Full paper published online: February 28, 2010 ISSN 1678-9199.
ABO blood groups and Helicobacter pylori cagA infection: evidence of an association
Mattos DE (1), Cintra JR (2), Brandão de Mattos CC (3), Nakashima F (4), Silva RCMA (5), Moreira HW (1), de Mattos LC (2, 3)
(1) Clinical Analysis Department, School of Pharmaceutical Sciences, São Paulo State University, UNESP, Araraquara, São Paulo State, Brazil; (2) Regional Blood Center of São José do Rio Preto, Regional Medical School Foundation, FUNFARME, São José do Rio Preto, São Paulo State, Brazil; (3) Immunogenetics Laboratory, Molecular Biology Department, São José do Rio Preto Medical School, FAMERP, São José do Rio Preto, São Paulo State, Brazil; (4) Biology Department, Institute of Biosciences, Letters and Exact Sciences, São Paulo State University, UNESP, São José do Rio Preto, São Paulo State, Brazil; (5) Specialized Gastroenterology Laboratory, Base Hospital, FUNFARME, São José do Rio Preto, São Paulo State, Brazil.
ABSTRACT: Diseases resulting from Helicobacter pylori infection appear to be dependent on a host of genetic traits and virulence factors possessed by this microorganism. This paper aimed to investigate the association between the ABO histo-blood groups and H. pylori cagA infections. Genomic DNA samples (n = 110) of gastric biopsies obtained from patients with endoscopic diagnosis of peptic ulcers (n = 25) and chronic active gastritis (n = 85) were analyzed by PCR using specific primers for the cagA gene. Of the samples, 66.4% (n = 73) tested positive and 33.6% (n = 37) negative for the gene. The cagA strain was predominant in peptic ulcers (n = 21; 84.0%) compared with chronic active gastritis (n = 52; 61.2%) (p = 0.05; OR 3.332; 95% CI: 1.050-10.576). Additionally, the cagA strain was prevalent in the type O blood (48/63; 76.2%) compared with other ABO phenotypes (25/47; 53.2%) (p = 0.01; OR 2.816; 95% CI: 1.246-6.364). These results suggest that H. pylori cagA infection is associated with the O blood group in Brazilian patients suffering from chronic active gastritis and peptic ulcers.
KEY WORDS: ABO blood groups, H. pylori infection, cagA strain, chronic active gastritis, peptic ulcers.
FINANCIAL SOURCE: BAP/FAMERP and CAPES.
CONFLICTS OF INTEREST: There is no conflict.
CORRESPONDENCE TO:
INTRODUCTION
Infection by Helicobacter pylori is linked to gastroduodenal disease and seems to be
dependent on genetic factors of the host and virulence factors presented by this
microorganism (1-3).
The involvement of blood group carbohydrates as facilitating factors for infection by
this bacillus has previously been reported (4). Some years ago we demonstrated that
infection by this Gram-negative bacillus is associated with ABO blood groups in
Brazilian patients submitted to upper gastrointestinal endoscopy (5).
Previous reports have shown that H. pylori strains that have the cagA gene infect
Brazilian patients suffering from various gastroduodenal diseases (6-8). The cagA
virulence factor seems to be involved in the induction of proinflammatory chemokines
expressed by host cells and plays a notable role in the onset and progression of
gastroduodenal diseases (2, 9).
H. pylori presents a remarkable allelic diversity and genetic variability (3). In addition
to readily binding to ABO blood group antigens in the South America Indian
population, infective cagA-positive H. pylori strains have also been associated with
the ABO histo-blood group in Lebanon and Iran (10-12).
Therefore, studies correlating infection by epidemiologically important
microorganisms such as H. pylori may provide relevant biological and clinical
contributions by identifying genetic risk factors of the host and the stages of virulence
and pathogenesis of the infectious agent (9). The aim of this study was to investigate
the association between the ABO histo-blood groups and H. pylori cagA infection.
MATERIALS AND METHODS
Type of Study
This cross-sectional study was carried out in a specialized gastroenterology
laboratory at the Base Hospital and the Immunogenetics Laboratory, Molecular
Biology Department, São José do Rio Preto Medical School (FAMERP), São José do
Rio Preto, São Paulo state, Brazil. After being informed about the experimental
nature of this study, written consent was obtained from all patients. The study was
Patient Selection
Patients with endoscopy diagnoses of peptic ulcers (PU) or chronic active gastritis
(CAG) and H. pylori infection were candidates for this study. H. pylori infection was
identified by routine urea breath and urease tests performed in the specialized
gastroenterology laboratory. Additionally, for all cases, whether positive, negative or
discordant, the presence of infection was confirmed by PCR using gastric biopsy
samples as described in our previous report (5). Patients who were pregnant, aged
less than 18 years, had gastrointestinal tract hemorrhages or acute gastritis, had
used a proton-pump inhibitor in the previous week or had used an H2 receptor
antagonist in the previous 24 hours were excluded from the study. A total of 110
Caucasian and non-Caucasian patients was included in this study.
Blood Sampling and ABO Blood Phenotyping
Five milliliters of whole blood was drawn from each patient and placed in vacuum
tubes with EDTA. The ABO blood group phenotypes were identified by
hemagglutination using commercial anti-A, anti-B and anti-A,B sera for forward typing
and standard A1 and B red blood cells for reverse typing (Fresenius Kabi, Brazil). A
drop of a red blood cell suspension in 5% sterile saline solution (0.9% NaCl)
prepared for each sample was mixed with a drop of each of the anti-A, anti-B and
anti-A,B sera to define the erythrocyte antigens. Two drops of blood plasma from
each sample were mixed with one drop of each of the standard 5% A1 and B red
blood cell suspensions to identify the anti-A and anti-B antibodies. The tubes were
centrifuged at 3400 rpm for 1.5 minutes with the interpretation of the results being
based on the presence or absence of hemagglutination. The recommendations of the
manufacturer of all reagents were strictly followed.
H. pylori cagA Genotyping
Genomic DNA samples were extracted from gastric biopsies obtained during
endoscopy examinations. The identification of the H. pylori strain, based on the
detection of the cagA gene, was achieved by PCR according to the previously published protocol (13). PCR reactions were carried out in a final volume of 25 μL containing Tris-HCl 10 mM (pH 8.3); MgCl2 1.5 mM; KCl 50 mM; 5 mM of each dNTP
(dATP, dTTP, dCTP, dGTP), 0.5 mM of each primer (forward:
0.5 U of Taq polymerase and 10 ng of genomic DNA. The amplification conditions
were: 35 denaturation cycles at 94°C for one minute, annealing at 55°C for two
minutes and extension at 72ºC for two minutes. Specific amplified fragments
containing 102 base pairs corresponding to the cagA gene were separated by
electrophoresis on a 2% agarose gel in TBE 1X and visualized using ultraviolet light
after ethidium bromide staining.
Statistical Analysis
The two-tailed Fisher’s exact test and odds ratio with a 95% confidence interval were
used to identify associations.
RESULTS
Of the 110 patients with H. pylori infection, 65.4% (n = 72) were Caucasians and
43.6% (n = 48) were non-Caucasians. There was a slight predominance of female (n
= 61; 55.4%) over male (n = 49; 44.6%) patients. Based on endoscopy results, there
were three times more patients suffering from CAG (n = 85; 77.3%) than those with
PU (n = 25; 22.7%). The O blood group (n = 63; 57.3%) was prevalent over non-O
blood groups (A: n = 33, 30.0%; B: n = 12, 10.9%; AB: n = 2, 1.8%).
The rate of H. pylori cagA infections was high (66.4%) but no statistically significant
difference was observed when cagA (+) and cagA (–) patients were compared by
gender and ethnic background. Table 1 summarizes the results in relation to
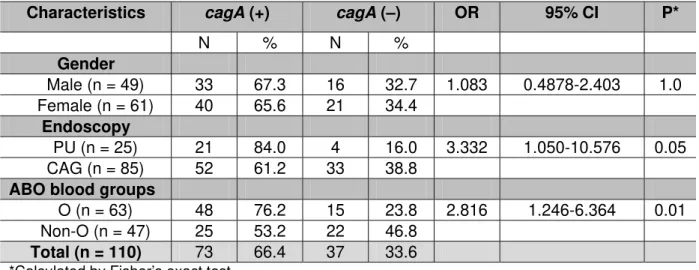
Table 1. Frequencies of O and non-O blood groups and H. pylori infection among
110 patients
Characteristics cagA (+) cagA (–) OR 95% CI P*
N % N % Gender
Male (n = 49) 33 67.3 16 32.7 1.083 0.4878-2.403 1.0
Female (n = 61) 40 65.6 21 34.4
Endoscopy
PU (n = 25) 21 84.0 4 16.0 3.332 1.050-10.576 0.05
CAG (n = 85) 52 61.2 33 38.8
ABO blood groups
O (n = 63) 48 76.2 15 23.8 2.816 1.246-6.364 0.01
Non-O (n = 47) 25 53.2 22 46.8
Total (n = 110) 73 66.4 37 33.6
*Calculated by Fisher’s exact test
DISCUSSION
Previous reports that suggesting that infections by the H. pylori bacillus may be
influenced by genetic traits of the host prompted us to test the hypothesis of an
association between the ABO histo-blood groups and cagA infections.
It is believed that studies on the involvement of this aggressive H. pylori strain in the
onset and progression of gastroduodenal diseases may provide information central to
the development of control and preventive measures against infection (9).
Some years ago we reported an association between the O blood group and H. pylori
infections in patients who were submitted to gastric endoscopy with our data being
subsequently verified by two studies involving adult and infant patients from different
regions of Brazil (5, 14, 15). Observations that emerged from these studies have
been supported by several publications over the last few years (1-3, 9, 16).
In this paper we demonstrate that in Brazil infection by H. pylori carrying the cagA
virulence factor is associated with the O blood group. The rate of infection by this
strain reported herein is similar to previous Brazilian studies of adult and infant
populations published in recent years (6-8).
The relation between infection by cagA-positive H. pylori strains and ABO histo-blood
groups has been investigated in different regions. A significant association between
this strain and the development of peptic ulcers was observed among Taiwanese
85% of patients suffering from PU and more than 60% of those with CAG were
infected by cagA-positive H. pylori strains.
Our study is in agreement with the Taiwanese study which revealed high rates of
infection by this strain among patients belonging to the O blood group with a
borderline significance level probably due to the small number of patients suffering
from PU.
Two recent reports evaluated the association between ABO histo-blood groups and
infection by cagA-positive H. pylori strains. One of them reported a significant
relationship in Lebanon among three factors, namely, infection by this strain, the A
blood group and the risk of gastric malignancy (11). The other one demonstrated that
the anti-cagA antibody was also slightly more prevalent among infected children with
A and O blood groups in Iran (12).
Our results are not concordant with the Lebanese study, but agree, at least in part,
with the Iranian study (11, 12). It is possible that additional differences in genetic
variability of some H. pylori strains infecting patients in the Middle East accounted for
the differences between these studies. The basis for this association is not totally
understood but the ability of H. pylori to bind to carbohydrate structures found in
receptors, especially those related to histo-blood groups, is attractive (4).
There is no evidence that cagA-positive H. pylori strains are able to directly recognize
the H antigen, expressed in the gastrointestinal tract of O blood group individuals, as
a receptor by using the cytotoxin associated antigen A (cagA) as ligand. However,
the co-expression of other virulence factors such as blood group adhesin binding A2
(BabA2), which binds to ABO blood group antigens, could explain, at least in part, the
association reported in this paper.
H. pylori infections by strains carrying the cagA, vacA, iceA and babA2 genes were
observed among Brazilian adults and children suffering from gastroduodenal
diseases (6-8, 18). Unfortunately these studies did not analyze the correlation
between ABO blood groups and infection by these strains.
It has been shown that some H. pylori strains infecting Amerindians present a certain
degree of adaptation that coincides with the high prevalence of the O blood group
among native South Americans. More than 95% of the BabA-positive H. pylori strains
are able to bind to ABO antigens and 60% of them present a specialized binding
The ABO blood group antigens are carbohydrate structures present in the
gastrointestinal tract epithelium while the H antigen is a fucosylated antigen
expressed by the majority of individuals belonging to the O blood group. This antigen
is not modified by glycosyltransferases coded by A or B genes of the ABO locus
responsible for the expression of A and B antigens while its level of expression is
quantitatively higher than that detected in non-O blood group individuals (19, 20).
Thus, these quantitative differences may contribute to the high prevalence of H.
pylori infections among Brazilian patients carrying the babA2 and cagA genes and
suffering from CAG and PU.
It is presumed that H. pylori co-evolved with its human host but it is not clear whether
its introduction into South America was due to waves of native Amerindians migrating
across the Central American isthmus or due to colonization by Europeans. The
suggestion that ancestral H. pylori was present in Peruvian Amerindians prior to the
arrival of European colonizers 500 years ago is attractive by virtue of the fact that
possible competition between Amerindian and European strains could contribute to
the diversification and high genetic variability in the genome of this bacillus in South
America (21). Therefore, the consequent acquisition of babA2 and cagA genes would
allow the H. pylori bacillus to adapt based on the prevalent ABO blood groups in
South America (10).
In conclusion, this paper presents evidence of an association between the O blood
group and cagA-positive H. pylori infections among Brazilian patients suffering from
PU and CAG. This association seems to be an important event which could elucidate
the epidemiological basis of the interaction between humans and H. pylori.
ACKNOWLEDGEMENTS
We thank BAP/FAMERP for the research grant (4291/2007) and CAPES for the
REFERENCES
1. Nguyen TN, Barkun AN, Fallone CA. Host determinants of Helicobacter pylori
infection and its clinical outcome. Helicobacter. 1999;4(3):185-97.
2. Atherton JC. The pathogenesis of Helicobacter pylori-induced gastroduodenal
diseases. Annu Rev Pathol. 2006;1:63-96.
3. Suerbaum S, Josenhans C. Helicobacter pylori evolution and phenotypic
diversification in a changing host. Nat Rev Microbiol. 2007;5(6):441-52.
4. Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori
to human gastric epithelium mediated by blood group antigens. Science.
1993;262(5141):1892-5.
5. Mattos LC, Cintra JR, Sanches FE, Silva RCMA, Ruiz MA, Moreira HW. ABO,
Lewis, secretor and non-secretor phenotypes in patients infected or uninfected by the
Helicobacter pylori bacillus. São Paulo Med J. 2002;120(2):55-8.
6. Leite KRM, Darini E, Canavez FC, Carvalho CM, Mitteldorf CATS, Camara-Lopes
LH. Helicobacter pylori and cagA gene detected by polymerase chain reaction in
gastric biopsies: correlation with histological findings, proliferation and apoptosis. São
Paulo Med J. 2005;123(3):113-8.
7. Gatti LL, Fagundes e Souza EK, Leite KR, De Souza Bastos EL, Vicentini LR, Da
Silva LC, et al. cagA, vacA alleles and babA2 genotypes of Helicobacter pylori
associated with gastric disease in brazilian adult patients. Diagn Microbiol Infect Dis.
2005;51(4):231-5.
8. Gatti LL, Lábio R, Silva LC, Smith MAC, Payão SLM. cagA positive Helicobacter
pylori in Brazilian children related to chronic gastritis. Braz J Infect Dis.
2006;10(4):254-8.
9. Kusters JG, van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori
infection. Clin Microbiol Rev. 2006;19(3):449-90.
10. Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, et al.
Functional adaptation of BabA, the H. pylori ABO blood group antigen binding
adhesin. Science. 2004;305(5683):519-22.
11. Sharara AI, Abdul-Baki H, ElHajj I, Kreidieh N, Kfoury Baz EM. Association of
gastroduodenal disease phenotype with ABO blood group and Helicobacter pylori
12. Jafarzadeh A, Ahmedi-Kahanali J, Bahrami M, Taghipour Z. Seroprevalence of
anti-Helicobacter pylori and anti-CagA antibodies among healthy children according
to age, sex, ABO blood groups and Rh status in south-east of Iran. Turk J
Gastroenterol. 2007;18(3):165-71.
13. Ito A, Fujioka T, Kodama K, Nishizono A, Nasu M. Virulence-associated genes as
markers of strain diversity in Helicobacter pylori infection. J Gastroenterol Hepatol.
1997;12(9-10):666-9.
14. Martins LC, de Oliveira Corvelo TC, Oti HT, do Socorro Pompeu Loiola R, Aguiar
DC, dos Santos Barile KA, et al. ABH and Lewis antigen distributions in blood, saliva
and gastric mucosa and H. pylori infection in gastric ulcer patients. World J
Gastroenterol. 2006;12(7):1120-4.
15. Rodrigues RV, Corvelo TC, Ferrer MT. Seroprevalence of Helicobacter pylori
infection among children of different socioeconomic levels in Porto Velho, State of
Rondônia. Rev Soc Bras Med Trop. 2007;40(5):550-4.
16. McNamara D, El-Omar E. Helicobacter pylori infection and the pathogenesis of
gastric cancer: a paradigm for host-bacterial interactions. Dig Liver Dis.
2008;40(7):504-9.
17. Lin CW, Chang YS, Wu SC, Cheng KS. Helicobacter pylori in gastric biopsies of
Taiwanese patients with gastroduodenal diseases. Jpn J Med Sci Biol. 1998;
51(1):13-23.
18. Mattar R, dos Santos AF, Eisig JN, Rodrigues TN, Silva FM, Lupinacci RM, et al.
No correlation of babA2 with vacA and cagA genotypes of Helicobacter pylori and
grading of gastritis from peptic ulcer disease patients in Brazil. Helicobacter.
2005;10(6):601-8.
19. Oriol R. ABO, Hh, Lewis and Secretion: serology, genetics and tissue distribution.
In: Cartron JP, Rouger P editors. Blood cell biochemistry: molecular basis of human
blood group antigens. New York: Plenum; 1995. 37-73 p.
20. Henry SM. Molecular diversity in the biosynthesis of GI tract glycoconjugates. A
blood group related chart of microorganism receptors. Transfus Clin Biol.
2001;8(3):226-30.
21. Devi SM, Ahmed I, Khan AA, Rahman SA, Alvi A, Sechi LA, et al. Genomes of
Helicobacter pylori from native Peruvians suggest admixture of ancestral and modern
lineages and reveal a western type cag-pathogenicity island. BMC Genomics.