w w w. s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Perezone,
from
the
gorgonian
Pseudopterogorgia
rigida
,
induces
oxidative
stress
in
human
leukemia
cells
Paula
A.
Abreu
a,b,
Diego
V.
Wilke
a,
Ana
J.
Araujo
a,c,
José
Delano
B.
Marinho-Filho
a,c,
Elthon
G.
Ferreira
a,
Carlos
Margo
R.
Ribeiro
d,
Leandro
S.
Pinheiro
d,
Juliana
W.
Amorim
d,
Alessandra
L.
Valverde
d,
Rosângela
A.
Epifanio
d,†,
Letícia
V.
Costa-Lotufo
a,e,
Paula
C.
Jimenez
a,f,∗aDepartamentodeFisiologiaeFarmacologia,UniversidadeFederaldoCeará,Fortaleza,CE,Brazil bINSERMU1113,StrasbourgUniversity,Strasbourg,France
cFaculdadedeMedicina,UniversidadeFederaldoPiauí,Parnaíba,PI,Brazil dInstitutodeQuímica,UniversidadeFederalFluminense,Niterói,RJ,Brazil eInstitutodeCiênciasBiomédicas,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil fDepartamentodeCiênciasdoMar,UniversidadeFederaldeSãoPaulo,Santos,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received1May2015
Accepted20July2015
Availableonline4September2015
Keywords:
Gorgoniancoral
Marinenaturalproducts
Cytotoxicity Quinone ROS
a
b
s
t
r
a
c
t
Fourbisabolanes1–4,includingperezone(1)andtriacetylperezone(2),wereisolatedthrougha bioassay-guidedfractionationoftheextractobtainedfromtheCaribbeangorgoniancoralPseudopterogorgiarigida collectedduringanexpeditioncruisetotheBahamas.Allisolatedcompoundsshowedtobecytotoxic towardpaneloffourhumantumorcelllines,asquantifiedbytheMTTassayafter72hincubation. Pere-zone(1),themostactiveone,wasfurtheranalyzed,showingtobecytotoxic,butnotselective,ina12-cell linepanelcomprisingtumorandnon-tumor,aswellashumanandmurinecells.Additionally,1was assayedforcytotoxicityagainstHL-60leukemiccells.Pre-treatmentwithanacutefreeradicalscavenger (l-NAC)beforeexposureofcellstoperezonevirtuallyeliminatedthegenerationofintracellularROSand lesseneditsseverecytotoxicity.Theprotectiveeffectdeliveredbyl-NACevidencesthatthemechanism ofperezone-inducedcytotoxicityispartiallyassociatedtoproductionofROSandaconsequentinduction ofoxidativestress.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Natural or synthetic quinones perform various functions in organisms,as someare tightlyinvolved in the cell’s biochemi-calmechanisms(Asche,2005).Nevertheless,quinoneshavebeen extensively studiedfor their cytotoxicpotential and, currently, areanimportantgroupofanticancerdrugs.Manyquinoid com-pounds,suchastheanthracyclinesdoxorubicinanddaunorubicin and the antibiotic mitomycin, are successfully applied in vari-ouscancerchemotherapyschemes(Asche,2005;Vásquezetal., 2010).
Perezone(1)wasfirstisolatedin 1852fromrootsof Perezia
mangroveplants(renamedAccourtia).Thisquinoneshowed insec-ticideactivityagainstLeptinotarsadecemlineataandMyzuspersicae
∗ Correspondingauthor.
E-mail:pcjimenez@unifesp.br(P.C.Jimenez).
†inmemorian.
(Burgue ˜no-Tapiaetal.,2008)andseveralpharmacological activ-ities,suchaslaxativeandanti-parasitic(Rubioetal.,1997).The studybyGarcíaetal.(1995)evaluatedtheeffectsof1on intesti-nalcontractilityofsmoothmuscleofratsand showedthat this quinoneseemstoincreasetheavailabilityofintracellularCa+2. Con-versely,DelaPe ˜naetal.(2001)disclosed1asaninhibitorofplatelet aggregationandsuggestedthatthisfeaturewasassociatedtothe impairmentinCa2+mobilizationofinternalstores.Furthermore,
1wasconsideredtobeahitina96druginvitroscreendirected againstneuroblastomacells,atumorthatremainsincurableinover 60%ofcases(Gheeyaetal.,2009).Morerecently,Sánchez-Torres etal.(2010)evaluatedthecytotoxicpropertiesofperezoneonK562 cellsanddemonstratedthattheapoptoticeffectsmaybedependent orindependentofcaspasesactivation,dependingonconcentration. The present study describes the isolation of 1, along with otherknowncompounds2–4,fromtheCaribbeangorgonian
Pseu-dopterogorgia rigida and the respective cytotoxic activity on a
paneloftumorcelllines.Additionally,weusedHL-60cells(acute promyelocyticleukemia) tocharacterizetherespectivemodeof
http://dx.doi.org/10.1016/j.bjp.2015.07.020
action,whichlargelyinvolvesthegenerationofreactiveoxygen species.
Materialsandmethods
Generalprocedures
High-performanceliquidchromatographywascarriedoutusing a semipreparative normal phase (silica gel) Dynamax column (15cmlength,i.d.10mm),aWatersM6000pumpandaWaters R401 differentialrefractometer. NMR spectrawere recorded in CDCl3solutiononaVarianUnity500MHzspectrometer.IRspectra wererecordedonaPerkin-Elmermodel1600(FTIR)spectrometer. MassmeasurementswereobtainedonaHP5989Aspectrometer. Vacuumliquidchromatography(VLC)wasperformedwithsilica gelforthinlayerchromatography.Isolationprocedureswere mon-itoredusingthin-layerchromatography(TLC)onpre-coatedsilica gelplates(Merck,Kieselgel60F-254)throughUVinspectionand H2SO4/heat.
Collectionandidentificationofbiologicalmaterial
Fourteen colonies of thegorgonian werecollected by scuba divingat15–20mdepthat SweatingCays,Bahamas, duringan expeditiononboardtheresearchvesselSewardJohnsonin June 1996. The colonies were labeledand air-driedfor 2h. A small amountofeachcolonywasextractedwithCH2Cl2,andtheextracts comparedbyTLCanalysiswerefurtherclassifiedasasinglespecies,
P.rigida,asidentifiedbyDr.FrederickM.Bayer(NationalMuseum
ofNaturalHistory,SmithsonianInstitution,andavoucher speci-men(ATPH/MO/57)hasbeendepositedattheHerbariumofthe DepartmentofPharmacognosyandChemistryofNaturalProducts, UniversityofAthens.
Extracts,fractionsandpurecompoundsfromP.rigida
Gorgonian tissues (300ml, about 25–30g of dry weight) were cut in to small pieces and extracted with a mixture of MeOH–CH2Cl2,1:1(once),andpureCH2Cl2(twice).Theextracts werecombinedand evaporatedunder reducedpressure afford-ingabrownishgum(12g).Thecrudeextractwasfractionatedby vacuumliquidsilicagelchromatography(VLC),employinga gra-dientrangingfrom0to100% ofEtOAcin (CH3)3CCH2CH(CH3)2 (TMP: 2,2,4-trimethylpentane) and MeOH toyield eleven frac-tions (F1–F11). Fraction 5 (2.67g) was purified by successive re-crystallizations using mixtures of hexane and ethyl acetate, yieldingpureperezone(1)(0.64g)asintenseyellowcrystalwith mp102◦C.Fraction7(1.56g)and11(39mg)werefurtherpurified bynormalphasesemi-preparativeHPLCusinghexaneorTMPand ethylacetatemixturestogive,respectively,compound2(10.7mg) andcompounds3(8mg)and4(9mg).
Compound 1 (perezone; (R
)-3-hydroxy-5-methyl-2-(6-methylhept-5-en-2-yl)cyclohexa-2,5-diene-1,4-dione).Mp102◦C, 1HNMR(CDCl
3,300MHz):␦1.19(3H,d,J=7.0Hz),1.53(3H,sl), 1.57(1H,m),1.65(3H,d, J=1.8Hz),1.82(2H,m),1.90(1H,m), 2.06(3H,d,J=1.8Hz),3.05(1H,m),5.07(1H,mt,J=7.2Hz),6.48 (1H,q,J=1.8Hz),7.00(1H,s);13CNMR(CDCl
3,75MHz):␦14.6 (CH3), 17.5(CH3), 18.1(CH3),25.6(CH3), 26.6(CH2), 29.2(CH),
34.0(CH2),124.5(CH),124.5(C),131.3(C),135.5(CH),140.4(C), 150.9(C),184.2(C),187.3(C).
Compound 2 (triacetyl perezone; (R
)-6-methyl-3-(6-methylhept-5-en-2-yl)benzene-1,2,4-triyl triacetate). Colorless oil, 1HNMR (CDCl
3,300MHz):1.20(3H,d, J=7.1Hz),1.54(3H, sl),1.59(2H, m),1.64(3H, d,J=1Hz), 1.87(2H,dd,J=15.0Hz; J=7.5Hz),2.14(3H,d, J=0.7Hz),2.29(3H, s),2.29(3H, s),2.28 (3H,s),2.82(1H,ddq,J=7.2Hz;J=7.2Hz;J=7.2Hz),5.04(1H,tdq, J=7.1Hz;J=1.4Hz,J=1.4Hz),6.82(1H,d,0.7Hz);13CNMR(CDCl
3, 75MHz): ␦ 16.0 (CH3), 17.5(CH3), 18.8(CH3), 20.2 (CH3), 20.3 (CH3),21.0 (CH3), 25.6(CH3), 26.2(CH2),30.9 (CH),35.2(CH2), 124.0(CH), 129.5(C),129.9(C),131.7(C),139.2 (C),141.4(C), 146.3(C),167.6(C),167.8(C),168.9(C).EI-MSm/z376(M+).
Compound 3 (2-((2R
)-5-hydroxy-6-methylhept-6-en-2-yl)-5-methylbenzene-1,4-diol). Yellowish oil. IV (film, CH2Cl2) max 3339, 2958, 1650, 1423, 1191 e 1006cm−1; 1H NMR (CDCl
3, 300MHz):␦1.20(3H,d,J=7.0Hz),1.26(1H,m),1.48–1.59(3H,m), 1.66(3H,s),2.16(3H,s),2.76(1H,sl),3.13(1H,m),4.14(1H,m), 4.81(1H,s),4.94(1H,s),6.58(1H,s)e6.60(1H,s);13CNMR(CDCl
3, 75MHz):␦15.5(CH3),17.6(CH3),21.1(CH3),30.8(CH),31.9(CH2), 34.8(CH2),76.8(CH),110.9(CH2),113.0(CH),118.4(CH),122.1(C), 131.5(C),146.8(C),147.6(C)e147.8(C);HR-ESIMS(positivemode) m/z273.1467[M+Na+](calcdforC
15H21O3,272.1388).
Compound 4 (2-((2R
)-5-hydroxy-6-methylhept-6-en-2-yl)-5-methylbenzene-1,4-diol). Yellowish oil. IV (film, CH2Cl2) max 3334, 2956, 1651, 1442, 12195 e1001cm−1; 1H NMR (CDCl
3, 300MHz):␦1.15(3H,d,J=7.0Hz),1.26(1H,m),1.42–1.55(3H,m), 1.66(3H,s),2.14(3H,s),2.66(1H,sl),3.03(1H,m),4.05(1H,m),4.82 (1H,s),4.93(1H,s)e6.57(2H,s);13CNMR(CDCl
3,75MHz):␦15.6 (CH3),17.9(CH3),21.2(CH3),31.7(CH),32.1(CH2),33.3(CH2),76.2 (CH),110.8(CH2),113.2(CH),118.1(CH),122.1(C),131.5(C),146.7 (C),147.2(C)e147.8(C);HR-ESIMS(positivemode)m/z273.1467 [M+Na+](calcdforC
15H21O3,272.1388).
Cytotoxicityassay
Human tumor cells (HL-60, SF 295, MDA MB435,MDA MB 231andMX1)andthemurinecelllineB16wereobtainedfrom theNationalCancerInstitute,Bethesda,USA.HCT-8celllinewas donatedbytheChildren’sMercyHospital,KansasCity,MO,USA. Non-tumor cells, L929 e J744, were kindly donated by Dr. C.I. Oliveira(CentrodePesquisaGonc¸aloMuniz,Fiocruz,Bahia,Brazil). CellsweremaintainedinRPMI1640mediumsupplementedwith 10%fetalbovineserum,2mMglutamine,100U/mlpenicillin,and 100g/mlstreptomycinat37◦Cwith5%CO
asthepercentageofthecontrolabsorbanceofthereduceddyeat 595nm.Dataarepresentedasmean±S.E.M.TheIC50valuesand their95%confidenceintervals(CI95%)wereobtainedbynon-linear regressionusingtheGraphPadSoftware4.0(IntuitiveSoftwarefor Science).
Phenotypiccharacterizationofperezoneeffects
Flowcytometrywasusedtofurthercharacterizethe cytotox-icity of perezone on the human promyelocitic cell line HL-60. Perezone(1)wastestedat2,4and8M.Doxorubicin0.5Mwas usedasthepositivecontrolandDMSO1%,thedilutionvehicleof allsamples,servedasthenegativecontrol.
Celldensityandviability
HL-60cells wereseededin a24-wellplate(3×105cells/ml), culturedfor24htoinitiatecellgrowthandtreated,aspreviously described.Cellswereharvestedafter3,6,12,24,28and72hof exposure, centrifugedand resuspendedin a 5g/ml propidium iodide(PI)solution(Sigma–Aldrich).After5minincubationinthe dark,fivethousandeventswereacquiredusingtheGuavaEasyCyte Miniflowcytometer(GuavaTechnologies)andtheGuavaExpress Plussoftwareonagatedregiontoexcludedebrisanddoubletsfrom theanalysis.Concentrationofcellsineachtreatmentand mem-braneintegritywereevaluatedandplottedinanXYgraphusing GraphPadSoftware4.0(IntuitiveSoftwareforScience).
CellcycleandDNAfragmentation
HL-60cells wereseededin a24-wellplate(3×105cells/ml), culturedfor24htoinitiatecellgrowthandtreated,aspreviously described.Afterexposurefor3or24h,cellswereharvested, cen-trifugedandresuspendedinasolutioncontaining5g/mlPI,0.1% citrateand0.1%TritonX–100.After30minincubationinthedark, fivethousandeventswereacquiredusingtheGuavaEasyCyteMini flowcytometer(GuavaTechnologies)andtheGuavaExpressPlus softwareonagatedregiontoexcludedebrisanddoubletsfromthe analysis.CellcyclehistogramswereacquiredusingtheFL3channel (excitationatmax488nmandemissionmax620nM)to cap-turetheredfluorescenceemittedfromPI–DNAcomplexes.Thecell cycleprofile(G1/G0–S–G2/M)wasanalyzedinlinearscalewiththe ModFitLT4.0software(VeritySoftwareHouse).Sub-G1cellswere ponderedashavingfragmentedDNA.Thedifferencesbetween neg-ativecontrolandexperimentalgroupsweredeterminedbyanalysis ofvariance (ANOVA)followedbyDunnetpost-testonGraphPad Software4.0(IntuitiveSoftwareforScience).Theminimal signifi-cancelevelwassetatp<0.05.
Analysisofthemechanismsinvolvedinthecytotoxicactivityof
perezone
Furtherexperimentswerecarriedouttoevaluatetheinfluence ofreactiveoxygenspecies(ROS)inthecytotoxicityinducedby1on humanpromyelociticcelllineHL-60.Perezonewastestedat2,4 and8M.-lapachone2Mwasusedasthepositivecontroland DMSO1%,thedilutionvehicleofallsamples,servedasthenegative control.Beforeexposure,cellswerepre-treatedwithl-NAC5M during1h.
MorphologicalanalysiswithMay–Grunwald–Giemsastaining
HL-60cellswereseededina24-wellplate(3×105cells/ml), cul-turedfor24htoinitiatecellgrowthandpre-treatedandtreated, aspreviouslydescribed.Afterexposurefor24htoalltreatment types,cellswereharvested, transferredtocytospinslides,fixed
withmethanolfor10sandstainedwithMay–Grunwald–Giemsa (Bioclin,Brazil).Slideswereexaminedformorphologicalchanges
vialightmicroscopy(Olympus,Tokyo,Japan).
Trypanblueexclusion
HL-60cellswereseededina24-wellplate(3×105cells/ml), cul-turedfor24htoinitiatecellgrowthandpre-treatedandtreated, aspreviouslydescribed.Afterexposurefor24htoalltreatment types,cellswereharvested,dyedwithTrypanblueand differen-tiallycountedforviable(notdyed)ornon-viable(dyed)counted in a Neubauer chamber. The differences between groups were determinedbyanalysisofvariance(ANOVA)followedbyStudent Newman–Keulspost-testonGraphPadSoftware4.0(Intuitive Soft-wareforScience).Theminimalsignificancelevelwassetatp<0.05.
Measurementofreactiveoxygenspeciesgeneration
HL-60cellswereseededina24-wellplate(3×105cells/ml), cul-turedfor24htoinitiatecellgrowthandpre-treatedandtreated,as previouslydescribed.Afterexposurefor1htoalltreatmenttypes, cellswereloadedwithH2-DCF-DAandincubatedfor30mininthe darkat37◦C.Cellswerethenharvested,washed,resuspendedin PBSandimmediatelyanalyzed.Fivethousandeventswereacquired usingtheGuavaEasyCyteMiniflowcytometer(Guava Technolo-gies)andtheGuavaExpressPlussoftwareonagatedregionto excludedebrisanddoubletsfrom theanalysis,usingexcitation andemissionwavelengthsof490and530nm,respectively.The differencesbetweengroupsweredeterminedbyanalysisof vari-ance(ANOVA)followedbyStudentNewman–Keuls post-teston GraphPadSoftware4.0(IntuitiveSoftwareforScience).The mini-malsignificancelevelwassetatp<0.05.
Results
Bioassay-guidedfractionationofP.rigida
TheCH2Cl2/MeOHextractofP.rigidaexhibitedastrong cyto-toxicitywithIC50valuesrangingfrom8.8g/mlinSF-295cellsto 43.2g/mlinMDA-MB-435cells(Table1).Crudeextractwas puri-fiedbyvacuumliquidchromatographyinsilicagelusingagradient ofincreasingpolarityofTMP/EtOAc/MeOH yieldingeleven frac-tions(F1–F11).FractionsF5andF6werethemostactive(Table1) andanalysisbyTLCand1HNMRrevealedthepresenceofanalmost purecompoundthatwhencrystallizedwithhexane/ethylacetate givepureintenseyellowcrystalswithmp102◦C.Spectrometric andspectroscopicdatarevealedthatthiscompoundisperezone(1). FromF8,themajorcomponentwasisolatedbysemi-preparative normalphaseHPLC(EtOAc/hexane),yieldingtriacethylperezone2
asacolorlessoil.IC50forthiscompoundrangedbetween23.1and 49.8Macrossafourhumantumorcelllinepanel(Table2).The mostpolarfraction,F11,wasalsopurifiedbynormalphaseHPLC (EtOAc/TMP),yieldingtwopurebisabolanes3and4.1Hand13C NMRdataofthesecompoundsarealmostidentical,andrevealed themtobeepimersatC-10stereochemistry.TheIC50value calcu-latedfor3variedfrom10.8to59.8M,while4retainednearlyhalf thecytotoxicitystrengthofhisepimer(Table2).
Cytotoxicityofperezone
Table1
IC50(CI95%)(g/ml)oftotalextractand11fractionsobtainedfromchemicalfractionationofaPseudopterorgiarigidaorganicextractontumorcelllinesinculturedetermined
bytheMTTassayover72hincubation.
Sample HL-60 HCT-8 SF-295 MDA-MB435
P.rigidacrudeextract 14.73 9.87–21.98
9.28 8.46–10.18
8.80 6.45–12.02
43.12 29.76–62.47
P.rigidaF1 11.06
n.d.
11.90 n.d.
13.36 9.87–18.08
12.38 7.93–19.33
P.rigidaF2 >100,0 >100,0 >100,0 >100,0
P.rigidaF3 >100,0 >100,0 >100,0 n.d.
P.rigidaF4 65.41
34.55–123.8
37.32 30.82–45.18
29.62 27.96–31.38
79.83 51.25–124.3
P.rigidaF5 4.81
4.27–5.43
4.85 4.31–5.47
2.09 1.86–2.35
8.31 7.72–8.94
P.rigidaF6 1.75
1.58–1.93
4.12 3.67–4.62
2.03 1.32–3.13
8.91 7.14–11.13
P.rigidaF7 13.10
9.42–18.22
14.49 11.86–17.70
7.12 5.878–8.62
33.49 18.56–60.44
P.rigidaF8 2.28
1.81–2.88
8.43 6.66–10.67
20.35 17.11–24.22
5.96 5.20–6.83
P.rigidaF9 7.53
5.61–10.10
28.25 22.99–34.72
32.63 27.88–38.18
20.89 19.38–22.52
P.rigidaF10 41.37
32.87–52.07
47.20 35.06–63.56
59.38 49.25–71.59
33.86 18.38–62.36
P.rigidaF11 15.91
13.59–18.62
39.39 33.15–46.79
21.00 16.71–26.38
13.98 12.38–15.79
n.d.,notdeterminedornotrated.
Table2
IC50(M)and95%confidenceintervalsofcompounds1–4obtainedfromaPseudopterorgiarigidaorganicextractontumorcelllinesinculturedeterminedbytheMTTassay
over72hincubation.TheresultsarepresentedasIC50(M)values,with95%confidenceintervals.-lapachonewasusedaspositivecontrol.
Celline Histotype IC50(95%CI)M
1 2 3 4 -lapachone
HL-60 Promyelocyticleukemia 8.7 7.5–10.2
33.2 n.d.
23.7 4.6–7.7
38.4 30.9–47.8
1.6 1.5–1.8
HCT-8 Colon 17.9
14.5–22.3
23.1 18.8–28.4
59.8 36.5–97.9
89.4 43.1–185.4
0.8 0.7–0.9
SF295 Brain 15.4
13.8–17.1
49.6 34.2–71.7
10.8 8.2–14.2
16.9 12.4–23.2
0.9 0.7–1.1 MDAMB435 Breastcancer 22.0
18.7–25.9
30.6 26.1–35.9
32.4 27.8–37.8
66.9 55.2–81.1
0.2 0.2–0.3 MDAMB231 Breastcancer 13.2
11.3–15.5
n.d. n.d. n.d. n.d.
MX1 Breastcancer 25.2
n.d.
n.d. n.d. n.d. n.d.
B16 Murinemelanoma 24.2
19.1–30.8
n.d. n.d. n.d. n.d.
J774 Murinemacrophage 11.5 9.8–13.5
n.d. n.d. n.d. n.d.
L929 Murinefibroblast 16.32 11.7–22.82
n.d. n.d. n.d. n.d.
n.d.,notdeterminedornotrated.
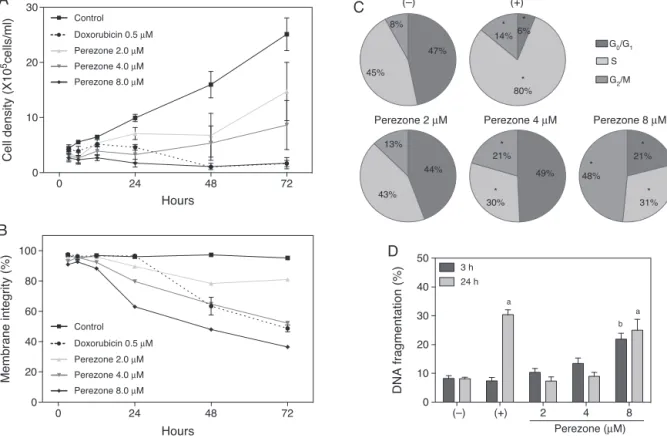
B).Precisely,duringthefirst24h,culturesexposedto1suffered a rapid decline in cell number and membrane integrity, espe-ciallyunderhigherconcentrations.Alterationsinthecellcycleof perezone-treatedcellswith4and8Mweredetectableafter24h, andshowedasignificantandratherstrikingincreaseoftheG2/M
phase,accompaniedbyadecreaseintheSphase(Fig.1C). Inter-nucleosomalfragmentation,however,wasdetectedonlyincells treatedwith1at8M(Fig.1D).
InvolvementofROSgenerationincytotoxicityinducedby
perezone
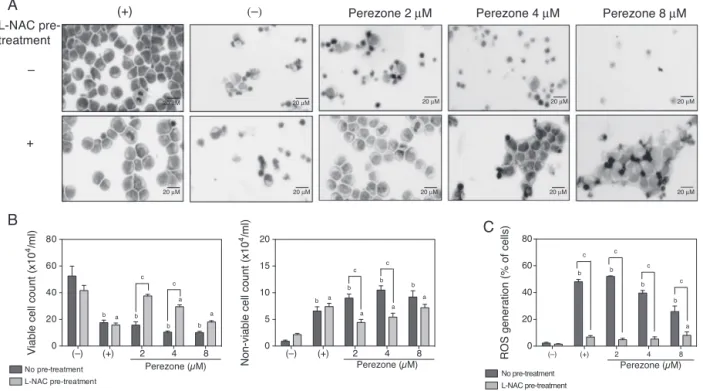
Interestingly, cytotoxic activity of 1 on HL-60 cells proved stronger aftera shorter exposuretime, as IC50 valueincreased a3.5-foldbetween24 and72h. Nevertheless,whenHL-60cells werepre-incubated withl-NAC,those became lesssensitiveto perezone-induced cytotoxicity,as IC50 increased about8 times
after 24htreatment and, after 72h, toxicity was undetectable (Table3).
SubstantialmorphologicalchangeswereobservedinHL-60cells treatedwith1or-lapachone,thelaterconsidered,herein,asthe positivecontrol(Fig.2A).In-lapachone-treatedcells,vacuolesin thecytoplasmcouldbeobserved,along withfurther character-isticsofapoptosis,suchasreducedvolume,membraneblebbing and nuclearfragmentation.Additionally, aconsiderablenumber cellsresemblingnecrosis,werealsoobservedafter24htreatment. Perezone-treatedcellsshowedvolumereductionandsomecellular aggregation.LittleDNAfragmentationandsomemembrane rup-tureweredetected.Moreover,picnoticcellswerewidespreadin slides.Suchcharacteristicsseamedtoretainconcentration depen-dency.Cellspre-treatedwithl-NACpriortoexposureto1showed fewersignsoftoxicity,especiallythoserelatedtoapoptosis,atall testedconcentrations.
8 4 2 (+) (–) 0 10 20 30 40 50
a
a b
Perezone (µM)
DNA fragmentation (%)
3 h 24 h
47%
45% 8%
(–)
44%
43% 13%
Perezone 2 µM
(+)
* 80% * 14%
* 6%
Perezone 8 µM
* 21% *
48% * 31% 49%
Perezone 4 µM
* 30%
* 21%
G0/G1
G2/M
S
D
72 48
24 0
0 20 40 60 80 100
Hours
Membrane integrity (%)
Doxorubicin 0.5 µM Control
Perezone 2.0 µM Perezone 4.0 µM Perezone 8.0 µM
B
72 48
24 0
0 10 20 30
Hours
Cell density
(X10
5cells/ml)
A
C
Doxorubicin 0.5 µM Control
Perezone 2.0 µM Perezone 4.0 µM Perezone 8.0 µM
Fig.1.Characterizationofperezone(1)cytotoxicityonHL-60cells.(A)Depictstheeffectsoncellgrowthafter3,6,12,24,48and72hexposure;(B)theeffectsonmembrane
integrityafter3,6,12,24,48and72h;(C)theeffectsoncellcycleafter24hexposure;and(D)theeffectsoninternucleossomalfragmentationafter3and24hexposure.
CellswereeithertreatedwithDMSO1%(−),0.5Mdoxorubicin(+),orwith1at2,4or8M,andanalyzedbyflowcytometryafterstainingwithPI.Dataarepresentedas
meanvalues±standarderrorofthemean(S.E.M.)fromthreeindependentexperiments,eachconductedintriplicate.Fivethousandeventswereacquiredforeachreplicate
andcomparedwiththerespectivecontrolsbyANOVAandfollowedbyDunnett’scomparisontest.In(C),*indicatesstatisticallysignificantlydifferentfrom(−)(p<0.05).In
(D),a,indicatesstatisticallysignificantlydifferentfrom(−)at24h(p<0.05);andb,indicatesstatisticallysignificantlydifferentfrom(−)at3h(p<0.05).
Table3
CytotoxicityofperezoneevaluatedbytheMTTassayinHL-60cellsafter24or
72hexposure,pre-treatedornotpre-treatedfor1hwithl-NAC.Theresultsare
presentedasIC50(M)values,withconfidenceinterval95%.
Treatment 24h 72h
Perezone 2.45 2.01–2.98 8.747.46–10.18 Perezone+NAC 20.3313.29–30.6 >25
increased the number of non-viable ones in a concentration-dependentmanner(Fig.2B). Pre-incubationwithl-NACfor 1h waseffectiveinlesseningperezone-inducedcytotoxicity,butnot sufficient to reverse the effects prompted especially by higher concentrationsonneitherviable andnon-viablecellcount.It is noteworthythatthepresentdatashowedareductioninthe num-berof viable cellsin control groups whenincubated onlywith l-NAC,whichindicatesthatthisantioxidantitselfhasamild cyto-toxicity.
Intracellular reactive oxygen species (ROS) accumulation was monitored using 2,7-dichlorodihydrofluorescein diacetate (H2-DCF-DA),whichisconvertedtothehighlyfluorescent dichlo-rofluorescein(DCF)inthepresenceofintracellularROS.Perezone (1)showedtobeastrongproducerofintracellularROS,andthis effectcouldbeperceivedaftermerely1hexposure(Fig.2C). Pre-incubationofcellswithl-NAC,asexpected,weakenedmostofthis effect.
Discussion
Marinesecondarymetabolitescontaininginteresting biomedi-calpropertieshavebeenisolatedfrommanyorganisms,including
bacteria, algae, sponges, bryozoans, mollusks, ascidians, and cnidarians(Costa-Lotufoetal.,2009).Fromachemicalstandpoint, withinthe cnidarians, thegreatest attentions in this groupare sessileand softbody organisms,suchascorals,gorgonians and zoanthids.MarineGorgonaceaOctocoralsareknowntobearich sourceofbiologicallyactiveterpenoids,whichareanimportant partinecologicalrelationships(MartinsandEpifanio,1998). Sev-eralstudiescarriedoutwithpseudopterosin,aditerpeneglycoside isolatedfromPseudopterogorgiaelisabethaespecies,demonstrated apotentanti-inflammatoryactivity(Ataetal.,2003;Mayeretal., 1998).
TheCaribbeangorgonianP.rigida(Bielschowsky,1929)belongs tothephylumCnidaria,classAnthozoaanditisknownfor produc-inganumberofsesquiterpenes,ofwhichmostarequinonesand hydroquinones(McEnroeand Fenical,1978;Freyer etal.,1997; D’Armaset al., 2000).Some of thesecompounds seem to play importantecologicalrolesinthehostorganism,suchasdefense against predation. In fact, curcuphenol and curcuhydroquinone proved to be responsible for the feeding deterrent activity of thecrudeextractobtainedfromP.rigidaagainstgeneralistfishes (Harvelletal.,1988).Inplants,bisabolanesarealsoallelopatic com-poundswithherbicidalandantifeedantactivities,hencerecruiting agrochemicalinterests(Burgue ˜no-Tapiaetal.,2008).
Perezone(1)hasbeenpreviouslyreportedinextractsofP.rigida
(Georganteaetal.,2013),besidesbeingisolatedfromthedryroots
ofPereziaspp.(Enrıquezetal.,1980).Itsspectraldataarealso
L-NAC pre-treatment
(+) (–)
–
+
Perezone 2 µM Perezone 4 µM Perezone 8 µM
8 4 2 (+) (–) 0 20 40 60 80
c
Perezone (µM)
Viable cell count (x10
4/ml)
c
a a
a b
b b
b
8 4 2 (+) (–) 0 5 10 15 20
a
a
a b
b
b b
a
Perezone (µM)
Non-viable cell count (x10
4/ml)
c c
8 4 2 (+) (–) 0 20 40 60 80
Perezone (µM)
c
b
a b b b
c c c
ROS generation (% of cells)
B
C
A
20 µM
20 µM
20 µM
20 µM
20 µM
20 µM
20 µM
20 µM
20 µM
20 µM
No pre-treatment L-NAC pre-treatment
No pre-treatment L-NAC pre-treatment
Fig.2.Influenceofreactiveoxygenspecies(ROS)onthecytotoxicityinducedbyperezone(1)onHL-60cellswithorwithout1hpre-treatmentwith5Ml-NAC5.(A)
DepictsrepresentativestructuraleffectsoncellmorphologybyMay–Grümwald–Giemsastainingafter24hexposure;(B)theeffectsoncellviabilitybyexclusionoftrypan
bluedyeafter24hexposure;and(C)theeffectsonROSgenerationbyH2-DCF-DAstainingandflowcytometryanalysisafter1hexposure.Cellswereeithertreatedwith
DMSO1%(−),2M-lapachone(+),orwith1at2,4or8M.Dataarepresentedasmeanvalues±standarderrorofthemean(S.E.M.)fromthreeindependentexperiments,
eachconductedintriplicate,andcomparedbyANOVAfollowedbytheStudentNewman–Keulstest.a,indicatesstatisticallysignificantlydifferentfrom(−)withl-NAC
pre-treatment(p<0.05);b,indicatesstatisticallysignificantlydifferentfrom(−)withoutl-NACpre-treatment(p<0.05);andc,indicatesstatisticallysignificantlydifferent
fromtheirrespectivetreatmentcounterpart(p<0.05).
fromthisspecieandtheNMRdataarealsoaccordingtothe litera-ture(Georganteaetal.,2014).
AnaturalproductscreeningcarriedoutbytheNationalCancer Institute(NCI)hasidentifiedthatquinonesareanimportant phar-macological elementwithcytotoxic activity(Pérez-Sacauet al., 2007).Accordingly, 1 showed tobe cytotoxicin a four-human tumorcelllinepanel.Furthermore,bisabolanes2–4alsoshowed thesamekindofactivity,howevertoalesserpotency(Table2). Perezone(1)wasnotselectivebetweentumorandnon-tumorcells, and itscytotoxicityseemedtoberelated toapoptosisinducing propertiesinleukemiacells.Thesefindingsareinagreementwith apreviousstudyfromSánchez-Torresetal.(2010)showingthat
1iscytotoxictoK562leukemiccellsandcancausemitochondrial depolarizationinaconcentrationdependentmanner.
Quinoidalstructuresarewidespreadinnatureandarehighly redox active molecules. Such reactivity leads to the produc-tion of ROS (reactive oxygen species), a normal mitochondrial metabolicby-product, which, in excess, triggers cellular oxida-tivestressthroughtheoxidationofmacromolecules(Boltonetal., 2000).These proprieties, nonetheless, can be explored for the developmentofnewdrugsincancertherapy.ProductionofROS is involved in induction of apoptosis and this death pathway is considered the most appropriate when it comes to provok-ing tumorcells death (Bironaite et al., 2004).One ofthe main features responsible for quinone cytotoxicity is the alkylation of cellular nucleophiles, suchas DNA, lipids and proteins, and therefore,theirabilitytogenerateROS.Theoneelectron reduc-tionfromaquinonecompoundproducesasemi-quinoneradical, which,inturn,generatesROSwhilealteringmitochondrial trans-membrane potential. This alteration can interfere in some cell signsassociatedwithdeathpathways(Tudoretal.,2003). Pere-zone(1)hasbeenshowntobeboth, anelectron-donor andan
electron-acceptor, and theseoxido-reductionproprietiesinhibit mitochondrialelectrontransportat50M(CarabezandSandoval, 1988).
Glutathioneisanacutefreeradicalneutralizerduetoitsrole asasubstratetointracellularROSscavengingenzymes(Circuand Aw,2008).l-NACisaprecursorofglutathioneand,therefore,an importantantioxidant.Thus,l-NAChasbeenwidelyusedastoolfor investigatingtheinvolvementofROSincytotoxicity(Sun,2010). Perezone (1) is a potent inducer of intracellular ROS in HL-60 cells,andthiseffectcanbeobservedafteraslittleas1hexposure (Fig.2C).Pre-treatment withl-NAC,ontheotherhand,showed competencetovirtuallyeliminatemeasurableROSinductionby1
and-lapachone.Actually,l-NACdesensitizedHL-60to perezone-inducedcytotoxicity,andIC50increasedatleast8-foldafter24h exposure(Table3).Theprotective effect deliveredby l-NACis evidencethatthemechanismofperezone-inducedcytotoxicityis partiallyassociatedtogenerationofROSandaconsequent induc-tionofoxidativestress.
Otherquinones,suchasthatisolatedfromtherootsofCordia
leucocephala,alsoinduceapoptosisandnecrosisbyamechanism
involvingoxidativestress.Thisnaphthoquinone,rapidlyinduced ROS-relateddeathinHL-60cellsafteronly3hexposure( Marinho-Filho et al., 2010). Studies also suggest that several cytotoxic agentsappliedclinicallyincancerchemotherapy,likedoxorubicin, bleomycinandcisplatin,inducestressthroughROSgenerationin tumorcells(Tiligadaetal.,2002).
Conclusion
Perezone(1),isolatedhereinfromthebisabolane-richtropical gorgonianP.rigida,isacytotoxicquinonewithlowselectivitythat inducescellsdeathtroughamechanismthatisassociated,though incompletely,withtheexcessivegenerationofintracellular reac-tiveoxygenspeciesandconsequentinductionofoxidativestress.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Authorcontribution
CMRR,LSP,JWA,ALVandRAEconductedtheextract fraction-ation,naturalproductisolationandstructureelucidationefforts, includingtheMS andNMR studies.PAA, PCJ,DVW,JDBMF, AJA andEGFconductedbiologicalassayswithfractionsandpure com-pounds, including cytotoxicity,flow cytometry and microscopy analysis.PCJ,ALV,CMRR,LVCLandRAEdevelopedtheprojectand interpretedtheresults.Allauthorscontributedtothewriting.
Acknowledgements
WethankDr.FrederickBayer,NationalMuseumofNatural His-torySmithsonianInstitution,forthegorgonianidentification,and thestaffattheMassSpectrometryCenter,Universityof California-Riverside,forprovidingHRMS.WealsothankDr.WilliamFenical, fromScrippsInstitutionofOceanography,UniversityofCalifornia SanDiego,forstructuralsupportduringthecollectionexpedition andplentifulscientificdiscussions.Thisworkwassupportedby grantsfromtheUnitedStatesNationalScienceFoundation(NSF) andtheBrazilianfundingagencyConselhoNacionalde Desenvolvi-mentoCientíficoeTecnológico(CNPq).
References
Asche,C.,2005.Antitumorquinones.MiniRev.Med.Chem.5,449–467.
Ata,A.,Kerr,R.G.,Moyab,C.E.,Jacobs,R.S.,2003.Identificationofanti-inflammatory
diterpenesfromthemarinegorgonianPseudopterogorgiaelisabethae. Tetrahe-dron59,4215–4222.
Bironaite,D.,Kalvelyte,A.V.,Imbrasaite,A.,Stulpinas,A.,2004.Theintracellular
antioxidantbalanceofHL-60cellsanditsimplicationintheapoptosisinduced byquinoidalcompounds.Biologia1,48–51.
Bolton,J.L.,Trush,M.A.,Penning,T.M.,Dryhurst,G.,Monks,T.J.,2000.Roleof
quinonesintoxicology.Chem.Res.Toxicol.13,135–160.
Burgue ˜no-Tapia,E., Castillo, L.,Gonzalez-Coloma, A., Joseph-Nathan, P., 2008.
Antifeedantandphytotoxicactivityofthesesquiterpenep-benzoquinone pere-zoneandsomeofitsderivatives.J.Chem.Ecol.34,766–771.
Carabez,A.,Sandoval,F.,1988.Theactionofthesesquiterpenicbenzoquinone
pere-zone,onelectrontransportinbiologicalmembranes.Arch.Biochem.Biophys. 260,293–300.
Circu, M.L., Aw, T.Y., 2008. Glutathione and apoptosis. Free Radic. Res. 42,
689–706.
Costa-Lotufo,L.V.,Wilke,D.V.,Jimenez,P.C.,Epifanio,R.A.,2009.Organismos
mar-inhoscomofontedenovosfármacos:histo´rico&perspectivas.Quim.Nova32, 703–716.
D’Armas,H.T.,Mootoo,B.S.,Reynolds,W.F.,2000.Anunusualsesquiterpene
deriva-tivefromtheCaribbeangorgonianPseudopterogorgiarigida.J.Nat.Prod.63, 1593–1595.
Enrıquez,R.,Ortega,J.,Lozoya,X.,1980.ActivecomponentsinPereziaroots.J.
Ethnopharmacol.2,389–393.
DelaPe ˜na,A.,Izaguirre,R.,Ba ˜nos,G.,Viveros,M.,Enriquez,R.G.,Fernández-G,
J.M.,2001.Effectofperezone,aminoperezoneandtheircorresponding
iso-mersisoperezoneandisoaminoperezoneuponinvitroplateletaggregation. Phytomedicine8,465–468.
Freyer,A.J.,Patil,A.D.,Kilmer,L.,Zuber,G.,Meyer,C.,Johnson,R.K.,1997.Rigidone,
asesquiterpeneo-quinonefromthegorgonianPseudopterogorgiarigida.J.Nat. Prod.60,309–311.
García,X.,Alcántara-Sarabia,G.,Cartas-Heredia,L.,Gijón,E.,1995.Actionsof
pere-zoneonratsmoothmuscle.Gen.Pharmacol.26,1741–1745.
Georgantea,P.,Ioannou,E.,Vagias,C.,Roussis,V.,2014.Bisabolaneandchamigrane
sesquiterpenesfromthesoftcoralPseudopterogorgiarigida.Phytochemistry. Lett.8,86–91.
Georgantea,P.,Ioannou,E.,Vagias,C.,Roussis,V.,2013.Perezoperezoneand
cur-cuperezone:bisabolanedimersfromthesoftcoralPseudopterogorgiarigida. TetrahedronLett.54,6920–6922.
Gheeya,J.S.,Chen,Q.R.,Benjamin,C.D.,Cheuk,A.T.,Tsang,P.,Chung,J.Y.,Metaferia,
B.B.,Badgett,T.C.,Johansson,P.,Wei,J.S.,Hewitt,S.M.,Khan,J.,2009.Screening
apanelofdrugswithdiversemechanismsofactionyieldspotentialtherapeutic agentsagainstneuroblastoma.CancerBiol.Ther.8,2386–2395.
Harvell,C.D.,Fenical,W.,Greene,C.H.,1988.Chemicalandstructuraldefensesof
Caribbeangorgonians(Pseudopterogorgiaspp).1.Developmentofaninsitu feedingassay.Mar.Ecol.Prog.Ser.49,287–294.
Joseph-Nathan,P.,González,M.P.,Rodríguez,V.M.,1972.TerpenoidsofPerezia
hebe-clada.Phytochemistry11,1803–1808.
Joseph-Nathan,P.,Mendoza,V.,García,E.,1977.Thereactionmechanismofthe
perezonepipitzoltransformation.Tetrahedron33,1573–1576.
Marinho-Filho,J.D.,Bezerra,D.P.,Araújo,A.J.,Montenegro,R.C.,Pessoa,C.,Diniz,
J.C.,Viana,F.A.,Pessoa,O.D.,Silveira,E.R.,Moraes,M.O.,Costa-Lotufo,L.V.,2010.
Oxidativestressinductionby(+)-cordiaquinoneJtriggersboth mitochondria-dependentapoptosisandnecrosisinleukemiacells.Chem.Biol.Interact.183, 369–379.
Martins,D.L.,Epifanio,R.A.,1998.AnewGermacraneSesquiterpenefromthe
BrazilianEndemicGorgonianPhyllogorgiadilatataEsper.J.Braz.Chem.Soc.9, 586–590.
Mayer,A.M.S.,Jacobson,P.B.,Fenical,W.,Jacob,R.S.,Glasei,K.B.,1998.
Pharmacolog-icalcharacterizationofthepseudopterosins:novelanti-inflammatorynatural productsisolatedfromtheCaribbeansoftcoral,Pseudopterogorgiaelisabethae. LifeSci.62,401–407.
McEnroe,F.J.,Fenical,W.,1978.Structuresandsynthesisofsomenewantibacterial
sesquiterpenoidsfromthegorgoniancoralPseudopterogorgiarigida. Tetrahe-dron125,1661–1664.
Ozben,T.,2007.Oxidativestressandapoptosis:impactoncancertherapy.J.Pharm.
Sci.96,2181–2196.
Pérez-Sacau, E., Díaz-Pe ˜nha, R.G., Estévez-Braun, A.E., Ravelo, A.G.,
García-Castellano,J.M.,Pardo,L.,Campillo,M.,2007.synthesisandpharmacophore
modelingofnaphthoquinone derivativeswithcytotoxicactivity inhuman promyelocyticleukemiaHL-60cellline.J.Med.Chem.50,696–706.
Rubio,M.,Ramirez,G.G.,Jimenez,F.G.,Salcedo,R.,Belmont,M.A.,1997.About
perezonederivatives,atheoreticalapproach.J.Mol.Struct.(Theochem)397, 239–248.
Sánchez-Torres, L.E., Torres-Martinez, L.A., Godínez-Victoria, M., Omar, J.M.,
Velasco-Bejarano, B., 2010. Perezone and its isomer isoperezone induce
caspase-dependentandcaspase-independent celldeath. Phytomedicine17, 614–620.
Sun,S-Y.,2010.N-acetylcysteine,reactiveoxygenspeciesandbeyond.CancerBiol.
Ther.9,109–110.
Tiligada,E.,Miligkos,V.,Delitheos,A.,2002.Cross-talkbetweencellularstress,cell
cycleandanticanceragents:mechanisticaspects.Curr.Med.Chem.Anti-cancer Agents2,553–566.
Tudor,G.,Gutierrez,P.,Aguilera-Gutierrez,A.,Sausville,E.A.,2003.Cytotoxicity
andapoptosisofbenzoquinones:redoxcycling,cytochromecrelease,andBAD proteinexpression.Biochem.Pharmacol.65,1061–1075.
Vásquez,D.,Rodríguez,J.A.,Theoduloz,C.,Calderon,P.B.,Valderrama,J.A.,2010.
Studiesonquinones.Part46.Synthesisandinvitroantitumorevaluationof aminopyrimidoisoquinolinequinones.Eur.J.Med.Chem.45,5234–5242.