ContentslistsavailableatSciVerseScienceDirect
Behavioural
Brain
Research
jo u r n al ho me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / b b r
Research
report
Social
modulation
of
brain
monoamine
levels
in
zebrafish
Magda
C.
Teles
a,b,
S.
Josefin
Dahlbom
c,
Svante
Winberg
c,
Rui
F.
Oliveira
a,b,∗aISPA-InstitutoUniversitário,UnidadedeInvestigac¸ãoemEco-Etologia,RuaJardimdoTabaco34,1149-041,Lisboa,Portugal bChampalimaudNeuroscienceProgramme,InstitutoGulbenkiandeCiência,RuadaQuintaGrande6,2780-156,Oeiras,Portugal cDepartmentofNeuroscience,UppsalaUniversity,Box593,Husargatan3,75124,Uppsala,Sweden
h
i
g
h
l
i
g
h
t
s
•Zebrafishwereexposedtodifferentfightingexperiences:winning,losingandmirror-fighting.
•Winnersshowhigherserotonergicanddopaminergicactivityinthetelencephalon.
•Losersshowhigherserotonergicintheoptictectum.
•Nosignificantchangesinmonoamineactivitywereobservedinmirrorfighters.
•Monoaminesaredifferentiallyregulatedbysocialinteractionsindifferentbrainregions.
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received21May2013
Receivedinrevisedform27June2013 Accepted1July2013
Available online 11 July 2013 Keywords: Aggressivebehaviour Behaviouralplasticity Neuromodulators Serotonin Dopamine Zebrafish
a
b
s
t
r
a
c
t
Insocialspeciesanimalstendtoadjusttheirsocialbehaviouraccordingtotheavailablesocial informa-tioninthegroup,inordertooptimizeandimprovetheironesocialstatus.Thischangingenvironment requiresforrapidandtransientbehaviouralchangesthatreliesprimarilyonbiochemicalswitchingof existingneuralnetworks.Monoaminesandneuropeptidesarethetwomajorcandidatestomediatethese changesinbrainstatesunderlyingsociallybehaviouralflexibility.Inthecurrentstudyweusedzebrafish (Daniorerio)malestostudytheeffectsofacutesocialinteractionsonrapidregionalchangesinbrain levelsofmonoamines(serotoninanddopamine).Abehaviouralparadigmunderwhichmalezebrafish consistentlyexpressfightingbehaviourwasusedtoinvestigatetheeffectsofdifferentsocialexperiences: winningtheinteraction,losingtheinteraction,orfightinganunsolvedinteraction(mirrorimage).We foundthatserotonergicactivityissignificantlyhigherinthetelencephalonofwinnersandintheoptic tectumoflosers,andnosignificantchangeswereobservedinmirrorfighterssuggestingthatserotonergic activityisdifferentiallyregulatedindifferentbrainregionsbysocialinteractions.Dopaminergicactivity itwasalsosignificantlyhigherinthetelencephalonofwinnerswhichmayberepresentativeofsocial reward.Togetherourdatasuggeststhatacutesocialinteractionselicitrapidanddifferentialchangesin serotonergicanddopaminergicactivityacrossdifferentbrainregions.
© 2013 Elsevier B.V. All rights reserved.
1. Introduction
Inordertooptimizethebenefitsofgrouplivingandto
min-imizeits costs,social animalsneed toadjust theexpression of
theirsocialbehaviouraccordingtodailychanges intheirsocial
environment. Thisabilityof anindividualtooptimizeits social
behaviourdependingonavailablesocial information(aka social
competence,[1]),dependsprimarilyonmechanismsthatallowfor
rapidandtransientbehaviouralchanges.Giventhespeedand
lia-bilityofthistypeofbehaviouralflexibility,suchmechanismsare
∗ Correspondingauthorat:ISPA-InstitutoUniversitário,UnidadedeInvestigac¸ão emEco-Etologia,RuaJardimdoTabaco34,1149-041,Lisboa,Portugal.
Fax:+351218860954.
E-mailaddress:ruiol@ispa.pt(R.F.Oliveira).
expectedtorelyonsociallydrivenbiochemicalswitchingof
exist-ingneuralnetworks,ratherthanonstructuralrewiringofneural
circuits[2].Inrecent yearsevidenceaccumulatedshowinghow
neuromodulatorscanchangetheactivityand even the
connec-tivity ofneuralcircuits ina waythat eachstructural circuit,as
representedbyitsconnectome,mayincludemultiplefunctional
circuits, withsomeofthem active andsome otherslatentat a
givenmomentintime[3].Differentneuromodulatoryagentsmay
interactwithspecific circuitsand alter theirfunctional
proper-ties,promotingeitherexcitatoryorinhibitorystates.Monoamines
andneuropeptidesareconsideredthetwomajorclassesof
neu-romodulators,andtheactionofbothonsocialbehaviouraswell
astheirsensitivitytoenvironmentalfactors,havebeenextensively
documented[4,5],whichmakesthemmajorcandidatesto
medi-atechangesinbrainstatesunderlyingsociallydrivenbehavioural
flexibility.
0166-4328/$–seefrontmatter © 2013 Elsevier B.V. All rights reserved. http://dx.doi.org/10.1016/j.bbr.2013.07.012
Monoamineshavebeenimplicatedintheregulationof
moti-vatedbehaviours andamong them therole oftheserotonergic
systemonthecontrolofaggressivemotivationhasbeen
demon-strated both in vertebrate and in invertebrate species [6,7].
Interestinglytheeffectsofserotonin(5-hydroxytryptamine,5-HT)
onaggressivebehaviouraretosomeextentparadoxical.While
sev-eralstudieshavepointedoutthatpharmacologicalmanipulations
thatincrease 5-HTinhibit aggressionin a wide range of
verte-brates,fromfishtohumans[8],otherstudies,incontrast,have
showedincreasedserotonergicactivityinspecificbrain regions
duringtheexpressionofaggressivebehaviour[8–10].Moreover,
the5-HT1Aand5-HT1Breceptorsexertfunctionallyopposingroles
invariousbehaviouralandphysiologicalprocessessuchasappetite,
sexuallibido,motoractivity,andthusitisreasonabletoconsider
thatthisdivergencemayalsobepresentinaggressivebehaviour
[11–13].Therefore, therole of5-HTontheregulation of social
behaviourcannotbeputsimplyintermsofpureinhibitionorpure
facilitationofaggression,butratherasafunctionof
environmen-talcontext.Theeffects ofdopamine(DA)onaggressionarealso
paradoxical.Forexampleinmammals,whileD1andD2dopamine
receptorantagonistsreduceaggression[14],D2receptorsinthe
medialpreopticarea(mPOA)andanteriorhypothalamusfacilitate
affectivedefensebehaviour[15].On theotherhand,the
meso-corticolimbicdopaminesystemhasbeenshowntobeinvolvedin
thepreparation andexecutionofaggressiveacts[16–20].These
neurochemicalstudies linkelevated dopamineand its
metabo-litesinprefrontalcortexandnucleusaccumbensnotonlytothe
initiationofattacksandthreats,butalsotodefensiveand
submis-siveresponsesinreactionofbeingattacked[19,21].Thetransition
betweenbehaviouralstates(e.g.inhibitionorpromotionof
aggres-sivebehaviours) inboth monoaminergic systemsappearstobe
sensitivitytodifferentsocialcontexts,whichmakethese
neuro-modulatorstremendously important in the regulationof social
interactions.
The highdiversity and plasticityof social behaviour among
teleostfishmakesthemexcellentmodelsforcomparativestudies
onthemechanismsofsocialplasticity[22].Inmanyfishspecies
social systems are characterizedby reversibledominance
hier-archies, where animals have to adjust the expression of their
socialbehaviourtotheirperceived socialstatus. Inthese social
systemsrapidchangesinbehaviouraloutputoccur,drivenbythe
assessmentthattheanimaldoesofthesocialinteractionsinwhich
itisinvolved.
Inthis paperwe usedzebrafish(Danio rerio)malestostudy
theeffectsofacutesocialinteractionsonrapidregionalchanges
inbrainlevelsofmonoamines.Zebrafishwerechoseasamodel
species given their increasing use in behavioural neuroscience
researchandtheirflexiblesocialbehaviour.Zebrafishisa
group-livingspeciesthatinnatureformshoals[23]butwhenallowedto
interactinpairs,formdominancehierarchies[24].Inthisspecies
aggressioniscommonlyusedbydominantindividualstogetaccess
tospawningsitesandtoprotecttheirsocialstatusfromcompetitors
[25].Recently,ourgroupdevelopedabehaviouralparadigmunder
whichmalezebrafishconsistentlyexpressfightingbehaviourand
characterizedthestructureofthesefightsinmaledyads[26].Here
thesameparadigmisusedtoinvestigatetheeffectsofdifferent
socialexperiences(i.e.individualsexperiencingavictory,adefeat
orfightinganunsolvedinteraction)onserotoninand dopamine
levelsindifferentbrainregions.
2. Materialsandmethods
2.1. Animalsandhousing
Allsubjectsusedinthisexperimentwereadultwild-type(AB)zebrafishbreed andheldatInstitutoGulbenkiandeCiência(IGC,Oeiras,Portugal).Fishwerekept inarecirculatingsystem(ZebraTec,93Tecniplast),at28◦Cwitha14L:10D
pho-toperiod.Watersystemwasmonitoredfornitrites(<0.2ppm),nitrates(<50ppm) andammonia(0.01–0.1ppm),whilepHandconductivityweremaintainedat7and 700Smrespectively.Fishwerefedtwiceadaywithcommercialfoodflakesinthe morningandArtemiasalinaintheafternoon,exceptonthedayoftheexperiments.
2.2. Experimentaldesign
Inthepresentstudyabehaviouralparadigmpreviouslydevelopedforthe studyofzebrafishaggressivebehaviourwasused[26].Thirty-twoadultmales (8ineachexperimentaltreatment)matchedforstandardlength(mean±SEM: 2.81±0.026cm)andbodymass(mean±SEM:0.350±0.009g)weregroupedin dyads.Therewerethreetypesofdyads:(1)realopponentfight:thefishfought withaconspecific;(2)mirrorfight:thefishfoughtwiththeirownmirrorimage; (3)nofight:thefishhadnoagonisticinteraction(Fig.1).Fromthesethreetypes ofdyads,cameoutfourexperimentalconditions:winningtheinteraction,losing theinteraction,fightinganunsolvedinteraction,orexperiencenointeraction (con-trolgroup).Subjectswerealwaystestedinpairs,inordertogivethemaccessto conspecificodours,whichwouldotherwiseonlybepresentinrealopponentdyads, thereforeavoidingconfoundingeffectsofputativechemicalcues.
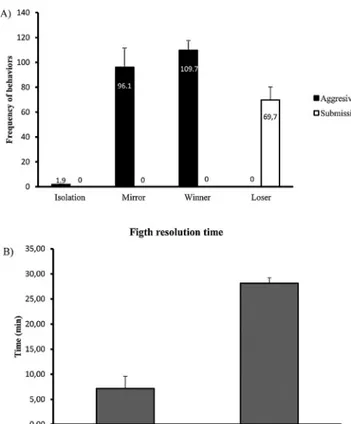
Fig.1.Experimentalprocedure:(A)overnightisolationtoelicitaggression.Eachfishpairwasplacedintheexperimentaltank,andisolatedvisually,butnotchemically,by aremovableopaquePVCpartition;(B)realopponentinteraction,fishfoughtwithaconspecific;(C)mirrorinteraction,fishfoughtwiththeirownmirrorimage(greybars); (D)controlgroup,noagonisticinteractionormirrorstimulation.
Prior to the experiment,each pair was placed in the experimental tank (20cm×14.5cm×12.5cm)wheretheywerekeptovernightinvisualisolationusing aremovableopaquePVCpartition.Previousstudieshadestablishedperiodsofsocial isolationof5days[24]and24h[26]aseffectivetoelicitaggressivebehaviour. How-ever,hereweestablishedthatovernightisolationwassufficienttopromotethe consistentexpressionofaggressivebehaviour.Aftertheisolationperiod,theopaque dividerwasremovedandthefishwereallowedtointeractforaperiodof30min. Behaviouralinteractionswerevideotaped(JVC-EverioSMemory camcorder-GZ-MS215)forsubsequentbehaviouralanalysis(seebelow).
2.3. Sampling
Inordertoavoidmonoaminedegradationduringthebrainmacro-dissectionand tokeepthetimeofsamplingafterthesocialinteractionsashomogeneousaspossible acrossdyads,onlyonefishfromeachdyadwasusedformonoaminequantification. Thesefishweresacrificedimmediatelyaftertheinteractionwithanoverdoseof tricainesolution(MS222,Pharmaq;500–1000mg/L)andthespinalcordsectioned. Thebrainwasmacrodissectedunderastereoscope(Zeiss;Stemi2000)intofive areas:OlfactorybulbandTelencephalon(OB/TL),Optictectum(OT),Diencephalon (DE),Cerebellum(CB),andBrainstem(BS).Immediatelyaftercollectionthebrain tissuewasplacedondryiceandstoredat−80◦Cuntilanalysis.
2.4. Analysisofbrainmonoaminesandmetabolites
Thefrozenmacroareaswerehomogenizedin4%(w/v)ice-coldperchloricacid containing100ng/ml 3,4-dihydroxybenzylamine(DHBA,theinternalstandard) usingaSonifiercelldisruptorB-30(BransonUltrasonics,Danbury,CT,USA)and wereimmediatelyplacedondryice.Subsequently, thehomogenized samples werethawedandcentrifugedat21,000×gfor10minat4◦C.Thesupernatant
wasusedforhighperformanceliquidchromatographywithelectrochemical detec-tion(HPLC-EC),analyzingthemonoaminesdopamine(DA)andserotonin(5-HT, 5-hydroxytryptamine)theDAmetaboliteDOPAC(3,4-dihydroxyphenylaceticacid) andthe5-HTmetabolite5-HIAA(5-hydroxyindoleaceticacid),asdescribedby Overlietal.[10].Inbrief,theHPLC–ECsystemconsistedofasolventdeliveryas systemmodel582(ESA,Bedford,MA,USA),anautoinjectorMidastype830(Spark Holland,Emmen,theNetherlands),areversephasecolumn(Reprosil-PurC18-AQ 3m,100mm×4mmcolumn,Dr.MaischHPLCGmbH,Ammerbuch-Entringen, Germany)keptat40◦CandanESA5200CoulochemIIECdetector(ESA,Bedford, MA,USA)withtwoelectrodesatreducingandoxidizingpotentialsof−40mVand +320mV.Aguardingelectrodewithapotentialof+450mVwasemployedbefore theanalyticalelectrodestooxidizeanycontaminants.Themobilephaseconsisted of75mMsodiumphosphate,1.4mMsodiumoctylsulphateand10MEDTAin deionizedwatercontaining7%acetonitrilebroughttopH3.1withphosphoricacid. Sampleswerequantifiedbycomparisonwithstandardsolutionsofknown con-centrations.TocorrectforrecoveryDHBAwasusedasaninternalstandardusing HPLCsoftwareClarityTM(DataApexLtd.,Prague,CzechRepublic).Theratiosof
5-HIAA/5-HTandDOPAC/DAwerecalculatedandusedasanindexofserotonergic anddopaminergicactivity,respectively.
Fornormalizationofbrainmonoaminelevels,brainproteinweightswere deter-minedwithBicinchoninicacidproteindetermination(Sigma–Aldrich,Sweden) accordingtothemanufacturer’sinstructions.TheassaywasreadonLabsystems multiskan352platereader(Labsystems,ThermoFisherScientific)wavelengthof 570nm.
2.5. Behaviouralobservations
Videorecordingswereanalyzedusingacomputerizedmulti-eventrecorder (ObserverXT,Noldus,Wageningen,TheNetherlands).Thezebrafishethogram[26] wasusedasareferenceandtheobservedbehavioursweredividedintoaggressive (bite,chaseandstrike)andsubmissive(freezeandflee).Aspreviouslydescribedin [26]dyadicmalefightshavetwodistinctphases:thepre-resolutionphasewhere thefightissymmetricandbothfishexhibitthesamerepertoireofbehaviours (dis-play,circle,andbite)andthepost-resolutionphasewhereallagonisticbehaviours areinitiatedbythewinnerwhereastheloseronlydisplayssubmissivebehaviours. Becausewewereonlyinterestedinthedifferentoutputofthefightswhich gener-atedifferentbehaviouralphenotypes(e.g.winnerandloser)weonlyanalyzedthe post-resolutionphase(i.e.thelast5minofthe30mininteraction).Wealso mea-suredthefightresolutiontime(timeforthesocialhierarchytobeestablished)in ordertocomparerealopponentwithmirrorinteractions.
2.6. Statisticalanalysis
StatisticalanalyseswereperformedwiththesoftwareSTATISTICAv.10 (Stat-Soft,Inc.,2011).Parametricstatisticwasusedgiventhatthevariablesmatchthe parametricparameters.Oneloserandonecontrolwereremovedfromtheanalysis, onebecausetheoutputofitsfightwasnotcompletelyclearandthesecondbecause mostofthetimeitwastrappedonthepartition,resultinginasamplesizeof7for losersandcontrolgroups,and8forwinnersandmirrorgroups.Inthebehaviour analyses,oneanimalfromthewinner,loserandmirrorgroupswasremovedfrom theanalysisduetoaproblemwiththevideorecordingswhichmadetheanalysis
impossible.AT-testwasusedtoaccessdifferencesbetweentypesofinteractions (realopponentvsmirror)andfightresolutiontime.Inthemonoaminesanalysis, foursamplesfromtheoptictectumwereexcludedduetoproblemsduringthe sam-plepreparation.Serotonin,dopaminelevelsandtherespectivemetabolites,5HIAA andDOPAC,aswellastheactivityofbothneurotransmittersasmeasuredbythe ratios5-HIAA/5-HTandDOPAC/DA,inbrainmacroareaswerelogtransformedin ordertomeettheassumptionofnormaldistribution.ArepeatedmeasuresANOVA (repeatedfactor:brainmacroareaswith5levels,independentfactor:malestatus with4levels,winner,loser,mirror,control)wasusedtoidentifythemaineffects andtheinteractionbetweenbrainareaandsocialstatusonthedifferentmonoamine measures,followedbyaposthoctestsandplannedcomparisonsofleastsquares meansbetweenthecontrolgroup(isolation)andeachofthedifferentsocialstatus. APCAanalysiswasusedtoreducethenumberofbehaviourvariablesinthereal opponentparadigm.Correlationsbetweenbehaviourandmonoamine concentra-tionswereobtainedwithPearsoncorrelationcoefficients.Alltestsweretwo-tailed andstatisticalsignificancewassetatp<0.05.
2.7. Ethicsstatement
Theanimalexperimentationproceduresusedinthisstudyfollowedthe Asso-ciation fortheStudy ofAnimal Behaviourand theAnimal BehaviourSociety guidelinesforthetreatmentofanimalsinbehaviouralresearchandteachingand wereapprovedbytheinternalEthicsCommitteeoftheGulbenkianInstituteof Sci-enceandbytheNationalVeterinaryAuthority(Direc¸ãoGeraldeAlimentac¸ãoe Veterinária,Portugal;permitnumber8954).
3. Results
3.1. Behaviour
Intherealopponentparadigmallpairsexceptone,developa
cleardominant/subordinaterelationship.Socialhierarchieswere
stableand thebehaviours exclusiveforeachphenotype. During
thepost-resolutionphase a winnernever becamea losernora
loserbecameawinner.Thebehavioursarestereotypedaccording
tosocialstatus,aggressivebehavioursinwinnersandsubmissive
Fig.2. Behaviouralresults.(A)Meannumberofaggressiveactsperformedinthe last5minofthe30minagonisticinteraction;errorbarsrepresentthestandard errorofthemean.(B)Fightresolutiontime,measuredasthetimeneededfora socialhierarchytobeestablishedinthefightingmaledyads(countingfromthefirst bitetothepost-resolutionphase);errorbarsrepresentthestandarderrorofthe mean(t-test:T=−6.39,p<0.0001).
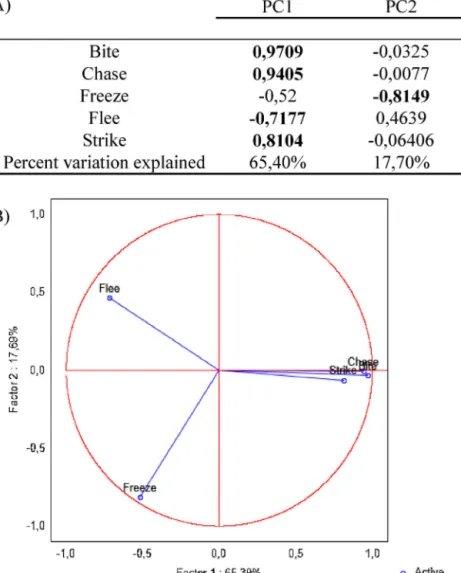
Fig.3. Principalcomponent(PC)analysisofaggressiveandsubmissivebehavioursintherealopponentparadigm.(A)Factorloadingsofthebehaviouralvariablesand varianceexplainedbyeachPC.(B)GraphicrepresentationoftheextractedPC’s:PC1representsaggressivebehaviourandPC2submissivebehaviour,whichcanbefurther dividedinactivesubmission(flee)onthepositivequadrantandpassive(freeze)onthenegativequadrant.
behaviours in losers. On theother hand,in mirror interactions
becausethefightis symmetricalong time theresulting
pheno-typeis not apparent, theynever behave likelosers orwinners,
andaggressivelevelsarekeptconstantduringthewhole
interac-tion(Fig.2).Thisdifferenceisobviousinthefightresolutiontime
(T=−6.39,p<0.0001;Fig.2B)wheremirrorfightersfightfor30min
whereas inthe realopponent interaction thefightis solved in
approximately7min,afterwhichapost-resolutionphaseis
estab-lished.
In order to reduce the number of behavioural variables in
subsequent analyses in the real opponent paradigm, a
Prin-cipal Component Analysis (PCA) was performed. Two factors,
thattogetherexplain83.1%ofthetotalvariance (Fig.3A),were
extractedthat showa clearseparation betweenaggressive and
non-aggressivebehaviours:PC1haspositiveloadingsfor
aggres-sivebehavioursandanegativeloadforsubmissivebehaviour(flee)
andexplains65.4%ofthevariation;PC2haspositiveloadingsfor
submissivebehaviourandnegativeloadingsforalltheaggressive
behaviours(bite,chase,strike) andexplains17.7% ofthe
varia-tion(Fig.3A).PC2allowsthesubsequentdivisionofsubmissive
behaviourintoanactive (flee,positive quadrant)and a passive
(freeze,negativequadrant)style(Fig.3B).Theseresultssupportthe
separationofaggressiveandsubmissivebehaviourinthereal
oppo-nentinteraction.Inthemirrorinteraction,becausethebehavioural
repertoireisrestrictedtotwobehaviours(bite,strike)noPCAwas
performed.
3.2. Brainmonoamines
Concentrationsofserotonin(5-HT),dopamine(DA) andtheir
mainmetabolites(i.e.5-HIAAandDOPAC,respectively)inthe
stud-iedbrainareasaregiveninTable1.
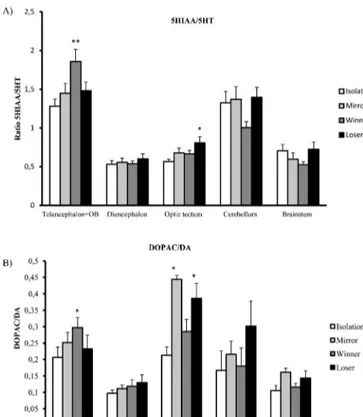
Therewas a treatmentand brain areamain effect for both
5-HT(repeated measuresANOVA; social treatment: F3,25=7.86,
p<0.001;brainarea:F4,100=79.39,p<0.0001,respectively)and
5-HIAA(repeated measuresANOVA;social treatment: F3,24=8.55,
p<0.001;brainarea:F4,96=50.36,p<0.0001).Theposthoc
anal-ysesrevealedthatsocialexperienceincreased5-HTand5-HIAA
levels in animals that foughtreal opponents (W/L)and mirror
imagewhencomparedtocontrolgroup.For serotonin,the
con-centrationwashigherinthediencephalon,followedbyolfactory
bulb/telencephalon,optictectumandbrainstemandthelowest
concentrationwasfoundinthecerebellum.Ontheotherhand,for
themetabolite5-HIAA,olfactorybulb/telencephalonhadthe
high-estconcentration,followedbydiencephalon,optictectum,brain
stemandfinallycerebellum.
ForDAandDOPACtherewasalsoamaineffectfortreatment
Table1
Monoamineandmetabolitesconcentrations(mean±SEM)indifferentbrainareas,anddifferenttreatments.Asterisk(*)inthemeanindicatessignificativedifferenceson specifictreatmentswhencomparedtocontrolgroup(repeatedmeasuresANOVA,*p<0.05).
Brainregion Monoaminesand metabolites
Treatment
Control Mirrorfighter Winner Loser Statistics
Telencepahlon 5-HT 3.83±1.02 6.40±0.85* 6.15±1.6 5.61±0.74 F(12,100)=2.48;p<0.01 5-HIAA 5.26±1.67 9.06±1.66* 9.98±1.64* 9.05±1.28* F(12,96)=1.17;p=0.32 DA 2.08±0.57 2.91±0.54 2.57±0.53 2.28±0.31 F(12,96)=3.11;p<0.001 DOPAC 0.46±0.16 0.78±0.20 0.68±0.08 0.54±0.11 F(12,100)=3.55;p<0.001 Diencephalon 5-HT 8.86±1.43 9.26±0.75 8.19±0.95 10.54±0.92 F(12,100)=2.48;p<0.01 5-HIAA 4.44±0.71 4.99±0.45 4.22±0.33 5.86±0.36* F(12,96)=1.17;p=0.32 DA 6.25±0.72 6.23±0.52 5.01±0.68 6.93±0.65 F(12,96)=3.11;p<0.001 DOPAC 0.53±0.08 0.67±0.06 0.52±0.04 0.83±0.09* F(12,100)=3.55;p<0.001 Optictectum 5-HT 4.19±0.34 4.11±0.36 4.04±0.22 3.55±0.28 F(12,100)=2.48;p<0.01 5-HIAA 2.37±0.25 2.11±0.39 2.69±0.26 2.87±0.26 F(12,96)=1.17;p=0.32 DA 0.92±0.07 1.13±0.17 1.01±0.06 0.83±0.08 F(12,96)=3.11;p<0.001 DOPAC 0.18±0.01 0.41±0.03* 0.28±0.02* 0.33±0.05* F(12,100)=3.55;p<0.001 Cerebellum 5-HT 0.32±0.06 1.72±0.51* 1.47±0.25* 1.08±0.26* F(12,100)=2.48;p<0.01 5-HIAA 0.92±0.49 2.17±0.60* 1.47±0.60* 1.75±0.42* F(12,96)=1.17;p=0.32 DA 0.20±0.02 1.63±0.63* 1.02±0.17* 0.72±0.17* F(12,96)=3.11;p<0.001 DOPAC 0.04±0.01 0.30±0.09* 0.15±0.04* 0.18±0.05* F(12,100)=3.55;p<0.001 Brainstem 5-HT 3.62±0.56 3.39±0.40 6.51±1.18* 4.43±1.43 F(12,100)=2.48;p<0.01 5-HIAA 2.37±0.32 2.26±0.18 3.15±0.36 3.03±0.38 F(12,96)=1.17;p=0.32 DA 2.63±0.41 2.23±0.21 4.23±0.66* 3.21±0.86 F(12,96)=3.11;p<0.001 DOPAC 0.27±0.06 0.35±0.03* 0.46±0.04* 0.41±0.08* F(12,100)=3.55;p<0.001
p<0.01)] and DOPAC levels [F3,25=8.31, p<0.001] in winners,
losers andmirrorfighters suggestingan activationofboth sys-temsinacuteinteractions.DA[F4,96=85.68,p<0.0001]distribution
acrossthebrainwasdistinct,withelevatedconcentrationsinthe diencephalon,thenolfactorybulb/telencephalonandbrainstem, andlastlyoptictectumandcerebellum.ForDOPAC[F4,100=39.09,
p<0.0001]olfactorybulb/telencephalonanddiencephalonexhibit thehighestconcentration,optictectumandbrainstemwereafter andcerebellumshowedthelowest.
Therewasa significantmain effect of brainareabut not of socialstatus intheratiosof both5-HIAA/5-HT(repeated meas-uresANOVA,brainareamaineffect:F4,88=83.38,p<0.0001;social
status main effect: F3,22=1.27, p=0.31) and DOPAC/DA (brain
area main effect: F4,68=28.53, p<0.00001; social status main
effect:F3,17=2.17, p=0.13). Theposthocanalysesrevealedthat
5-HIAA/5-HT ratios were significantly higher in the olfactory bulb/telencephalon,followedbythecerebellum,thenoptictectum andbrainstemandlastlybythediencephalon.DOPAC/DAratios weresignificantlyhigherintheoptictectum,followedbyolfactory bulb/telencephalon,thencerebellum,anddiencephalonandlastly inthebrainstem.Contrastanalysisof5-HIAA/5-HTandDOPAC/DA activityofanareabyareabasisrevealedthat5-HIAA/5-HTlevels weresignificantlyhigherinwinners’olfactorybulb/telencephalon (F=18.43, p<0.001), and losers optic tectum (F=9.92, p<0.01;
Fig.4A). Regardingthe DOPAC/DA,winners had higheractivity
levelsintheolfactorybulb/telencephalon(F=6.32,p<0.05),and
mirrorandlosersintheoptictectum(F=12.05,p<0.01andF=6.67,
p<0.05respectively;Fig.4B).Therewasalsoamarginally
non-significanttendencyforloserstohaveincreasedDOPAC/DAratios
inthecerebellum(F=3.96,p=0.06).
3.3. Relationshipbetweenmonoaminesandbehaviour
Correlationsanalysesbetweenbehaviourand monoaminein
differentbrainareasrevealedthatintherealopponentparadigm
therewerenegativecorrelationsbetween5-HIAAlevels(r=−0.70,
N=12,p<0.05)andDOPAClevelsinthediencephalon(r=−0.58,
N=13,p<0.05)andaggressivebehaviour,andbetween5HIAA/5HT
ratio in the diencephalon and submissive behaviour (r=−0.69,
N=12, p<0.05). Positive correlations were found between DA
levels in the diencephalon and submissive behaviour (r=0.60,
N=13,p<0.05)and DAlevelsin thecerebellumand aggressive
behaviour(r=0.76,N=12,p<0.01).
Inthemirrorfightingtreatmenttherewerepositive
correla-tionsbetweenbitefrequencyand5-HIAAlevelsintheoptictectum
(r=0.81,N=7,p<0.05),the5HIAA/5HTratiosinthediencephalon
(r=0.90, N=7,p<0.01)and optictectum(r=0.83,N=7,p<0.05)
andDOPAC/DAratiointhediencephalon(r=0.76,N=7,p<0.05).
Strikefrequencywasnegativelycorrelatedwith5-HTandDOPAC
Fig.4.Monoaminergicactivityindifferentbrainareasfollowinganacutesocial interaction:(A)HIAA/5-HTratio;(B)DOPAC/DAratio.Errorbarsrepresentthe standarderrorofthemean(repeatedmeasuresANOVA,*p<0.05and**p<0.01).
levelsinthecerebellum(r=−0.79,N=7,p<0.05;r=−0.79,N=7,
p<0.05)andpositivelycorrelatedintheoptictectumwithDAlevels
(r=0.77,N=7,p<0.05).Allothercorrelationswerenonsignificant.
4. Discussion
Inthecurrentstudyitisshownthatfollowinganacute
ago-nisticencounterzebrafishmalesexpresstwodistinctbehaviour
profilesdepending onthesocial status achieved: losers exhibit
exclusivelysubmissivebehaviours,whereaswinnersexpressonly
aggressivebehaviours (Fig.2A). Aftertherelative fighting
abil-ityhasbeenestablished,thedifferentbehaviouralrepertoiresfor
each social status are stable over time (at least up to5 days,
R.F.Oliveiraandco-workers,unpublisheddata).Foranimalsthat
foughttheirownmirrorimageonlyaggressivebehaviourswere
observed,withafrequencythatwasnotsignificantlydifferentfrom
thatobservedinwinnersofrealopponentfights(T-test:T=−0.84,
p=0.42).However,amajordifferencebetweenwinnersandmirror
fightersispresent,notontheirbehaviouraloutput,butratheron
thebehaviourobservedintheopponent,sinceinmirrorfightsthe
opponent(i.e.ownimageonthemirror)neverdisplayssubmissive
behaviours.Asaconsequencemirrorfightswereunsolvedfights,
ascanbedemonstratedbythefactthattheexpressionof
aggres-sivebehaviourtypicalofthepre-resolutionphaselastedforthe
wholedurationofthetrial(30min),whereasinrealopponentfights
theencounterwasresolvedinapproximately7min(afterwhich
post-resolutionbehaviouralprofileswereobserved).Therefore,the
experimentaldesignusedsuccessfullyproducedfourtypesofsocial
phenotypes:winners,losers,individualsthatexpressedaggressive
behaviourbutdidnotexperienceeitherawinoraloss(i.e.
mir-rorfighters),andindividualsthatdidnotexpressorperceivedany
socialbehaviour(control=socialisolation).Therefore,the
compar-isonofmonoaminelevelsinregionsofinterestinthebrainacross
thesefoursocialphenotypesallowstheinvestigationofthe
short-termeffectsofacutesocialinteractionsdependingonperceived
outcomebytheparticipants.
Formonoamines,we foundthat5-HTlevelsaresignificantly
higherinthetelencephalonofmirrorfighters,inthebrainstem
ofwinnersandinthecerebellumofallexperimentalgroups.The
increasein5-HTbrainlevelsinthetelencephalonandbrainstem
suggeststhatmirrorfightersandwinnersarethegroupswherethe
serotonergicsystemisfirstactivatedinresponsetoasocial
interac-tionandalthoughtheybehavesimilarly,thebrainareasactivated
aredistinctwhichmayindicatedifferentperceptionofthecontext.
Wealsofoundabrainarea(i.e.cerebellum)thatrespondstoacute
stressindependentoftheinteractionstype(i.e.anincreaseinall
groupswasseencomparedtocontrols).
For5-HTmetabolite(5-HIAA),significantincreaseswerefound
inthetelencephalonandinthecerebellumofalltreatments
(win-ner,losers, and mirrorinteraction), and in thediencephalon of
losers.Interestingly,5-HIAAlevelsinthediencephalonwere
neg-ativelycorrelatedwithaggressivebehaviourintherealopponent
paradigmsupportingthediencephalonenrolmentintheregulation
ofaggressivebehaviour.Ontheotherhand,aggressivebehaviour
(bitefrequency)waspositivelycorrelatedwith5-HIAAintheoptic
tectumformirrorfighters.Thislatercorrelationmaybeprimarily
associatedwithincreasedvisualstimulationinmirrorfighters.
Ourresultssuggestthatacuteinteractionactivated
serotoner-gicsystemincreasing5-HTand5-HIAAbrainlevelsinresponseto
differentsocialconditions.
Serotonergicactivityinturn,issignificantlyhigherinthe
tele-ncephalonofwinnersandintheoptictectumoflosers,andno
sig-nificantchangeswasobservedinmirrorfighters.Moreover,inreal
opponentfightsserotonergicactivityinthediencephalonwas
neg-ativelycorrelatedwithsubmissivebehaviourandinmirrorfights
serotonergicactivityboth in thediencephalon andin theoptic
tectumispositivelycorrelatedwithovertaggression(i.e.bites).
Giventhatsocialinteractiondidnotaffect5-HTlevelsinthesebrain
areas,5-HTactivitywasmainlydeterminedbymetabolitelevels.
Theseresultssuggestthatserotonergicactivityisdifferentially
reg-ulatedindifferentbrainregionsbysocialinteractions.Inzebrafish
threeclustersofserotonergicneuronshavebeendescribed:the
raphenuclei,theposteriortuberculum/hypothalamicpopulations
andthepretectalarea.Thetelencephalon(includingtheolfactory
bulbs)receivesprojections fromthedorsal cellsofthesuperior
raphe[27,28].Mostofthe5-HT-irfibresterminateindorsolateral
partsoftherostraltelencephalonandaminorpartcontinues
ven-trallyintotheolfactorybulb[29].Thus,theobservedincreasein
telencephalonandolfactorybulbserotonergicactivityinwinners
mayreflectanactivationofthesuperiorrapheprojectionsinthis
socialcondition.Alternativelythisincreaseintelencephalic
sero-tonergicactivitymaybedue topre-synaptic stimulationofthe
terminalareas,whichhasbeendemonstrated,bydisinhibitionof
GABAergicinterneurons,increasedglutamatergiclocalstimulation,
andglucocorticoidinfusion[30,31].
Mostof theserotonergicfibres in theoptictectum seemto
originatefromserotonergicneuronsofthepretectalcluster[29].
Pretectalnuclei,aswellastheoptictectum,havebeenimplicated
intheregulationofvisualandmotorbehaviour,multimodal
sen-soryintegration[32]andescaperesponses[33],whichmayexplain
thesignificantincreasedin subordinates orloser conditions,as
observedinthepresentstudy.Inmammals,avoidanceresponses
areobtainedfromstimulationsinaregionofthesuperior
collicu-lusthatappearstorepresenttheuppervisualfield[34].Finally,
serotonergicactivityin thediencephalonwhich mustrepresent
theactivationoftheposteriortuberculum/hypothalamic5-HT
neu-ronalpopulationswaspositivelycorrelatedwithovertaggression
(i.e.bites)inthemirrorfightsandnegativelycorrelatedwith
sub-missivebehaviourin realopponentfights,suggesting arole for
theseserotonergicpopulationsinthebalancebetweenaggressive
andsubmissivebehaviour.
Theactivationoftheserotonergicsysteminresponsetosocial
interactionshadbeenpreviouslydemonstratedforotherspecies.In
earlystagesofhierarchyformationtheserotonergicsystemappears
tobeactivatedin both dominantsand subordinates.For
exam-ple,5-HTlevelswereelevatedafter10minofsocialinteraction
inthelimbicregionsandinthelocuscoeruleusofdominantand
subordinatefightinglizardmales(inAnolis carolinensis)[35].In
rainbowtrout(Oncorhynchusmykiss)bothdominantsand
subordi-natesincreased5-HTactivityinthetelencephalonandoptictectum
3haftertheinteraction[10].Similarly,inthebicolordamselfish
(Stegastespartitus),afterachronicinteractionof5ddominantsas
wellassubordinatesshowedhigherlevelsof5-HTactivityinthe
telencephalon[36].Otherstudieshave shownthat serotonergic
activityhassimilarpatternsindominantsand subordinatesbut
thispatternseemstobetemporallyadvancedindominants[35].
Ourdatadoesnotallowsuchcomparisonsinceweonlycollectone
timepointbutwecanspeculatethatthedifferencesbetweensocial
statusinthebrainareduetoatimelinethatisactingatdifferent
speedsdependingonsocialstatus,giventhatdominantsand
sub-ordinatesexhibitalreadydifferentialpatternsof5-HTactivationa
shorttimeaftertheresolutionofthefight.
Inthedopaminergicsystemtherewasasignificantincreasein
DAlevelsinthecerebellumforallgroups,andinthebrainstemof
winners.IntherealopponentparadigmDAlevelswerepositively
correlated with aggressive behaviourin thecerebellum and in
thediencephalon withsubmissivebehaviour.For DOPAC, there
wasa significantincrease for allgroups in severalbrain areas;
optictectum,cerebellumandbrainstemandinthediencephalon
oflosers.We alsofoundanegativecorrelationofDOPACinthe
thecontributionofdiencephalonintheregulationofsubmissive
behaviour. For mirror fighters DOPAC levels in the cerebellum
werepositivelycorrelatedwithstrikes.
On the other hand, dopaminergic activity was significantly
higherinthetelencephalonofwinnersandintheoptictectumof
bothlosersandmirrorfightersandtheseincreasesweremainly
determinedbythemetabolitelevels.Moreover,theexpressionof
aggressivebehaviourwaspositivelycorrelatedwithdopaminergic
activityinthediencephaloninmirrorfights.Togethertheseresults
suggestaninvolvementofthediencephalicmonoaminergic
sys-temintheregulationofaggressiveandsubmissivebehavioursin
differentsocialconditions.Thishypothesisisfurthersupportedby
theknownroleofdifferentdiencephalicnucleiintheregulation
ofspecies-specificbehavioursacrossvertebrates.Forexample,in
thebluegillfish(Lepomismacrochirus)stimulationofthe
preop-ticregioninhibitsaggressivebehavioursandevokecourtship,and
stimulationofaregionsurroundingthelateralrecesselicits
aggres-sivebehaviourandfeeding[37].Similarly,ingoldenhamstersand
rats,theanteriorhypothalamus[38]andthenucleusaccumbens
[16]respectively,havebeenimplicatedintheregulationof
aggres-sivebehaviours,andinSyrianhamsters(Mesocricetusauratus)the
nucleusaccumbensisinvolvedinconditioneddefeat[39].
Dopaminereleaseappeartobeaffectedalsoinotherbrainareas,
asthecerebellumandbrainstem,buttherewerenosignificant
dif-ferencesinDOPAC/DAratiossinceboththeneurotransmitterand
themetabolitelevelsincreasedinparallelindicatinganincreasein
monoaminergicactivity.
Theincreaseddopaminergicactivityinthetelencephalonwhen
males successfully achieve dominant status (i.e. winners) may
be representative of social reward. A similar pattern hasbeen
previously observed in salmonids where dominant individuals
showedhigherDAactivityintelencephalonthansubordinatefish
[40].However,incontrasttoamniotes,wherethedopaminergic
mesolimbicrewardsystemislocatedintheventraltegmentalarea
(VTA),thatprojectrostrallytothenucleusaccumbens,amygdala
andcorticalareas(e.g.prefrontalcortexinmammals),fishdonot
presentamidbraindopaminergicpopulationhomologoustothe
VTA [41].In contrasts,in fishtheDA inputstothe
telencepha-lonoriginateina localsubpallial DAsystemandinDAneurons
intheventraldiencephalon,inparticularintheposterior
tuber-culum,that projecttowardsthe subpallium[42–44]. Therefore,
althoughevolutionaryitcannotbeconsideredashomologousto
themammalianVTADAneurons,infishthisascendingDApathway
maybeplayingasimilarroleinrewardbehaviourasthe
mam-malianmesostriatalDApathway.Ontheotherhand,theincreased
DAactivityobservedinlosersandmirrorfightersmustbea
con-sequenceofthedifferentialactivationofanotherDAsubsystem.
ApretectalDAcellgroup(alarplateofp1)isconsistentlyfound
inbonyfishes,amphibians,andmostamniotesexceptmammals
[41].Thesepretectalneuronsareprojectingmostlyontheoptic
tectum,inalayer-specificfashionandtheymayplayaroleinthe
modulationoftheretino-tectalvisualinput[45].Inthisregardit
isextremelyinterestingtonotethatthesimilaroptictetctumDA
activationinmirrorfightersandlosers,despitethedissimilarities
oftheirbehaviouralprofile(i.e.mirrorfightersareasaggressiveas
winners,andlosersincontrast,aresubmissive),suggeststhatwhat
isdrivingtheDAactivationinthisregionistheperceptionofthe
interaction,whichissimilarinmirrorfightersandlosers(i.e.both
areexposedtoanaggressiveopponent),ratherthatthebehavioural
outputofthefocalindividual.
Insummarythedatapresentedhereconfirmsthatacutesocial
interactions elicit rapid and differential changes in
serotoner-gic and dopaminergic activity across differentbrain regions in
zebrafish. Further studies are needed to elucidate the specific
rolesof differentneuromodulatorysubsystemin theregulation
ofsocialbehaviour.Finally,theabilityofzebrafishreportedhere
torespondtoexperimentalmanipulationsofits social
environ-ment,combinedwiththefactthatitisaspecies thatexpresses
both gregarious (shoaling) andterritorial behaviour,makesit a
promisingmodelorganisminsocialneuroscience.Incomparison
tootherestablishedmodelsinthisfield,suchascichlidfish(e.g.
Astatotilapiaburtoni[46]),zebrafishhastheaddedvalueofhaving
alargegenetictoolboxavailablethatcanbeusedtogenetically
dissectthemechanismsinvolvedinsocialdecision-making.
Acknowledgements
ThisstudywasfundedbythePortugueseFoundationforScience
and Technology (FCT, grants PTDC/PSI/71811/2006 and
PEst-OE/MAR/UI0331/2011toRFO).DuringthisstudyMTwasbeing
sup-portedbyaPh.D.studentfellowshipbyFCT(SFRH/BD/44848/2008).
Wethanktothetwoanonymousrefereeswhocontributedtothe
improvementofthefinalmanuscript.
References
[1]TaborskyB,OliveiraRF.Socialcompetence:anevolutionaryapproach.Trends EcolEvol2012;27:679–88.
[2]ZupancGKH,LamprechtJ.Towardsacellularunderstandingofmotivation: structuralreorganizationandbiochemicalswitchingaskeymechanismsof behavioralplasticity.Ethology2000;46:467–77.
[3]BargmannCI.Beyondtheconnectome:howneuromodulatorsshapeneural circuits.Bioessays2012;34:458–65.
[4]LibersatF,PflugerHJ.Monoaminesandtheorchestrationofbehavior. Bio-science2004;54:17–25.
[5]Goodson JL, Thompson RR. Nonapeptide mechanisms of social cogni-tion, behavior and species-specific social systems. Curr Opin Neurobiol 2010;20:784–94.
[6]KravitzEA.Serotoninandaggression:insightsgainedfromalobstermodel systemandspeculationsontheroleofamineneuronsinacomplexbehavior. JCompPhysiolA2000;186:221–38.
[7]HuberR.Aminesandmotivatedbehaviors:asimplersystemsapproachto com-plexbehavioralphenomena.JCompPhysiolANeuroetholSensNeuralBehav Physiol2005;191:231–9.
[8]SummersCH,KorzanWJ,LukkesJL,WattMJ,ForsterGL,OverliO,etal.Does serotonininfluenceaggression?Comparingregionalactivitybeforeandduring socialinteraction.PhysiolBiochemZool2005;78:679–94.
[9]WinbergS,NilssonGE.Rolesofbrainmonoaminesneurotrannsmittersin agonisticbehaviour andstressreactions,withparticularreference tofish. CompBiochemphysiol1993;106:597–614.
[10]OverliO,HarrisCA,WinbergS.Short-termeffectsoffightsforsocial domi-nanceandtheestablishmentofdominant-subordinaterelationshipsonbrain monoaminesandcortisolinrainbowtrout.BrainBehavEvol1999;54:263–75. [11]OlivierB.Serotoninandaggression.AnnNYAcadSci2004;1036:382–92. [12]DulawaSC,GrossC,StarkKL,HenR,GeyerMA.Knockoutmicerevealopposite
rolesforserotonin1Aand1Breceptorsinprepulseinhibition. Neuropsy-chopharmacology2000;22:650–9.
[13]JenckF,BroekkampCL,VanDelftAM.Effectsofserotoninreceptorantagonists onPAGstimulationinducedaversion:differentcontributionsof5HT1,5HT2 and5HT3receptors.Psychopharmacology(Berl)1989;97:489–95.
[14]SanchezC,ArntJ,HyttelJ,MoltzenEK.Theroleofserotonergicmechanismsin inhibitionofisolation-inducedaggressioninmalemice.Psychopharmacology (Berl)1993;110:53–9.
[15]SweidanS,EdingerH,SiegelA.D2dopaminereceptor-mediatedmechanismsin themedialpreoptic-anteriorhypothalamusregulateeffectivedefensebehavior inthecat.BrainRes1991;549:127–37.
[16]VanErpAM,MiczekKA.Aggressivebehavior,increasedaccumbaldopamine, anddecreasedcorticalserotonininrats.JNeurosci2000;20:9320–5. [17]FerrariPF,vanErpAM,TornatzkyW,MiczekKA.Accumbaldopamineand
serotonininanticipationofthenextaggressiveepisodeinrats.EurJNeurosci 2003;17:371–8.
[18]MosJ,VanValkenburgCF.Specificeffectonsocialstessandaggressionon regionaldopaminemetabolisminratbrain.NeurosciLett1979;15:325–7. [19]Puglisi-Allegra S, Cabib S. Effects of defeat experiences on dopamine
metabolism in different brain areas of the mouse. Aggress Behav 1990;16:271–84.
[20]LouilotA,LeMoalM,SimonH.Differentialreactivityofdopaminergicneurons inthenucleusaccumbensinresponsetodifferentbehavioralsituations.An invivovoltammetricstudyinfreemovingrats.BrainRes1986;397:395–400. [21]TideyJW,MiczekKA.Socialdefeatstressselectivelyaltersmesocorticolimbic
dopaminerelease:aninvivomicrodialysisstudy.BrainRes1996;721:140–9. [22]OliveiraRF.Socialplasticityinfish:integratingmechanismsandfunction.JFish
Biol2012;81:2127–50.
[23]SpenceR,SmithC.Maleterritorialitymediatesdensityandsexratioeffectson ovipositioninthezebrafish,Daniorerio.AnimBehav2005;69:1317–23.
[24]LarsonET,O‘MalleyDM,MelloniJrRH.Aggressionandvasotocinare associ-atedwithdominant–subordinaterelationshipsinzebrafish.BehavBrainRes 2006;167:94–102.
[25]PaullGC,FilbyAL,GiddinsHG,CoeTS,HamiltonPB,TylerCR.Dominance hierar-chiesinzebrafish(Daniorerio)andtheirrelationshipwithreproductivesuccess. Zebrafish2010;7:109–17.
[26]OliveiraRF,SilvaJF,SimoesJM.Fightingzebrafish:characterizationof aggres-sivebehaviorandwinner–losereffects.Zebrafish2011;8:73–81.
[27]LillesaarC,TannhauserB,StigloherC,KremmerE,Bally-CuifL.Theserotonergic phenotypeisacquiredbyconverginggeneticmechanismswithinthezebrafish centralnervoussystem.DevDyn2007;236:1072–84.
[28]LillesaarC,StigloherC,TannhauserB,WullimannMF,Bally-CuifL.Axonal pro-jectionsoriginatingfromrapheserotonergicneuronsinthedevelopingand adultzebrafish,Daniorerio,usingtransgenicstovisualizeraphe-specificpet1 expression.JCompNeurol2009;512:158–82.
[29]KaslinJ,PanulaP.Comparativeanatomyofthehistaminergicandother amin-ergicsystemsinzebrafish(Daniorerio).JCompNeurol2001;440:342–77. [30]SummersTR,MatterJM,McKayJM,RonanPJ,LarsonET,RennerKJ,etal.Rapid
glucocorticoidstimulationandGABAergicinhibitionofhippocampal seroto-nergicresponse:invivodialysisinthelizardAnoliscarolinensis.HormBehav 2003;43:245–53.
[31]BarrJL,ForsterGL.Serotonergicneurotransmissionintheventralhippocampus isenhancedbycorticosteroneandalteredbychronicamphetaminetreatment. Neuroscience2011;182:105–14.
[32]Bally-CuifL,VernierP.Organizationandphysiologyofthezebrafishnervous system.In:PerrySF,EkkerM,FarrellAP,BraunerCJ,editors.Zebrafish. Amster-dam:Elsevier;2010.
[33]HerreroL,RodriguezF,SalasC,TorresB.Tailandeyemovementsevoked byelectricalmicrostimulationoftheoptictectumingoldfish.ExpBrainRes 1998;120:291–305.
[34]SahibzadaN,DeanP,RedgraveP.Movementsresemblingorientationor avoid-ance elicitedby electricalstimulationofthesuperiorcolliculus inrats.J Neurosci1986;6:723–33.
[35]SummersCH,SummersTR,MooreMC,KorzanWJ,WoodleySK,RonanPJ,etal. Temporalpatternsoflimbicmonoamineandplasmacorticosteroneresponse duringsocialstress.Neuroscience2003;116:553–63.
[36]WinbergS,MyrbergJrAA, NilssonGE.Agonisticinteractionsaffectbrain serotonergic activity inan acanthopterygiianfish: thebicolor damselfish (Pomacentruspartitus).BrainBehavEvol1996;48:213–20.
[37]Demski LS, Knigge KM. The telencephalon and hypothalamus of the bluegill (Lepomis macrochirus): evokedfeeding, aggressive and reproduc-tivebehaviorwithrepresentativefrontalsections.JCompNeurol1971;143: 1–16.
[38]CraigFF,MelloniJrRH,KoppelG,PerryKW,FullerRW,DelvilleY. Vaso-pressin/serotonininteractionsintheanteriorhypothalamuscontrolaggressive behavioringoldenhamsters.JNeurosci1997;17:4331–40.
[39]Luckett C, Norvelle A, Huhman K. The role of the nucleus accumbens in theacquisitionandexpressionofconditioneddefeat.BehavBrainRes 2012;227:208–14.
[40]WinbergS,NilssonGE,OlsenKH.Socialrankandbrainlevelsofmonoamines andmonoaminemetabolitesinArcticcbarr,SMvelinusMpinus(L.).JComp PhysiolA1991:241–6.
[41]SmeetsWJ,GonzalezA.Catecholaminesystemsinthebrainofvertebrates: newperspectivesthroughacomparativeapproach.BrainResBrainResRev 2000;33:308–79.
[42]Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascendingtothesubpallium(striatum)islocatedinthebasaldiencephalon (Posteriortuberculum).BrainRes2001;889:316–30.
[43]RinkE,WullimannMF.Developmentofthecatecholaminergicsysteminthe earlyzebrafishbrain:animmunohistochemicalstudy.BrainResDevBrainRes 2002;137:89–100.
[44]TayTL,RonnebergerO,RyuS,NitschkeR,DrieverW.Comprehensive cat-echolaminergic projectome analysis reveals single-neuron integration of zebrafish ascendinganddescendingdopaminergic systems. NatCommun 2011;2:171.
[45]Smeets WJ, Reiner A. Catecholamines in the CNS of vertebrates: cur-rent conceptsof evolutionand functional significance. In:Smeets WJAJ, Reiner A,editors. Phylogenyand developmentofcatecholaminesystems in theCNSofvertebrates. Cambridge:CambridgeUniversityPress; 1994. p.463–81.