Universidade Federal de Uberlândia
Instituto de Biologia
INTERAÇÃO PREDADOR-PRESA ENTRE A ARANHA
Loxosceles gaucho (SICARIIDAE) E O OPILIÃO Mischonyx
cuspidatus (GONYLEPTIDAE)
Júlio do Monte Gonzalez de Segovia
Júlio do Monte Gonzalez de Segovia
INTERAÇÃO PREDADOR-PRESA ENTRE A ARANHA
Loxosceles gaucho (SICARIIDAE) E O OPILIÃO Mischonyx
cuspidatus (GONYLEPTIDAE)
Dissertação apresentada à Universidade Federal de Uberlândia, como parte das exigências para
obtenção do título de Mestre em “Ecologia e Conservação de Recursos Naturais”.
Orientador
Prof. Dr. Kleber del Claro Co-orientador
Prof. Dr. Rodrigo Hirata Willemart
Júlio do Monte Gonzalez de Segovia
INTERAÇÃO PREDADOR-PRESA ENTRE A ARANHA
Loxosceles gaucho (SICARIIDAE) E O OPILIÃO Mischonyx
cuspidatus (GONYLEPTIDAE)
Dissertação apresentada à Universidade Federal de Uberlândia, como parte das exigências para
obtenção do título de Mestre em “Ecologia e
Conservação de Recursos Naturais”.
APROVADO em 28 de Fevereiro de 2014
______________________________________ Prof. Dr. Marcelo Oliveira Gonzaga UFU
______________________________________
Prof. Dr Michael Hrncir UFERSA
_______________________________________________ Prof. Dr. Kleber de Claro
(Orientador)
________________________________________________ Prof. Dr. Rodrigo Hirata Willemart
(Co-orientador)
DEDICATÓRIA
Dedico esta dissertação ao meu Pai, que me contava entusiasmado sobre cada novo bicho que avistava. Em nossa última conversa, me perguntou sobre este projeto, os objetivos do trabalho e fez comentários bastantes motivantes, que para mim serviram como reconhecimento e me deram força para seguir trabalhando após o seu falecimento. Com muito carinho e enorme saudade.
AGRADECIMENTOS
- Agradeço à minha Mãe, por toda ajuda, apoio, exemplos e ensinamentos que me deu ao longo da vida e durante o período de Mestrado. Uma pessoa que possui um valor imensurável para mim e certamente foi fundamental para minha formação, em todos os sentidos.
- Agradeço à Andressa, por todo seu o apoio durante o mestrado, pelas muitas ajudas e por sua presença, durante parte do período experimental, que foi decisiva para que eu conseguisse conduzir os experimentos em determinados momentos. Bem como à sua família pelo apoio, confiança e pelo trato sempre gentil em relação a mim.
- Agradeço aos meus irmãos Caio e Vitor pela amizade, apoio e pelos momentos agradáveis da vida que compartilhamos.
- Agradeço ao meu professor Lito, por tudo que com ele aprendi, que certamente influenciou de forma positiva minha personalidade.
- Agradeço a todo pessoal do LESCA: Nathy, Thay e prof. Rodrigo, por ajudarem nas coletas iniciais da fase de experimentos pilotos, a todos os membros do laboratório pelas sugestões e críticas, sempre com muito boa vontade. Ao Gabriel por me ajudar na filmagem dos pilotos. Ao Nortão pela ajuda nas coletas de opiliões, sempre com muito boa vontade. À Elene e ao Gui por terem sido muito legais quando entrei no laboratório.
- Agradeço ao pessoal do LECI pelas sugestões e críticas e pela gentileza durante as reuniões, em especial a Alexandra, Estevão, Gudryan e Vanessa.
- Ao prof. Kleber Del Claro por ter aceito me orientar, pela forma como me recebeu e incentivou no momento em que o procurei, por ter acreditado em mim e pela orientação. - Agradeço o prof. Rodrigo Willemart, pela co-orientação. Por toda a ajuda no
desenvolvimento do projeto, nos experimentos, pelas dicas, correções gerais e de inglês. Por permitir que eu desenvolvesse o trabalho em seu laboratório e até mesmo pelos Papers que me mandava às pencas aos 44 do segundo tempo. Todo este suporte foi fundamental para o desenvolvimento deste trabalho.
- Agradeço o professor Heraldo Vasconcelos pelos ensinamentos durante a disciplina de estatística, que foi uma das disciplinas que mais contribuíram para minha formação. - Agradeço o Professor Luiz Andrioli pelo empréstimo do equipamento de CO2.
- Agradeço o professor Michael Hrncir por ter aceito fazer parte desta banca.
- Agradeço a técnica de laboratório Ana Machado pelo auxílio com os cuidados dos opiliões e ser muito gentil em todas as ajudas adicionais.
- Agradeço a Vanessa Penna do instituto Butantan pelas valiosas dicas de cuidado com as Loxosceles.
- Agradeço ao Elmo e Giancarlo pela convivência pacífica e agradável, pelas discussões e trocas de experiências durante o Mestrado. Ao Rafael pelas discussões de artigos e Ciência, algumas vezes calorosas, mas certamente produtivas. A estes três também agradeço pelas brincadeiras e risadas, que ajudaram a deixar este período mais leve.
- Agradeço a toda minha turma de Mestrado pelos momentos que compartilhamos e pelas contribuições de cada um em minha formação, mesmo que em alguns casos de maneira inconsciente.
- Agradeço à secretária da pós graduação, Maria Angélica, pelas muitas ajudas ao longo do Mestrado de forma sempre muito eficiente e educada.
- Agradeço ao Instituto de Biologia da UFU e ao Programa de Pós Graduação em Ecologia e Conservação de Recursos Naturais por terem me proporcionado este período de aprendizado. - Agradeço pelo apoio FAPESP 2010/00915-0, ao laboratório (LESCA) coordenado pelo professor Rodrigo H. Willemart.
ÍNDICE
RESUMO GERAL ... 01
Introdução geral ... 02
Referências ... 04
FIRST CHAPTER ... 06
Abstract ... 06
Introduction ... 07
Methods ... 09
Species studied ... 09
Laboratorial conditions ... 10
Experiment 1: the role of silk in predation ... 10
Experiment 2: substrate vibration as information for predators and predator behavior with distinct prey ... 11
Experiment 3: chemicals of prey as information for the predator ... 12
Results ... 13
Experiment 1: the role of silk in predation ... 13
Experiment 2: substrate vibration as information for predators and predator behavior with distinct prey ... 13
Experiment 3: chemicals of prey as information for the predator ... 15
Discussion ... 20
References ... 23
SECOND CHAPTER ... 29
Abstract ... 29
Introduction ... 30
Material and methods ... 31
Species studied ... 31
Collection and maintenance ... 32
1 - Behaviour of the harvestman in an intermediate threat situation ... 33
2- Behaviour of the harvestman in a high threat situation ... 34
3 - Potential lethality of a harvestman defence under high threat ... 35
Results ... 36
1 - Behaviour of the harvestman in an intermediate threat situation ... 36
2- Behaviour of the harvestman in a high threat situation ... 36
3- Potential lethality of a harvestman defence under high threat ... 38
Discussion ... 39
References ... 42
1 RESUMO GERAL
A interação entre presas e predadores é uma relação ecológica assimétrica e antagônica visto que, para predadores, capturar presas é vantajoso devido ao ganho de energia e, para presas, ser capturado, frequentemente significa morrer. A interação predador-presa é tida como uma pressão evolutiva que tende a aperfeiçoar tanto os mecanismos de caça de predadores como as defesas das presas. Para esta dissertação, utilizamos como modelo de predador a aranha Loxosceles gaucho e como presa o opilião Mischonyx cupidatus, ambos noturnos e sintópicos. No primeiro capítulo investigamos o uso de modalidades sensoriais por L.gaucho na busca por um sítio adequado de forrageio e na detecção de presas, bem como qual comportamento possibilita que estas aranhas consumam opiliões, presas com uma cutícula altamente
esclerotizada. Foram testadas as seguintes hipóteses: (1) L. gaucho passa mais tempo em áreas com químicos de suas presas; (2) a vibração é uma informação essencial no processo de predação por L. gaucho; (3) o lençol de teia permite que L. gaucho prede opiliões. Realizamos também uma análise comportamental em que foi descrito um etograma e um fluxograma. Todas as hipóteses foram rejeitadas. O que aparentemente possibilita que L. gaucho capture esta presa é sua habilidade de encontrar e morder os pontos fracos do corpo do opilião, como juntas e partes distais das pernas. Contrariando vários estudos que demonstram que aranhas rejeitam opiliões Laniatores, este foi o primeiro estudo que demonstrou uma alta taxa de sucesso de predação de aranhas sobre opiliões e, além disso, demonstrou evidências comportamentais das estratégias que permitem a predação. No segundo capítulo,
investigamos os comportamentos defensivos de M. cuspidatus em uma situação de risco intermediário (frente aos químicos dos predadores) e alto risco (frente aos predadores), visto que a teoria conhecida como "Threat sensitive hypothesis" postula que para as presas é vantajoso modular comportamento defensivo de acordo com o nível de ameaça.
Adicionalmente, testamos se lesões no abdômen de L. gaucho, provocadas por apófises do fêmur da perna IV de um opilião, podem influenciar na sobrevivência da aranha. Encontramos comportamentos defensivos nos opiliões apenas na situação de alto risco. A sobrevivência das aranhas não foi alterada após serem lesionadas pelas apófises dos opiliões. Surpreendentemente, não observamos comportamentos de defesa muito conhecidos entre opiliões (ex. defesa química, retaliação com quelícera e pedipalpos) em uma situação de alto risco. Por fim, destacamos a importância de abordagens comportamentais descritivas de comportamento para compreender especificidades das interações entre predadores e presas.
2 INTRODUÇÃO GERAL
A interação entre presas e predadores é um tema que desperta grande interesse, provavelmente por estar diretamente relacionado à fronteira entre continuar vivo ou morrer, seja por meio da manutenção da vida (por meio da ingestão de presas) ou de evitar ser morto por predadores. Dentro das ciências biológicas, a interação predador-presa é estudada em diversos campos, tais como: ecologia de populações, ecologia de comunidades, ecologia comportamental e evolução. Devido a abrangência deste tipo de interação, livros-texto básicos de várias áreas discutem o tema (por exemplo Begon et al., 2007; Ricklefs, 2003; Davies & Krebs, 2012;Ridley, 2004;e outros), além de existirem títulos dedicados especialmente aos mecanismos de defesa contra predadores (Edmunds, 1974) ou à ecologia deste tipo de interação (Barbosa & Castellanos, 2013).
No que diz respeito à ecologia de populações, trabalhos clássicos demonstram oscilações sincronizadas nos ciclo populacionais de presas e predadores (Elton, 1924). Entretanto, o impacto da predação em uma população de presas pode variar dependendo do tipo de predador, uma vez que diferentes estratégias de captura podem resultar em uma taxa de sucesso de captura distinta (veja Cresswell & Quinn, 2010). Outro ponto importante é que os predadores podem exibir preferências em sua dieta. A preferência por presas pode se basear em fatores como: o seu valor energético, a proporção peso do predador em relação ao tamanho da presa e em experiências anteriores com as presas (Spitz et al., 2010, McQuaid, 1994,Pearson & Rypstra, 2000; Punzo, 2002). Além destes fatores, podem existir mudanças ontogenéticas no tipo de dieta (Cardona et al., 2010).
Os predadores possuem um importante papel na estruturação de comunidades, pois podem possibilitar que espécies competidoras coexistam prevenindo que determinadas espécies superiores na hierarquia competitiva monopolizem os recursos. Este fenômeno é conhecido como coexistência mediada pelo predador (Paine, 1966). Uma meta-análise realizada por Gurevich et al., (2000), que comparava o efeito da competição na presença e ausência de predadores, demonstrou que de fato os competidores tiveram uma maior influência em parâmetros como o crescimento, sobrevivência e massa, quando não havia predadores do que quando os predadores estavam presentes. No entanto, determinar o efeito da predação em uma comunidade natural pode ter complicadores, como os efeitos
3 A interação entre presas e predadores ao longo do tempo dá origem a uma relação
coevolutiva conhecida como “corrida armamentista” (e.g. Thompson, 2013e refêrencias neste). Esta relação possui um caráter assimétrico e antagônico, pois para os predadores, capturar presas é vantajoso devido ao ganho de energia. Para as presas ser capturado significa morrer (Dawkins & Krebs, 1979). Assim, a pressão da predação seleciona presas com
melhores mecanismos defensivos, e predadores com melhores estratégias de caça (Davies & Krebs, 2012).
Os predadores apresentam várias adaptações para capturar presas, como estruturas sensoriais bem desenvolvidas, adaptações para escolha do sítio de forageio, mecanismos de aproximação, habilidades motoras, estratégias e estruturas ofensivas (Davies & Krebs, 2012). As presas, por sua vez, possuem adaptações morfológicas como um tegumento espesso e espinhos, além de mecanismos comportamentais como aposematismo, anacorese, tanatose, camuflagem e defesas químicas (Edmunds, 1974; Eisner, 2005; Davies & Krebs, 2012). Outra estratégia de alto valor adaptativo conhecida entre as presas é a capacidade de modular os comportamentos defensivos de acordo com o nível de ameaça imposto pelo predador (Helfman, 1989).
Esta breve introdução objetivou tratar sucintamente alguns dos domínios nos quais a interação presa-predador está direta ou indiretamente ligada. A presente dissertação aborda este tipo de interação sob uma perspectiva ecológico-evolutiva, buscando compreender quais as estratégias que possibilitam que a aranha Loxosceles gaucho consuma opiliões, que são conhecidos por sua secreção defensiva e por possuírem um tegumento espesso bastante esclerotizado (Primeiro capítulo, formatado de acordo com as normas da revista Animal behaviour). O Segundo capítulo (que segue as normas da revista Behaviour) investiga como o opilião Mischonyx cuspidatus reage a este predador em uma situação de risco moderado (frente aos químicos do predador) e em uma situação de alto risco (frente à aranha L.gaucho). Adicionalmente, este estudo contribuirá para o conhecimento das aranhas Loxosceles, um gênero de importância médica (Veter, 2008), uma vez que, até onde temos conhecimento, este será o primeiro estudo a descrever o comportamento de captura de presas em uma das
4 REFERÊNCIAS
Barbosa, P. & Castellanos, I. (eds) (2013). Ecology of predator–prey interactions. Oxford, UK: Oxford University Press.
Begon, M., Townsend, C. R., & Harper, J. L. (2007). Ecologia: de indivíduos a ecossistemas. (4th ed.) Porto Alegre, Brazil, Artmed.
Cardona L., Campos P., Levy Y., Demetropoulos A. & Margaritoulis D. (2010). Asynchrony between dietary and nutritional shifts during the ontogeny of green turtles (Chelonia mydas) in the Mediterranean. Journal of Experimental Marine Biology and Ecology, 393, 83–89.
Cresswell W & Quinn J.L. (2010). Attack frequency, attack success and choice of prey group size for two predators with contrasting hunting strategies. Animal Behaviour, 80, 643-648. Davies, N. B., Krebs, J. R., & West, S. A. (2012). An introduction to behavioural ecology.
(4th ed.) Chichester, UK: Wiley-Blackwell.
Dawkins, R., & Krebs, J. R. (1979). Arms races between and within species. Proceedings of the Royal Society of London. Series B. Biological Sciences, 205, 489-511.
Edmunds, M. (1974). Defence in animals: a survey of anti-predator defences. London, UK: Longman.
Eisner, T., Eisner, M., & Siegler, M. V. (2005).Secrets Weapons: Defenses of Insects, Spiders, Scorpions, and Others Many-legged Creatures. Cambridge, USA: Harvard University Press.
Elton, C. S. (1924). Periodic fluctuations in the numbers of animals: their causes and effects. Journal of Experimental Biology, 2, 119-163.
Fenk, L. M., Hoinkes, T., & Schmid, A. (2010). Vision as a third sensory modality to elicit attack behavior in a nocturnal spider. Journal of Comparative Physiology A, 196, 957-961. Gurevitch, J., Morrison J. A., & Hedges L. V. (2000). The Interaction between Competition
and Predation: A Meta analysis of Field Experiments. The American Naturalist, 155, 435-45.
Helfman, G. S. (1989). Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behavioral Ecology and Sociobiology, 24, 47-58.
5 Paine, R.T. (1966). Food Web Complexity and Species Diversity. The American Naturalist,
100: 65-75.
Persons, M. H. & Rypstra A. L. (2000). Preference for chemical cues associated with recent prey in the wolf spider Hogna helluo. (Araneae: Lycosidae) Ethology, 106 27-35.
Punzo, F. (2002). Early Experience and Prey Preference in the Lynx Spider, Oxyopes salticus Hentz (Araneae:Oxyopidae). Journal of the New York Entomological Society. 110, 255-259.
Ridley, M. Evolution. (2004). (2nd ed.), USA, Oxford University Press.
Ricklefs, R. E. (2003). A economia da natureza. Rio de Janeiro, Brazil, Guanabara-Koogan. Sih, A., Englund, G., & Wooster, D. (1998). Emergent impacts of multiple predators on
prey. Trends in Ecology & Evolution, 13, 350-355.
Spitz J., Mourocq E., Leauté J.P., Quéro J.C. & Ridoux. (2010). Prey selection by the common dolphin: fulfilling high energy requirements with high quality food. Journal of Experimental Marine Biology and Ecology, 390, 73–77.
Thompson, John N. (2013). Relentless evolution. Chicago, USA: University of Chicago Press. Vetter, R. S. (2008). Spiders of the genus Loxosceles (Araneae, Sicariidae): a review of
6
FIRST CHAPTER
Delicate fangs, smart killing: the predation strategy of the brown recluse spider overcomes heavily sclerotized prey
ABSTRACT
Consuming prey involves finding an adequate site to forage, detecting and manipulating it. We investigated these three steps in interactions between the delicate recluse
spider Loxosceles gaucho (Araneae) and a heavy bodied and armored harvestman, here represented by the harvestman Mischonyx cuspidatus (Opiliones). The hard integument of such harvestmen had been previously reported to protect them from spiders larger than Loxosceles. We tested the following hypotheses: (1) spiders prefer areas with cues from their prey, (2) vibratory cues are essential information in the predatory process and (3) the web sheet allows handling prey adequately so that the vulnerable spots of the prey can be bitten. To understand exactly how a delicate predator can overcome the defenses of a heavy bodied and well-defended prey, we also quantitatively described the spider´s behavior. To test hypothesis (1), we compared the time spent in areas with harvestmen, crickets and no cues. For hypothesis (2), we compared latency to bite and number of bites in the presence or absence of vibratory cues, and for hypothesis (3) we compared latency to detect, to capture and predation success. All hypotheses were rejected. Loxosceles gaucho seems to be exceptional among spiders by not needing its web, indirect prey chemical cues, or prey´s substrate borne vibrations to hunt it. What seems to enable Loxosceles to prey upon M. cuspidatus is its totally different hunting strategy, compared to previously studied spiders attempting to eat these armored harvestmen. The spider quickly touches the prey with its tarsi, finding and biting several times the weak parts of the prey body, such as joints and distal parts of the legs. With several previous papers reporting spiders rejecting these harvestmen, this is the first that describes how a spider overcomes the defenses of an armored harvestman.
7 INTRODUCTION
Prey-predator interactions shape each other so that both prey defences and predatory strategies improve in evolutionary time in a process known as arms races (Dawkins & Krebs 1979). The predatory process encompasses the phases of search, capture and handling prey (Davies et al 2012). The search phase begins with the choice of a foraging site. Distinct pieces of information are used to assess the quality of a spot, such as the presence of conspecifics (review in Danchin et al 2004; Valone 2007), environmental characteristics that potentially attract prey, the presence of prey, prey cues or physical characteristics that facilitates prey capture (Chien & Morse 1998; Clark et al 2000; Hopcraft et al 2005; Johnson 2011; Hanna & Eason 2013).
After picking an adequate foraging site, predators need to detect prey using distinct sensory modalities. These include vision in frogs (González-Bernal et al 2011); the perception of substrate-borne vibration in scorpions (Mineo & Del Claro 2006); echolocation in bats (Schnitzler & Kalko 2001); tactile sense in star-nosed moles and shrews (Catania & Remple 2005; Anjum et al 2006); thermal sensitivity in snakes (Buning 1983) or chemoreception in salamanders (Placyk & Graves 2002), for example. Very often, multiple sensory modalities are important for prey detection (Piep et al 2008).
After detecting prey, predators must capture and handle them, which involve distinct structures and behaviors. To give a few examples, snakes use venom or constriction (Greene 1978; Kardong 1986), chameleons rapidly extend their sticky tongues (Herrel et al 2000; de Groot & Leween 2004), insects may move over the prey and directly bite quickly (De la Mora et al 2008), or use raptorial legs (Correte 1990; Betz & Mumm 2001). Among arachnids, the structures used to capture and handle prey may be chelicerae, pedipalps (Casper 1985; de Andrade & Gnaspini 2002; Ladle & Velandes 2003; Beccaloni 2009; Del Claro & Tizo-Pedroso 2009; Santer & Hebets 2009; Willemart et al 2011), and adhesive setae on the legs (Rovner 1980), sometimes with the help of venom in scorpions, pseudoscorpions and spiders (Weygoldt 1969; Bub & Bowerman 1979; Foelix 2011).
8 & Rypstra 2000; Jackson et al 2002). In the following stage, when the prey is close and
specifically in the phase of capture, substrate-borne vibrations have a fundamental role in web spiders, wandering spiders and even in spiders that capture prey on aquatic environment (Hergenröder & Barth 1983; Bleckmann & Barth 1984; Masters 1984; Barth 2002).
Among the several species eaten by spiders, harvestmen are certainly an interesting prey from a behavioral point of view. Harvestmen exhibit many kinds of defences such as cripsis, thanatosis, anachoresis, aposematism, mimicry, deimatic behavior and fleeing (see Gnaspini & Hara 2007). Chemical defence has been the most studied, with harvestmen releasing defensive secretions through glandular openings positioned latero-dorsally on the body (Eisner et al 2004; Hara & Gnaspini 2003; Hara et al 2005; Machado & Pomini 2008; Pomini et al 2010). Such chemicals have been shown to be efficient against spiders (eg. Machado et al 2005), but they do not seem to be always used. Instead, a thick cuticle of adults seems to be protective against predatory some spiders (Souza & Willemart 2011; Dias & Willemart 2013). Unless they are strong enough to pierce the thick cuticle, spiders are left only a few vulnerable spots to bite, such as the distal parts of legs, mouth and articulations (Souza & Willemart 2011). Wandering spiders previously tested with heavy bodied laniatorid harvestmen as prey have a low success rate (Eisner et al. 2004; Souza & Willemart 2011; Carvalho et al 2012; Dias & Willemart 2013) despite their large size or ability to spit venom. The large Enoploctenus cyclothorax, for example, did not feed on the harvestman Mischonyx cuspidatus even after sharing a small terrarium with it for about 70 days and with no
alternative food source (Willemart & Pellegatti-Franco 2006). In contrast, we often find dead harvestmen on the horizontal sheets of web of recluse spiders (Loxosceles) (Fischer et al 2006; Fig. 1). Despite being world famous for causing severe skin wounds and being of medical importance (Cardoso et al 2009), there are no detailed studies on how recluse spiders hunt their prey. Because these spiders have delicate body and chelicerae, Carvalho et al (2012) hypothesized that they could prey upon harvestmen because the sheet of web would help immobilizing the prey, allowing the spiders to bite the vulnerable parts of the harvestman body.
9 detect their prey: we hypothesized that vibratory cues would be essential information in the predatory process. We also tested the hypothesis of Carvalho et al (2012), that the web sheet would allow to handle prey adequately so that the vulnerable spots would be bitten. In order to understand exactly how a delicate predator can overcome the defences of a heavy bodied and well-defended prey that is rejected by much larger predators (Souza & Willemart 2011; Dias & Willemart 2013), we also quantitatively described the spider´s behavior.
METHODS
Species studied
The harvestmen Mischonyx cuspidatus (Roewer 1913) and spiders of the genus Loxosceles are active mainly at night (Pereira et al 2004; Fischer et al 2006) and can be found under tree trunks, dead palm fronds and anthropized spots, often under bricks (Mestre & Pinto-da-Rocha 2004; Fischer & Vasconcelos-Neto 2005). Loxosceles spiders are polyphagous, feeding on a wide variety of arthropods (Fischer et al 2006).
In our study, the spiders Loxosceles gaucho Gertsch 1967 were collected in Mairiporã city, São Paulo State Brazil (23◦19 S, 46◦35 W), within building material, between November 2012 and February 2013. We found individuals of M. cuspidatus on the exact same bricks where the spiders were collected, but not in enough quantity for the experiments. The harvestmen M. cuspidatus used in the experiments were therefore collected at the "Parque Ecológico do Tietê - São Paulo city, São Paulo State, Brazil (23◦25 S, 46◦28 W) between December, 2012 and May 2013, under tree trunks.
Laboratorial conditions
10 Figure 1.The recluse spider Loxosceles sp. in the field, in a dead palm frond, with three harvestmen carcasses (white setae). In this case the web sheet where the spider and prey carcasses are on is very thin and can hardly be seen.
Experiment 1: chemicals of prey as information for the predator
This experiment was conducted to test whether L. gaucho uses chemicals left on substrate by its prey when foraging. We predicted that the spiders would spend more time in places with chemicals of harvestmen (M. cuspidatus) and crickets (Gryllus sp.) than on white controls.
The spiders used were previously fed on crickets and harvestmen to minimize biases, because the previous feeding experience can influence future decisions about what to eat (Persons & Rypstra 2000). The food deprivation period before the trials was between 37-38 days.
11 To impregnate the filter paper with chemicals of prey (harvestmen or crickets), 15 individuals were left for 24 hours in a recipient similar to the arenas, with two pieces of filter paper on the substrate. The filter papers were removed immediately before the tests.
The spider was introduced in the central neutral place in a vial (5cm diameter x 6 cm height) and acclimated for 3 minutes. After releasing the spider we covered the arena with a sheet of glass. We recorded the animals for 30 minutes in each test. After each trial, we horizontally turned the arena 45° clockwise to minimize biases related to the position of the arena in relation to the laboratory. We used a Sony Handycam HDXR550V in nightshot mode to register the time spent in each side of the arena. The arena was cleaned with 70% ethanol between trials and each piece of filter paper was only used once. The data were collected in May 2013, between 8 pm and 5 am. We used paired t-tests to analyze the data, and therefore measures of central tendency presented here are for the differences between the time spent in each side of the arena.
Experiment 2: substrate vibration as information for predators and predatory behavior with
distinct prey
In this experiment we tested whether substrate-borne vibrations are important in the predatory process for L. gaucho with harvestmen and crickets, tested separately. We predicted that predatory success would be higher and latency to attack would be lower in spiders tested in a substrate that transmits vibration (filter paper) than in spiders tested in granite, a substrate that greatly reduces the transmission of substrate-borne vibrations (Elias et al 2004; Hebets 2004). Because harvestmen have fewer vulnerable spots to bite than crickets (Souza & Willemart 2011) so that spiders would not be able to pick body regions more sensitive to venom, we also predicted that handling time would be larger and more bites would be used against harvestmen.
12 We tested the spiders offering prey in four different situations, all of them in a
circular arena (18 cm diameter x 6 cm height) made of plastic walls, open on the bottom, placed either on the filter paper or on the granite block: (i) harvestman on filter paper
substrate (N = 14, all females); (ii) harvestman on granite substrate (N = 14, all females); (iii) cricket on filter paper substrate (N = 14, all females); and (iv) cricket on granite substrate (N = 14, 13 females and 1 male);. Each spider was tested only once. Both spider and prey were introduced in the arena and acclimated for 3 minutes in separate recipients (5 cm diameter x 6 cm height) and in opposite sides of the arena. The animals were released together and the arena was covered with a sheet of glass.
The experiments were recorded with a camera Sony Handycam HDXR550V, in nightshot mode. The time of recording was standardized in 40 minutes, starting when contact was established. If prey and predator did not touch each other after 40 min, we considered there was no predation. The trials were conducted in April 2013, from 8PM to 5AM. We compared predation success between substrates with a Chi-Square, latencies to attack with a Mann-Whitney, time between first bite and long bite and number of bites with a t-test
Experiment 3: the role of silk in predation
This experiment was conducted to test the hypothesis that the sheet of silk of L. gaucho is necessary to capture the harvestmen. We predicted that the spiders tested with the sheet of silk (control group) would have a greater success in capturing and that the latency to detect and capture prey would be shorter in the absence of silk (treatment group). Spiders in both the no silk group (nsg) (N = 19, 17 females and 2 males) and the silk group (sg) (N = 19, 13 females and 6 males) were offered 10 females and 9 male harvestmen, totalizing 38
13 remove remnants of silk and then put new soil in it. Changing the soil was necessary to
completely remove the silk of the terrarium. We then reintroduced the spider. In both groups, we allowed the spider to walk freely in the terrarium for 15 minutes before we introduced a harvestman as far as possible from the spider.
We registered the trials using a Sony Handycam HDXR550V in the nightshot mode. We started recording after introducing the harvestman and recorded for 40 minutes after the predatory process started (defined as first bite). Each spider was tested only once. We collected the data between February and March 2013 at night time (Between 9 PM and 5 AM). We compared capture success with a Chi-Square, latencies between behavioral categories with a Mann-Whitney and number of bites with a t-test.
RESULTS
Experiment 1: chemicals of prey as information for the predator
The time (in seconds) spent in each side of the arena with chemicals of
harvestmen/crickets did not differ (median of the difference harvestmencricket: 127; min: -1800, max: 1219; t = 0.190; df = 18; P = 0.852). For the combination harvestmen/blank there was also no difference (median of the difference harvestmen-blank: 255; min: -1071, max: 1518; t = 0.977; df = 16; P = 0.343). But spiders spent slightly more time in blank filter paper than on cricket cues (median of the difference cricket-blank: -217, min: -897, max: 1122; t = 2.387; df = 16; P = 0.03).
Experiment 2: substrate vibration as information for predators
Ten out of 14 harvestmen were preyed upon in each of the substrates (χ² = 0; df = 1; P = 1). Among crickets, nine were preyed upon in filter paper (fp) and seven on granite (g) (χ² = 0.583; df = 1; P = 0.699) (N = 14 for each treatment). The latency to bite compared between the substrates of filter paper (median = 268.5; min: 111, max 745, N = 10) and granite (median = 156; min: 21, max 643, N = 11) was not different within the harvestmen (U = 45;
14 (median = 157; min: 52, max 1358, N = 9) and granite (median = 411; min: 48, max 950, N = 9) (U = 43; N1fP = 9; N2g = 9; P = 0.825).
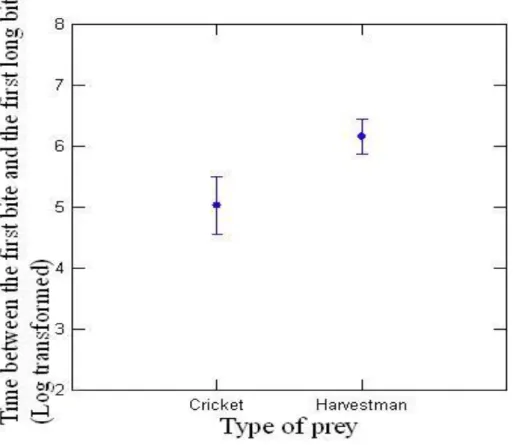
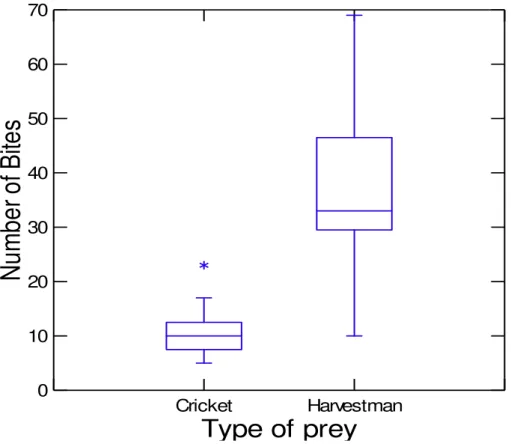
Because substrate was unimportant, we pooled the data from granite and filter paper to compare the predation rate between crickets and harvestmen and found no differences (χ² = 1.244; df = 1; P = 0.265). However, the time between the first bite and the first long bite was longer for harvestmen (t = 2.116 df = 28 P = 0.043) (Figure 2), and the number of bites on harvestmen was higher (U = 27.500 Ncricket = 18 Nharvestman = 21; p < 0.001) (Figure 3).
Because there were no differences in the predation rate of harvestmen among treatments, we used the videos of both substrate groups to analyse where the spiders bit the harvestmen's body (Figure 4).
Figure 2. Time between the first bit and the first long bit in harvestmen (N = 15) and crickets (N = 15). The data was transformed in logarythm to suit the premisses of parametrical
15 Cricket Harvestman
Type of prey
010 20 30 40 50 60 70
Nu
m
be
r
of
B
ite
s
Figure 3. Number of bites performed by Loxosceles gaucho when preying upon crickets (N = 16; max = 23; min = 1) and harvestmen (N = 20; max = 69; min = 1).
Experiment 3: the role of silk on predation
Contrary to our expectations, the presence of silk did not influence the capture success of harvestmen by the spider L. gaucho. We described 17 behavioral categories of the spider when interacting with the harvestman (Table 1). A typical capture sequence (Figure 6) starts
with prey detection either at a distance or after “Passive contact”, with either the prey or both walking. The spider would then “Approach”) and/or “Touch prey". After biting the prey
16 Seventeen spiders preyed upon the harvestmen in the silk group (N = 19) and 14 in the no silk group (N = 19) (χ² = 1.576; df = 1; P = 0.209). The number of bites performed by the spiders (X̄sg = 24.8 ± 11.9; min = 9; max = 52; X̄nsg = 20.5 ± 9.1; min = 1; max = 34; t =
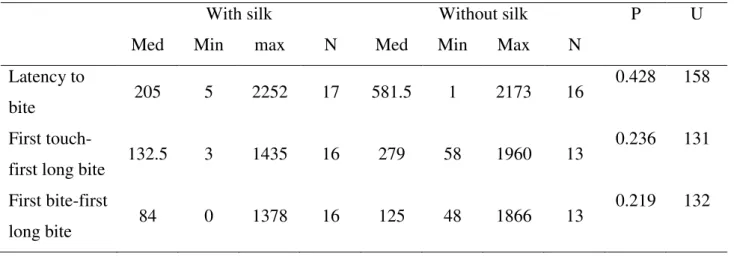
1.136; df = 30; P = 0.265) did not differ between the groups. Latency to bite, the time between the first touch and the first long bite, and the time between the first bite and the first long bite also did not differ between the two groups (Table 2).
17 Table 1
Behavioral repertory of the spider Loxosceles gaucho when interacting with the harvestman Mischonyx cuspidatus.
Behavioral category Definition
Active contact Spider moves mainly its legs I and II and touches the prey with the distal portion
Approach Spider moves towards the prey, either following a straight way or in a zigzag manner
Detection at a distance The spider raises any of its legs in a slow dorso-ventral movement, often of small amplitude and without touching the substrate. A variation of this category is to quickly move the legs
Drag The spider drags the harvestman with their first pair of legs or the chelicerae, holding the prey by its legs
Handling silk Spider performs repeated movements with its first, second or third pair of legs, extending and flexing them sideways, in a movement with short amplitude. Some variations of this category include rubbing legs I, II e III between them in dorso-ventral movements and quick antero-posterior movements with pedipalps
Leg-threading Spider passes the leg of the second pair between its chelicerae
Long bite Spider pinches the prey with the fangs of chelicerae for ten or more seconds (the spider can hold the prey for minutes) Sometimes the spider weaves silk threads and/or apparently handle threads of silks often with its third pair of legs (Figure 5)
18 Move pedipalps
quickly
Fast dorso-ventral movements of pedipalps
Motionless Not moving the body or the locomotory appendages
Orient to prey To rotate the body without displacement, ending with the anterior portion of the body facing the prey
Passive contact While walking a prey touches a spider
Short bite Spider pinches the prey with the fangs of the chelicerae for less than 10 seconds. This behavior can happen while the spider is touching the prey (mainly with its legs) and/or while the spider taps the harvestmen and/or weaving threads of silk
Touch with legs Spider maintains the legs on the body of the harvestman
Sometimes the spider taps the prey with its legs and/or pedipalps
Tremble Spider performs movements of small amplitude and flexion of
legs without withdrawing the legs of the ground
Wa-zari Spider pulls the harvestman mainly with its first pair of legs grabbing it by the fourth pair of legs close to the patella or to the femur-trochanter joint and slowly throws the harvestman with the dorsum on the floor
Weave silk Spider weaves silk threads, sometimes while maintaining the appendages (mainly the legs) on the harvestmen body
19 Table 2
Time (in seconds) spent to some acts of predation by Loxosceles gaucho when preying upon Myschonix cuspidatus
With silk Without silk P U
Med Min max N Med Min Max N
Latency to
bite 205 5 2252 17 581.5 1 2173 16
0.428 158
First
touch-first long bite 132.5 3 1435 16 279 58 1960 13
0.236 131
First bite-first
long bite 84 0 1378 16 125 48 1866 13
0.219 132
20 Figure 6. Fluxogram showing behavioral sequence of spiders that preyed upon harvestmen in the silk group (arrows and percentage in black – N = 17) and in the no silk group (arrows and percentage in grey – N = 14). Arrows represent the percentage of times behaviour A was followed by behaviour B. For the sake of clarity, only behavioral sequences with frequencies higher than 25% are included (except the final categories "Long bite" and "Ignore"). The behaviors "Move pedipalps quickly", "Drag " and "Tremble" were omitted because they occurred just in one group or in both groups.
DISCUSSION
Loxosceles gaucho do not use harvestmen´s or cricket‟s chemical cues to choose
where to forage, do not rely on vibratory cues to detect the tested prey and exhibits a high predation rate of heavy bodied laniatorid harvestmen. These spiders are not dependent on the sheet of silk to capture harvestmen and behave very similarly in the presence or absence of silk, at least against this specific prey.
21 clearly show that the presence of the sheet of silk is not decisive. Moreover, we found a high predation rate, showing that the spider L. gaucho do prey upon harvestmen, contrasting with much more robust spiders such as Enoploctenus cyclothorax (Bertkau 1880) and Ctenus ornatus (Keyserling, 1877) (Willemart & Pellegatti-Franco 2006; Souza & Willemart 2011; Dias & Willemart 2013) or the spitting-spider Scytodes globula Nicolet 1849 that cannot prey on the harvestman (Carvalho et al 2012).
In the fluxogram, a strong association between the categories "Touch with legs" and "short bite" was found in the silk and no silk groups. This behavior probably allows the spider to find the weak spots of the harvestman´s body, such as joints and the distal portions of the tarsi (Souza & Willemart 2011). Corroborating the suggested correlation between these two behavioral categories, C. ornatus and E. cyclothorax do not choose where to bite and do not
display “Touch with legs” (Souza & Willemart 2011; Dias & Willemart 2013). Loxosceles gaucho seem therefore to have a behavioral strategy that allows it to prey upon well armored prey, differently from some spiders of the genus Dysdera (Dysderidae) which, in addition to distinct behavior, also have modified chelicerae that allow them to prey upon armored
woodlouse (Isopoda: Oniscoidea) (Řezáč et al 2008). The fluxogram also show that similar behavioral sequences are efficient to subdue the harvestmen in the presence or absence of silk. We suggest this is due to the fact that these harvestmen are not fast runners or jumpers, so that silk is not necessary for the venomous bites to take place at the exact spots.
Because we did not find differences in the latency to attack in a substrate that transmits vibration compared to one that does not, we rejected our hypothesis that substrate-borne vibrations are fundamental information in the capture process of L. gaucho. This was quite interesting not only because this is a main sensory modality among spiders
22 Our results show that the time between the first bite and first long bite and the number of bites performed by the spiders was greater in harvestmen than in crickets. These results are probably related to the barrier imposed by the thick cuticle of the harvestmen (Souza & Willemart 2011; Dias & Willemart 2013), which can prevent the spiders to access more vulnerable areas such as the main areas of the nervous system. The spider would compensate that by biting more, maximizing the chances of immobilizing the prey.
Due to the greater time of handling and greater number of bites it is probable that L.gaucho spends more energy attacking harvestmen than crickets, potentially leading to prey preference. But our experimental set up was testing prey separately and in this case both prey were equally eaten. The same set up with a less intense starvation period could also increase preference for crickets. That could be related to the low proportion of harvestmen found on the webs of L.intermedia, in spite of the great number of harvestmen using the same
microhabitat of the spider (Fischer et al 2006).
No preference for substrates with prey chemicals were detected in our study. This was similar to results found for salticid spiders (Hoefler et al 2002) but contrasts with the results obtained for lycosid and oxyopid spiders (Persons & Uetz 1996; Punzo & Kukoyi 1997; Persons & Rypstra 2000). It is possible that the use of chemicals as a source of information to find prey is restricted to some spider species. On the other hand, our results showed that the time spent in the area with no chemicals was greater that the time spent in the area with cricket chemicals. In this way, apparently these spiders can detect chemicals on the substrate, but for some reason do not use this information to pick a foraging spot.
23 REFERENCES
Anjum, F., Turni, H., Mulder, P. G., van der Burg, J., & Brecht, M. (2006). Tactile guidance of prey capture in Etruscan shrews. Proceedings of the National Academy of Sciences, 103, 16544-16549.
Barth, F. G. (2002). A spider's world: senses and behavior. Springer.
Betz, O., & Mumm, R. (2001). The predatory legs of Philonthus marginatus (Coleoptera, Staphylinidae): functional morphology and tarsal ultrastructure. Arthropod Structure & Development, 30, 77-97.
Bleckmann, H., & Barth, F. G. (1984). Sensory ecology of a semi-aquatic spider (Dolomedes triton). Behavioral Ecology and Sociobiology, 14, 303-312.
Bub, K., & Bowerman, R. F. (1979). Prey capture by the scorpion Hadrurus arizonensis Ewing (Scorpiones: Vaejovidae). Journal of Arachnology, 7, 243-253.
Buning, T. D. C. (1983). Thermal sensitivity as a specialization for prey capture and feeding in snakes. American Zoologist, 23, 363-375.
Cardoso, J. L. C., França, F. D. S., Wen, F. H., Malaque, C. M. S., & Haddad Jr, V. (2003).
Animais peçonhentos no Brasil: biologia, clínica e terapêutica dos acidentes.Sarvier
(Almed).
Carvalho, L.A., Souza, E.S. & Willemart R.H. (2012). Behavioral analysis of the interaction between the spitting spider Scytodes globula (Araneae: Scytodidae) and the harvestman Discocyrtus invalidus (Opiliones: Gonyleptidae). Journal of Arachnology, 40, 332-337. Casper, G. S. (1985). Prey capture and stinging behavior in the emperor scorpion, Pandinus
imperator (Koch) (Scorpiones, scorpionidae). Journal of Arachnology, 13, 277-283. Catania, K. C., & Remple, F. E. (2005). Asymptotic prey profitability drives star-nosed moles
to the foraging speed limit. Nature, 433, 519-522.
Chien, S. A. & Morse, D. H. (1998). The roles of prey quality in the choice of hunting sites by adult male crab spiders Minusena vatia (Araneae, Thomisidae). The Journal of
Arachnology, 26, 238-23.
24 Clark, R. J., Jackson, R. R., & Cutler, B. (2000). Chemical cues from ants influence predatory
behavior in Habrocestum pulex, an ant-eating jumping spider (Araneae, Salticidae). Journal of Arachnology, 28, 309-318.
Danchin, E., Giraldeau, L. A., Valone, T.J. & Wagner, R.H. (2004). Public information: from nosy neighbors to Cultural evolution. Science, 305, 487-491.
Davies, N. B., Krebs, J. R., & West, S. A. (2012). An introduction to behavioural ecology. (4th ed.) Chichester, UK: Wiley-Blackwell.
Dawkins, R., & Krebs, J. R. (1979). Arms races between and within species. Proceedings of the Royal Society of London Series B. Biological Sciences, 205, 489-511.
de Andrade, R., & Gnaspini, P. (2002). Feeding in Maxchernes iporangae (Pseudoscorpiones, Chernetidae) in captivity. Journal of Arachnology, 30, 613-617.
De la Mora, A., Pérez-Lachaud, G., & Lachaud, J. P. (2008). Mandible strike: The lethal weapon of Odontomachus opaciventris against small prey. Behavioural processes, 78, 64-75
Del-Claro, K., & Tizo-Pedroso, E. (2009). Ecological and evolutionary pathways of social behavior in Pseudoscorpions (Arachnida: Pseudoscorpiones). Acta Ethologica, 12, 13-22. de Groot, J. H., & van Leeuwen, J. L. (2004). Evidence for an elastic projection
mechanism in the chameleon tongue. Proceedings of the Royal Society of London-B, 271, 761.
Dias, B.C. Willemart, R.H. (2013). The effectiveness of post-contact defenses in a prey with no pre-contact detection. Zoology, 116, 168-174.
Eisner, T., Rossini, C., González, A., & Eisner, M. (2004). Chemical defense of an opilionid (Acanthopachylus aculeatus). Journal of Experimental Biology, 207, 1313-1321.
Fenk, L. M., Hoinkes, T., & Schmid, A. (2010). Vision as a third sensory modality to elicit attack behavior in a nocturnal spider. Journal of Comparative Physiology, 196, 957-961. Fischer, M. L., & Vasconcellos-Neto, J. (2005). Microhabitats Occupied by Loxosceles
intermedia and Loxosceles laeta (Araneae: Sicariidae) in Curitiba, Paraná, Brazil. Journal of Medical Entomology, 42, 756-765.
25
Fischer, M. L., Čokl, A., Ramires, E. N., Marques-da-Silva, E., Delay, C., Fontana, J. D., Donatti, L., Schneider, V.F. & Marques, F. D. A. (2009). Sound is involved in multimodal communication of Loxosceles intermedia Mello-Leitão, 1934 (Araneae;
Sicariidae). Behavioural Processes, 82, 236-243.
Foelix, R. (2011). Biology of spiders. Oxford University Press.
Gnaspini, P. & Hara, M. R. 2007. Defense mechanisms. In: Harvestmen: the Biology of Opiliones (Ed. by R. Pinto-da-Rocha, G. Machado & G. Giribet), pp. 374e399. Cambridge, Massachusetts: Harvard University Press.
González-Bernal, E., Brown, G. P., Cabrera-Guzmán, E., & Shine, R. (2011). Foraging tactics of an ambush predator: the effects of substrate attributes on prey availability and predator feeding success. Behavioral Ecology and Sociobiology, 65, 1367-1375.
Greene, H. W., & Burghardt, G. M. (1978). Behavior and phylogeny: constriction in ancient and modern snakes. Science, 200, 74-77.
Hanna, C. J., & Eason, P. K. (2013). Juvenile crab spiders (Mecaphesa asperata) use indirect cues to choose foraging sites. Ethology Ecology & Evolution, 25, 161-173.
Hara, M. R., & Gnaspini, P. (2003). Comparative study of the defensive behavior and morphology of the gland opening area among harvestmen (Arachnida, Opiliones,
Gonyleptidae) under a phylogenetic perspective. Arthropod Structure & Development, 32, 257-275.
Hara, M. R., Cavalheiro, A. J., Gnaspini, P., & Santos, D. Y. (2005). A comparative analysis of the chemical nature of defensive secretions of Gonyleptidae (Arachnida: Opiliones: Laniatores). Biochemical Systematics and Ecology, 33, 1210-1225.
Herberstein, M. E. (Ed.). (2011): Spider behaviour: flexibility and versatility. Cambridge University Press.
Hergenröder, R., & Barth, F. G. (1983). Vibratory signals and spider behavior: How do the sensory inputs from the eight legs interact in orientation?. Journal of comparative physiology, 152, 361-371.
Herrel, A., Meyers, J. J., Aerts, P., & Nishikawa, K. C. (2000). The mechanics of prey prehension in chameleons. Journal of Experimental Biology, 203, 3255-3263.
26 Hoefler, C. D., Taylor, M., & Jakob, E. M. (2002). Chemosensory response to prey in
Phidippus audax (Araneae, Salticidae) and Pardosa milvina (Araneae, Lycosidae). Journal of Arachnology, 30, 155-158.
Jackson, R. R., Clark, R. J., & Harland, D. P. (2002). Behavioural and cognitive influences of kairomones on an araneophagic jumping spider. Behaviour, 139, 749-776.
Johnson, A., Revis, O., & Johnson, J. C. (2011). Chemical prey cues influence the urban microhabitat preferences of Western black widow spiders, Latrodectus hesperus. Journal of Arachnology, 39, 449-453.
Kardong, K. V. (1986). Predatory strike behavior of the rattlesnake, Crotalus viridis oreganus. Journal of Comparative Psychology, 100, 304.
Klopsch, C., Kuhlmann, H. C., & Barth, F. G. (2013). Airflow elicits a spider's jump towards airborne prey. II. Flow characteristics guiding behaviour. Journal of The Royal Society Interface, 10.
Ladle, R. J., & Velander, K. (2003). Fishing behavior in a giant whip spider. Journal of Arachnology, 31, 154-156.
Machado, G., Carrera, P. C., Pomini, A. M., & Marsaioli, A. J. (2005). Chemical defense in harvestmen (Arachnida, Opiliones): Do benzoquinone secretions deter invertebrate and vertebrate predators? Journal of Chemical Ecology, 31, 2519-2539.
Machado, G., & Pomini, A. M. (2008). Chemical and behavioral defenses of the neotropical harvestman Camarana flavipalpi (Arachnida: Opiliones). Biochemical Systematics and Ecology, 36, 369-376.
Masters, W. M. (1984). Vibrations in the orbwebs of Nuctenea sclopetaria (Araneidae). Behavioral Ecology and Sociobiology, 15, 207-215.
Mestre, L. A. M., & Pinto-da-Rocha, R. (2004). Population dynamics of an isolated population of the harvestman Ilhaia cuspidata (Opiliones, Gonyleptidae), in Araucaria Forest (Curitiba, Paraná, Brazil). Journal of Arachnology, 32, 208-220.
Mineo, M. F., & Del Claro, K. (2006). Mechanoreceptive function of pectines in the Brazilian yellow scorpion Tityus serrulatus: perception of substrate-borne vibrations and prey
detection. Acta Ethologica, 9, 79-85.
27 Persons, M. H., & Uetz, G. W. (1996). The influence of sensory information on patch
residence time in wolf spiders (Araneae: Lycosidae). Animal Behaviour, 51, 1285-1293. Persons, M. H., & Rypstra, A. L. (2000). Preference for chemical cues associated with recent
prey in the wolf spider Hogna helluo (Araneae: Lycosidae). Ethology, 106, 27-35.
Piep, M., Radespiel, U., Zimmermann, E., Schmidt, S., & Siemers, B. M. (2008). The sensory basis of prey detection in captive-born grey mouse lemurs, Microcebus murinus. Animal Behaviour, 75, 871-878.
Placyk Jr, J. S., & Graves, B. M. (2002). Prey detection by vomeronasal chemoreception in a plethodontid salamander. Journal of Chemical Ecology, 28, 1017-1036.
Pomini, A. M., Machado, G., Pinto-da-Rocha, R., Macías-Ordóñez, R., & Marsaioli, A. J. (2010). Lines of defense in the harvestman Hoplobunus mexicanus (Arachnida: Opiliones): Aposematism, stridulation, thanatosis, and irritant chemicals. Biochemical Systematics and Ecology, 38, 300-308.
Punzo, F., & Kukoyi, O. (1997). The effects of prey chemical cues on patch residence time in the wolf spider Trochosa parthenus (Chamberlin) (Lycosidae) and the lynx spider Oxyopes salticus Hentz (Oxyopidae). Bulletin of the British Arachnological, Society, 10, 323-326.
Řezáč, M., Pekár, S., & Lubin, Y. (2008). How oniscophagous spiders overcome woodlouse
armour. Journal of Zoology, 275, 64-71.
Rinaldi, I. M. P., & Stropa, A. A. (1998). Sexual behaviour in Loxosceles gaucho Gertsch (Araneae, Sicariidae). Bulletin of the British Arachnological Society, 11, 57-61
Rovner, J. S. (1980). Morphological and ethological adaptations for prey capture in wolf spiders (Araneae, Lycosidae). Journal of Arachnology, 8, 201-215.
Santer, R. D., & Hebets, E. A. (2009). Prey capture by the whip spider Phrynus marginemaculatus CL Koch. Journal of Arachnology, 37, 109-112.
Schnitzler, H. U., & Kalko, E. K. (2001). Echolocation by Insect-Eating Bats. Bioscience, 51, 557-569.
Souza, E. D. S., & Willemart, R. H. (2011). Harvest-Ironman: heavy armature, and not its defensive secretions, protects a harvestman against a spider. Animal Behaviour, 81, 127-133.
28 Willemart, R. H., & Pellegatti-Franco, F. (2006). The spider Enoploctenus cyclothorax
(Araneae, Ctenidae) avoids preying on the harvestman Mischonyx cuspidatus (Opiliones, Gonyleptidae). Journal of Arachnology, 34, 649-652.
Willemart, R.H. Santer, R.D., Spence, A.J & Hebets, E.A. (2011). A sticky situation:
29 SECOND CHAPTER
Defences of a Neotropical harvestman against different levels of threat by the recluse
spider
ABSTRACT
The threat sensitive hypothesis predicts that animals modulate the defensive behaviour with the level of threat. Therefore, responses to predator cues may differ from responses to the actual predator in close range. Also, in high threat situations, prey would be expected to use their most dangerous defences. The recluse spider Loxosceles gaucho (Araneae, Sicariidae) is known to prey upon well defended harvestmen such as the laniatorid Mischonyx
cuspidatus (Opiliones, Gonyleptidae), which has been reported to use tanathosis, chemical defences, pinching with sharp apophyses on legs, chelicerae and pedipalps. Because of harvestmen´s dependence on chemical stimuli, we tested if M. cuspidatus would change its locomotory behaviour in the presence of chemicals of the recluse spider (medium threat situation; spider vs blank vs chemical control; one at a time). Subsequently, we tested harvestmen behaviour in the presence of the spider in close range, a high-threat situation. Finally, we looked at the survival rate of spiders after being pierced by sharp apophyses M. cuspidatus that have on the legs. The harvestmen only showed defensive behaviours in the high threat situation. Surprisingly, their mostly known defensive behaviours (chemical defence, tanathosis, pinching with chelicerae and pedipalps) were not seen even in the high threat situation. This is the first evidence that these behaviours are not used against a natural predator that has an almost 80% predation success when attacking harvestmen. Pinching with the sharp legs IV apophyses may perforate but do not kill the spiders. We highlight the importance of the traditional descriptive approach with natural predators to understand the specificities of defensive behaviours against different types of predator.
30 INTRODUCTION
According to the threat sensitive hypothesis, modulating the defensive behaviour with the level of threat is evolutionarily advantageous (Helfman, 1989), and previous studies have indeed shown that many prey react differently depending on the threat imposed: these
reactions can be physiological responses of stress (Monclús et al., 2009), increased vigilance time (Mathoth et al., 2009), thanatosis (Gyssels & Stocks, 2005) and distinct behaviours according to the distance of the predator (Kleindorfer et al., 2005). Prey can exhibit a threat sensitive behaviour related to the size of the predator, for example by avoiding only larger individuals (Chivers et al., 2001). In mites, latency to lay the first egg and the total number of eggs laid can also be affected by the level of threat by the presence of predator cues as well as to the predator species (Ferrari & Schausberger, 2013). Finally, some monkeys can modulate their behaviour according to the threat imposed by human presence (Papworth et al., 2013).
When animals face a moderate threat like chemical cues of predators, they can avoid the site, increase locomotion, decrease movements and flee (Kats & Dill, 1998 for a review). After the predator gets closer or grasp the prey, defensive behaviours such as retaliation come into play (Edmunds, 1974). Among the weapons used to retaliate predators, some seem to have evolved from mechanisms of prey capture, others from functions related to intra-specific interactions and others are supposed to have evolved only for defensive reasons (Edmunds, 1974).
Harvestmen, arachnids belonging to the order Opiliones, have several anti-predator behaviours. When facing an intermediate threat situation such as the presence of predator chemical cues, Eumesossoma roweri (Eupnoi: Sclerosomatidae) alters its locomotory pattern (Chelini et al., 2009). A presumably high threat situation such as a human attempt to hold it causes harvestmen to release defensive secretions produced by glands that open dorso-ventrally, to autotomise locomotory appendages, to shake the body, to flee, and to pinch the potential predator with apophyses on the legs, the so called 'nipping behaviour' (the harvestman flexes legs IV so that the apophyses of the femur of the fourth pair of legs pinch against the body of the attacker) (Gnaspini & Hara, 2007).
31 Ctenidae) feeds on harvestmen of the subfamily Goniosomatinae (Opiliones, Gonyleptidae) (Gnaspini, 1996; Machado et al., 2000) but there are no detailed studies on this prey-predator system. The only pairs of spiders-harvestmen that have been studied in details were spiders that do not usually eat harvestmen. These were carried out exactly with the purpose of
understanding the proximal causes of rejection (Eisner et al., 2004; Souza & Willemart, 2011; Carvalho et al., 2012; Dias & Willemart, 2013; see also Machado et al., 2005). It has been shown that the hard integument is a highly important defence in these harvestmen belonging to the suborder Laniatores, and overcoming or avoiding it somehow is a prerequisite to feed on these animals. We have shown that the delicate recluse spider Loxosceles gaucho (Gertsch, 1967) (Sicariidae) bites such prey at the few vulnerable spots of the integument and is a very efficient predator of such harvestmen (JMGS, KDC and RHW, unpublished data). It seemed therefore to be an excellent system to understand how prey react to different levels of threat by predators. In addition, we expected that most known defensive behaviours in laniatorid harvestmen would be revealed in interactions with such efficient predators. We therefore conducted a detailed behavioural analysis of the interaction between the recluse spider and the syntopic harvestman Mischonyx cuspidatus (Roewer, 1913) (Gonyleptidae). We first tested whether these harvestmen changed their locomotory behavioural pattern when exposed to chemical cues of dangerous predators (a situation of intermediate threat). Then we looked at the defensive behaviours of the harvestmen when the spider is approaching, after spider's touches and after spider's bites (all situations considered to be of high threat based on unpublished data by JMGS, KDC and RHW (unpublished data). Since preliminary data showed that the sharp spines of legs IV in M. cuspidatus may perforate the abdomen of a spider and because prey are expected to use their most dangerous weapons in situations of high threat, we also tested the hypothesis that such sharp spines are lethal when the abdomen of the spider is perforated.
MATERIAL AND METHODS
Species studied
32 KDC and RHW, unpublished data). It typically builds a web sheet in which it is usually found, but it can also leave the web and capture prey out of it (Fischer et al., 2006). In laboratory experiments of predation under different substrate conditions and in the presence or absence of their web sheets, the success rate of prey capture was 77.3% with pooled data, combining substrates and presence/absence of web (N = 66) (JMGS, KDC and RHW, unpublished data).
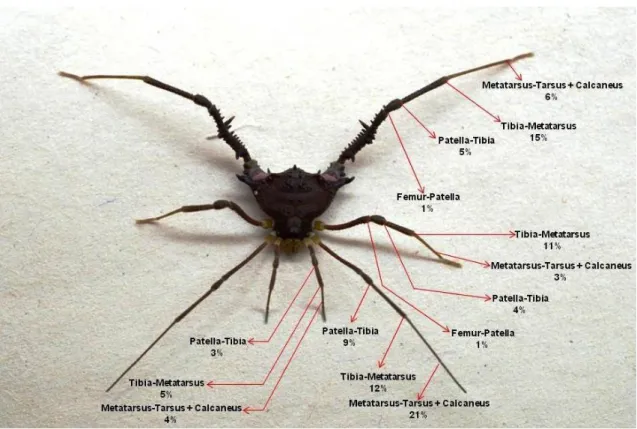
Mischonyx cuspidatus has defensive behaviours that include the release of defensive secretions by the ozopores (Hara et al., 2005), pinching with sharp apophyses on legs IV (Figure 1), thanatosis by flexing the legs and keeping them close to the body (Pereira et al., 2004) and vibrating legs II, a putative defensive behaviour recently described (ILT or intense leg tapping, see; Dias et al., 2014). In the field, dead carcasses have been found on the webs of Loxosceles gaucho and theridiid spiders (Mestre & Pinto da Rocha 2004).
Collection and maintenance
Loxosceles gaucho was collected in a terrain located in the city of Mairiporã-SP, within a pile of bricks (23◦19 S, 46◦35 W). They were collected between 24 November of 2012 and 26 February of 2013. Mischonyx cuspidatus was also found in that same area, but for this study they were collected at Parque Ecológico do Tietê- São Paulo (23◦25 S, 46◦28 W) between 20 December of 2012 and 8 May of 2013. Both species co-occur in several locations (e.g. Dias and Willemart, 2013).
In the laboratory, they were both individually maintained in plastic recipients (12x8x4 cm height) with soil on the bottom. Water was provided with a wet cotton ball. We fed the spiders with larvae of the tenebrionid beetle Zophobas sp. and nymphs of crickets Gryllus sp. according to the schedule of the experiments, and harvestmen once a week with moistened dog food.
33 Figure 1. A harvestman Mischonyx cuspidatus (Arachnida, Opiliones) male, dorsal view. Black arrows show the sharp apophyses of the femur of the fourth pair of the legs.
Behaviour of the harvestman in an intermediate threat situation
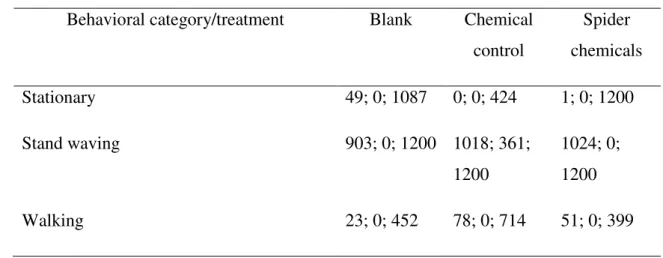
This experiment was conducted to test the hypothesis that harvestmen react to chemicals left on the substrate by dangerous predators. We predicted that the harvestmen would change their locomotory behaviour in the presence of the spider chemicals (see Chelini et al., 2009). To test it, the harvestmen (N = 20) were exposed to filter paper in the following conditions, with one single stimulus per trial: (1) chemicals of spider; (2) a chemical control (mate tea) and (3) a blank control. Each harvestman was tested three times in a design of repeated measures with interval of 24 hours between the tests for each individual. Individuals in all treatments were tested in distinct hours of the night.
To impregnate the filter paper with the spider cues we maintained one adult female of L. gaucho (5-10 days after it received its last meal) for 24 hours in a recipient (8 cm
34 paper with a thin layer of mate tea and maintained it there for 24h, removing it immediately before the trials. In the blank control, the filter paper was not impregnated. In each group the recipient was closed while impregnating (the 24h before the test).
Tests were run in the same recipient where we impregnated the filter paper with one of the chemicals (or control with no cues). We placed the filter paper on the bottom and a neutral central disc of filter paper (4 cm diameter) in the center. We first acclimated the harvestman in a vial (4 cm diameter x 7.5 cm height) placed in the center of arena (in the neutral disk). After a 2 min, the harvestman was released and we recorded it for 20 minutes with a Sony Handycam HDXR550V in nightshot mode. The harvestmen were submitted to each of the three treatments in a systematic alternated order.
We compared three behavioural categories between the treatments: the time
harvestmen spent: 1- stationary (Motionless), 2- stand waving (stationary but leg waving, and 3- walking (displacing around the arena) (cf Chelini et al., 2009). We used the Friedman test (because the data did not follow a normal distribution), followed by post hoc test Student Neuman Keuls.
Behaviour of the harvestman in a high threat situation
To describe the behaviour of the harvestmen in a high threat situation, we reanalyzed videos used in an experiment for another project, which were conducted to test, from the spider point of view, the role of silk on predation (JMGS, KDC and RHW, unpublished data). Spiders in both the “no silk” group (N = 19, 17 females and 2 males) and the “silk group” (N = 19, 13 females and 6 males) were offered 10 females and 9 male harvestmen (total = 28 harvestmen used). Twenty five to 29 days before the trials (following Souza & Willemart, 2011), we offered one cricket nymph (Gryllus sp.) (≅1.5 cm) and one beetle larvae
(Zophobas sp.) simultaneously to the spiders, to standardize hunger. Only spiders that ate at least one prey were tested. Prey uneaten after 48h were removed. The terraria of the
35 new soil in it. This was done because removing silk was not possible without removing the soil. We then reintroduced the spider. In both groups, we allowed the spider to walk freely in the terrarium for 15 minutes before we introduced a harvestman as far as possible of the spider.
We registered the experiments using a Sony Handycam HDXR550V in the nightshot mode. We recorded for 40 minutes after the predatory process (defined as first bite) and the recording time was usually one hour. Each spider was tested only once. We collected the data between 21 February and 07 March of 2013, between 9 pm and 5 am. We used both the videos with the presence and absence of silk because the predation success between the two treatments was the same (JMGS, KDC and RHW, unpublished data). We only analyzed videos in which the spider bit the harvestman. The behavioural analysis started about 5
seconds before the spider touched the harvestman and finished 2 minutes after the first bite by the spider. We looked at the behavioural categories displayed by the harvestmen. Preliminary analyses of ten videos (5 silk group and 5 no-silk group) showed that increasing this time did not result in more behavioural categories observed.
Potential lethality of a harvestman defence under high threat
Nipping behaviour has been observed in interactions between M. cuspidatus and the spiders Ctenus ornatus (Keyserling, 1891) (Ctenidae) and L. gaucho (Dias & Willemart, 2013; this paper). This behaviour is potentially dangerous for a predator since M. cuspidatus has a sharp apophysis on the retrolateral portion of the femur of both legs IV. When they rapidly flex their legs at the coxa-trochanter articulation (= 'nipping'), the two apophyses pinch what is in between the legs. By experimentally holding the spider by the prosoma and allowing a male M. cuspidatus to naturally pinch the abdomen of the spider with these
apophyses, we found that the harvestman is capable of perforating the abdomen of L. gaucho, which loses hemolymph (Figure 2). Because losing hemolymph seem to potentially cause death in arachnids (e.g. Willemart, 2002), we tested the hypothesis that a perforation caused by these apophyses negatively affects the survivorship of the spiders L. gaucho. We predicted that perforated spiders would have a lower survival rate than the spiders that were not
36 We used two groups in this experiment. In the treatment group (N = 10) we
anesthetize the spiders with CO2 by having them in a closed vial with a small aperture on the
top in which the gas was injected for 1 minute and a half. We then held the spider within the femora of a male adult harvestman so that we could manually flex its legs, pinch and perforate the spider´s abdomen. We used a different harvestman for each spider and the same person held the harvestmen in every test. In the control group (N = 10), we used the same procedures, but we pinched the abdomen with a cushioned forceps, again always by the same person. The experiment was carried out in May 2013. The harvestmen were then maintained in plastic recipients (12 x 8 x 4 cm height) with soil on the bottom. Water was provided with a wet cotton ball. In the 5 subsequent days we verified the survivorship of the spiders between 10 and 11 pm and after 40 days we monitored again. We compared the survival rate at the end of the experiment using a X2.
RESULTS
Behaviour of the harvestman in an intermediate threat situation
There were no differences when comparing the controls (blank and mate tea) and the treatment group (spider chemicals) in the time that the harvestman spent: stationary (p = 0.071), stand waving (p = 0.170) or walking (p = 0.744). The animals spent more time stand waving in all groups (p < 0.05): mate tea, blank and spider chemicals (Table 1). No defensive behaviour was observed.
Behaviour of the harvestman in a high threat situation
We found behaviours that can be considered as potentially defensive in M.
cuspidatus in different phases, as detailed below (see Table 2 - for descriptions). The results are divided in 'no-silk group' (N = 15) and 'silk group' (N = 17).
37 Phase 2- post contact, before biting:In the no-silk group, we have observed 'Move away from the spider' (N = 3); 'Motionless' (N = 2); 'ILT' (N = 1) and 'Move toward the spider' (N = 1). In the silk group we have observed 'Motionless' (N = 4); 'ILT' (N = 1); 'Move away from the spider' (N = 1) and 'Move pedipalps' (N = 1).
Phase 3- post contact after biting:In the no-silk group we have observed 'Move toward the spider' (N = 13); 'Move away from the spider' (N = 8); ' Motionless' (N = 2); 'Move pedipalps'
(N = 1); 'ILT' (N = 1) and „Nipping' (N = 1). In the silk group we have observed 'Pull the leg' (N = 9); 'Motionless' (N = 2); 'Move away from the spider' (N = 2) and 'Nipping' (N = 1).We did not observe the release of the defensive secretions, thanatosis, and pinching with
pedipalps and chelicerae in any phase analyzed.
Table 1. Time spent by the harvestman Mischonyx cuspidatus on three behavioral categories when on filter paper without stimuli; with mate tea (chemical control) or with predator chemicals. The harvestmen were recorded for 1200 seconds. Each treatment was tested separately. MIN = minimum; MAX = maximum.
time spent: median; MIN and MAX (seconds)
Behavioral category/treatment Blank Chemical
control
Spider chemicals
Stationary 49; 0; 1087 0; 0; 424 1; 0; 1200
Stand waving 903; 0; 1200 1018; 361;
1200
1024; 0; 1200