.
Initially, the antimicrobials were tested against
azole-derivative-resistant C. albicans and C. tropicalis strains and all of them effectively inhibited the growthof planktonic Candida spp.,withMICsrangingfrom0.5mg/mLto2mg/mL.Althoughall testedantibacterialdrugswereabletoinhibittheinvitrogrowthof planktonicCandida,theMICssurpassedthedesirabletherapeutic bloodconcentrationsforalloftheseantibacterialdrugs[11–14]. Hence,theycannotbeusedtoeffectivelytreatCandidainfections.
The mechanisms through which these antibacterial drugs
inhibited fungal growth remain unknown. Only a few studies
havebeenperformedtacklingtheeffectsofantibacterialdrugson Candidabiofilmsandnoneofthemhaveaddressedpossible mech-anismsof action[5,6].However,it canbesuggested thatthese drugsactthroughunspecificmechanisms,withoutspecifictarget molecules,sinceextremelyhighconcentrationsofthefourtested drugswererequiredtocausethisgrowthinhibition.Thus,
consid-eringthis newpotentialuseofcefepime, meropenem,TZP and
vancomycin,itisnecessarytodesignspecificexperimental proto-colstoelucidatethemechanismsbehindtheobservedantifungal effect.
On the other hand, the results demonstrate that antibacte-rialconcentrationsaslowasMIC/10werecapableofsignificantly reducingthebiofilmcellularmetabolicactivitybothofgrowingand matureCandidabiofilms.Themostinterestingfindingofthisstudy wasthatantibacterialconcentrationslowerthantheMICsagainst
planktoniccellswereabletosignificantlydecreasetheinvitro via-bilityofgrowingandmatureCandidabiofilms.
Clinically,thesefindingsmaybeground-breakingsinceallof thetestedantibacterialdrugs(cefepime,meropenem,TZPand
van-comycin)arecommonlyusedinpatientswithhospital-acquired
infections[4]andarealsousedinantibiotic-locktherapy[15].This techniqueinvolvestheprolongedinstillationofasolution contain-ingextremelyhighconcentrationsofantimicrobialorantiseptic agents,100–1000-foldhigherthanthoseusedsystemically,within aninfectedintravascularcatheterasinanattempttosterilisethe interiorofthecatheterandcontrolbloodstreaminfections[5,15]. Thiskindoftreatmentappearstobeaviablealternativeinthose special cases in which the salvage of the catheter is desirable, althoughremovingthedeviceisthetreatmentofchoicefor per-sistentorcomplicatedbacteraemiaorfungaemiarelatedtoitsuse
[15].
Itis importanttoemphasisethattheuseof systemic broad-spectrum antibiotics is an important risk factor for developing
candidaemia and that biofilms play a major role in
main-taining bloodstream Candida infections [10]. Therefore,
consid-ering the low minimum antibacterial concentrations (MIC/10;
50–200g/mL) required to significantly decrease the viability of Candida biofilm cells, we believe that the use of cefepime,
meropenem,TZPandvancomycinintheantimicrobial-lock
solu-tionmightnotonlyaidthepatientinthemanagementofbacterial infectionassociatedwithindwellingcatheters,butalsocontrolthe formationofCandidabiofilmswithinthesemedicaldevicesduring antibacterialtherapy.However,morestudieswillbenecessaryto establishtheeffectsofthistherapyonCandidabiofilmsinsidethe patientduringtreatment.
5. Conclusion
Theantibacterial drugscefepime, meropenem,TZP and
van-comycinareabletodecreasetheinvitroviabilityofgrowingand
matureCandida biofilms. These resultsmay bring the
perspec-tiveofobtaininganadditionalbenefitwithantibiotic-locktherapy, namelythecontroloffungalbiofilmswhenusingthesedrugsin hospitalisedpatients.
Funding
ThisstudywassupportedbyCNPq, Brazil[PROTAX562296/
2010-7;504189/2012-3;307606/2013-9]andCAPES,Brazil[PNPD
J.J.C.Sidrimetal./InternationalJournalofAntimicrobialAgents45(2015)420–423 423
Competinginterests
Nonedeclared.
Ethicalapproval
Notrequired.
References
[1]SardiJCO,ScorzoniL,BernardiT,Fusco-AlmeidaAM,MendesGianniniMJS. Candida species:current epidemiology, pathogenicity, biofilm formation, naturalantifungalproductsandnewtherapeuticoptions.JMedMicrobiol 2013;62:10–24.
[2]FanningS,MitchellAP.Fungalbiofilms.PLoSPathog2012;8:e1002585.
[3]WilleMP,GuimarãesT,FurtadoGHC,ColomboAL.Historicaltrendsinthe epidemiologyofcandidaemia:analysisofan11-yearperiodinatertiarycare hospitalinBrazil.MemInstOswaldoCruz2013;108:288–92.
[4]TorresA,FerrerM,BadiaJR.Treatmentguidelinesandoutcomesof hospital-acquiredandventilator-associatedpneumonia.ClinInfectDis2010;51(Suppl 1):S48–53.
[5]KuTSN,PalanisamySK,LeeSA.SusceptibilityofCandidaalbicansbiofilmsto azithromycin,tigecyclineandvancomycinandtheinteractionbetween tige-cyclineandantifungals.IntJAntimicrobAgents2010;36:441–6.
[6]VogelM,KöberlelM,ScäfflerH,TreiberM,AutenriethIB,SchumacherUK. RifampicininducedvirulencedeterminantsincreaseCandidaalbicansbiofilm formation.F1000Research,vol.2;2013.p.106,http://dx.doi.org/10.12688/ f1000research.2-106.v1.
[7]CordeiroRA,TeixeiraCE,BrilhanteRSN,Castelo-BrancoDS,PaivaMA,Giffoni LeiteJJ,etal.MinimuminhibitoryconcentrationsofamphotericinB,azoles andcaspofunginagainstCandidaspeciesarereducedbyfarnesol.MedMycol 2013;51:53–9.
[8]Clinical and LaboratoryStandards Institute. Reference method for broth dilutionantifungalsusceptibilitytestingofyeasts;approvedstandard.In: Doc-umentM27-A3.3rded.Wayne,PA:CLSI;2008.
[9]ChatzimoschouA,KatragkouS,SimitsopoulouM,AntachopoulosC,Georgiadou E,WalshTJ,etal.Activitiesoftriazole–echinocandincombinationsagainst Can-didaspeciesinbiofilmsandasplanktoniccells.AntimicrobAgentsChemother 2011;55:1968–74.
[10]Cuéllar-CruzM,López-RomeroE,Villagómez-Castro JC,Ruiz-BacaE. Can-didaspecies:newinsightsintobiofilmformation.FutureMicrobiol2012;7: 755–71.
[11]LamothF, BuclinT,PascualA,Vora S,Bolay S,DecosterdLA,etal. High cefepimeplasmaconcentrations andneurological toxicityinfebrile neu-tropenicpatientswithmildimpairmentofrenalfunction.AntimicrobAgents Chemother2010;54:4360–7.
[12]AmericanSocietyofHealth-SystemPharmacists.Therapeuticmonitoringof vancomycininadultpatients:aconsensusreviewoftheAmericanSociety ofHealth-SystemPharmacists,theInfectiousDiseasesSocietyofAmerica, andtheSocietyofInfectiousDiseasesPharmacists.AmJHealthSystPharm 2009;66:82–98.
[13]NicolauDP.Pharmacokineticandpharmacodynamicpropertiesofmeropenem. ClinInfectDis2008;47:32–40.
[14]Burgess DS, Waldrep T. Pharmacokinetics and pharmacodynamics of piperacillin/tazobactamwhenadministeredbycontinuousinfusionand inter-mittentdosing.ClinTher2002;24:1090–104.