ContentslistsavailableatScienceDirect
Catalysis
Today
jo u rn al h om ep a g e :w w w . e l s e v i e r . c o m / l o c a t e / c a t t o d
Alcoxycle:
A
novel
route
for
glycerol
reform
into
H
2
and
CO
x
in
separate
stages
Fabiano
Gomes
Ferreira
de
Paula
a,
Marcelo
Gonc¸
alves
Rosmaninho
b,
Ana
Paula
de
Carvalho
Teixeira
a,
Patterson
Patrício
de
Souza
c,
Rochel
Montero
Lago
a,∗ aUniversidadeFederaldeMinasGerais(UFMG),Av.AntônioCarlos,6627,BeloHorizonte,31270-901,MinasGerais,BrazilbUniversidadeFederaldeOuroPreto(UFOP),MorrodoCruzeiro,Bauxita,OuroPreto,35400-000,MinasGerais,Brazil cCentroFederaldeEducac¸ãoTecnológica(CEFET),Av.Amazonas,5253,BeloHorizonte,30421-169,MinasGerais,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received16May2016
Receivedinrevisedform14June2016 Accepted15June2016
Availableonlinexxx
Keywords: Glycerol Sodiumhydroxide Alkoxide Hydrogen Syngas
a
b
s
t
r
a
c
t
Inthispaper,itisproposedanewroute,named“Alkoxycle”,fortheconversionofglycerolfrombiodiesel productionintoH2andCO/CO2indifferentandseparatestages.Inthisprocess,glycerolfirstreactswith NaOHtoformanalkoxidethat,inasecondstep,undergoescontrolledthermaldecomposition.Analyses ofglycerol:NaOHprecursormixtures(molarratiosof1:1,1:2,1:3and1:5)byTG,XRD,SEM,TEM,Raman, totalcarbonandGC–MSshowedthatthedecompositionofthealkoxideat400◦Cleadstotheformation
ofthreefractions:liquid,gasandsolid.Theliquidproductswereformedonlyinverysmallamounts whereasanimportantgasfraction(12–16wt%)wasproducedconsistingmainlyofH2(95%selectivity). Inthesecondstage,thesolidproducts(70–86wt%)consistingofNa2O,carbonandmostlyNa2CO3can bedecomposebyheatingat700◦CtoprocuceCO
2andespeciallyCO.AttheendoftheAlcoxycleprocess, theNaOHusedinthereactionandalsotheNaOHpresentinthebiodieselglycerolcanberecoveredand reused.
©2016PublishedbyElsevierB.V.
1. Introduction
Newstrategieshavebeenintensivelyinvestigatedtodevelop processestousethedifferentbiodiesel by-products.Oneofthe mainby-productisglycerolformedduringthetransesterification reaction,whichcontains10–15%impuritiesbasedonwater,oil, carboxylicacid/soapsand5–10%ofcatalystwaste,typicallyNaOH [1].
Differentroutestoconvertglycerolintoseveralproductshave beenstudiedinthelastyears[2–6],e.g.1,3-propanedioland dihy-droxyacetone, organic acids from biological route [7], acrolein [8–10],fueladditives[11],polymers[12]andoligomerswith dif-ferentapplicationssuchasdustsuppressor[13].Theconversion ofglycerolintosyngas,H2 andCO,hasbeenreceiving
consider-ableattentionlately[14,15].Differentreformstrategieshavebeen investigated suchas steam,liquid phase, supercritical, dry and autothermal.Thesteamreformconsistsinthereactionofglycerol withwater(Eq.(1))[16,17].
C3H8O3(g)+ 3H2O(g)→ 3CO2(g)+ 7H2(g) (1)
∗Correspondingauthor.
E-mailaddress:rochel@ufmg.br(R.M.Lago).
Thisreactionisendothermicandusuallytemperatureshigher than500◦C areusedand sidereactionssuchaswater-gas shift
andmethanationcommonlyoccur.ThisprocessproducesH2:CO
ratiogenerallyhigherthan10,whichisfarfrom2,theideal syn-gasratioforFischer-Tropsch[18,19].Analternativerouteisthe aqueous phase reform which demands moderate temperatures (200–300◦C)buthighpressures,e.g.28–55atm[20]affording
rel-ativelylowyields.Thereforminsupercriticalwaterconsistsinthe samechemicalprocesses(Eq.(1))butthetemperatureandpressure mustbehigherthanthecriticalpoint(T>374◦CandP>218atm)
[21].Also,ingeneral,thesereformprocessesproduceconsiderable amountsofundesirablechemicalintermediates,e.g.acrolein[21]. Thedryreformisdoneintheabsenceofwater,usingCO2asan
oxidant(Eq.(2))[19].
C3H8O3(g)+ CO2(g)→ 4CO(g)+ 3H2(g)+ H2O(g) (2)
Theautothermalreformisthecombinationoftwoprocesses, thesteamreformandpartialoxidation(Eq.(3)).Thisprocessoffers anenergeticadvantageoncetheexothermicpartialoxidation out-balancestheendothermicsteamreform[22,23].
C3H8O3(g)+ air+ steam→ carbonoxides+ hydrogen (3)
theneedofarelativelyexpensivemetalbasedcatalystwhicheasily deactivatesunderthereactionconditions[14,24].
Inthiswork,anewroutetotransformglycerolintoH2andCOis
proposedwithsignificantadvantagessuchas(i)theH2isproduced
inadifferentstepfromCO production,beingpossibletoobtain thesegasesseparatelybyacontrolofthetemperatureand(ii)the NaOHusedascatalystinthetransesterificationreaction(present asacontaminationintheglycerol)canberecovered.
2. Materialsandmethods
Allchemicalswereusedwithoutprevioustreatment.The pre-cursorwerepreparedreactingglycerol(Synth,99,5%)withNaOH (Vetec,99%)inmolarratiosof1:1,1:2,1:3and1:5glycerol:NaOH (Glyc:1Na,Glyc:2Na, Glyc:3Na and Glyc:5Na, respectively). The resultantmixturewastreatedat60◦Cfor24handuseddirectly
in the further experiments. For the thermal decomposition, 80–120mgoftheprecursorswereplacedinatubularquartz reac-torunderastaticargonatmosphereandheated(10◦min−1)ina
ceramicfurnaceuntil400,550and900◦Cfor1heach.Three
frac-tionswereobtained:solid,liquidandgaseous.
Thecompositionofthesolidfractionwasdeterminedusing sev-eraltechniques.TheX-raydiffraction(XRD)wasperformedina ShimadzuequipmentmodelXRD-7000,withCuK␣radiation,using
thepowdermethod.TheRamanspectrawasobtainedinaBruker equipment,modelSenterraequippedwithaCCDdetector.Also,it iscoupledwithanopticalmicroscopy(OLYMPUSBX51)tofocus thelaserbeamandtocollectthebackscatteredlight.Thespectra wasdoneusingthe633nmlaserwith2mWofpotency,integration timeof10sand10co-additions.TheSEMimageswereacquiredin aQuanta200FEGequipment.TheTEMimageswereobtainedin aTecnaiG2-20SuperTwinFEI200kV.Thermogravimetricanalysis (TG)wasdoneinaShimadzumodelDTG–60Hwithanitrogenor airfluxof50mLmin−1upto900◦Candheatingrateof10◦Cmin−1.
Thetotalcarbon (TC)in solutionwasperformedin aShimadzu equipmentmodelTOC-V-CPH.Forthisanalysis,deionizedwater wasaddedtothecrudesolidandthemixturewasthenfilteredto obtainasolution.
Theliquidcondensedatthetrapwerecollecteddissolvingitat acetone(Quimex,99,5%).Then,theproductswerecharacterized usinggaschromatographyinanAgilent®modelGC-7890coupled
withamassspectrometerAgilent®model5975Cwithaquadrupole
analyzer.TheproductswereseparatedonaHP-5column. Thegasproducedweresampledandanalyzedwithgas chro-matographyinaShimadzumodelGC-2010.Thepermanentgases wereseparatedonaCarboxen®-1010columnandanalyzedina
thermalconductordetector(TCD).
Thehydrocarbonsgaseswereseparatedinthesamecolumnand analyzedwithaflameionizationdetector(FID).TheCGwas cali-bratedwithastandardgasmixturecontainingH2,CO,CO2,CH4,
C2H6,C2H4eC2H2inN2.Thegasproductswereobtainedin%molar
andindicatedthevariationofgascompositionwithrespecttothe precursorthermallydecomposed.
3. Resultsanddiscussion
The alkoxide intermediates were prepared by the sim-ple reaction of glycerol with sodium hydroxide with different NaOH/glycerolmolarratios,i.e.1,2,3and5(Eq.(4)).
C3H5(OH)3(l)+ 3NaOH(s)→ C3H5(O−Na+)3(s)+ 3H2O(l) (4)
Theformationofthesealkoxideshasbeendescribedbefore[25]. Theseprecursorsareveryhygroscopicand,afterdryingat60◦Cfor
24h,theywereuseddirectlyfortheexperiments.
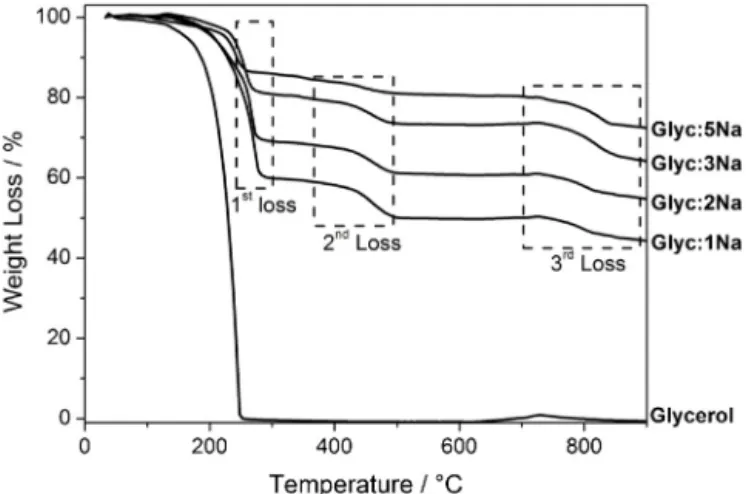
Fig.1.TGanalyses,inargonatmosphere,oftheprecursorsGlyc:Naandglycerol.
Fig.2.Massbalanceofthesolid,liquidandgasfractionsobtainedforthe decom-positionofthedifferentprecursorsat400◦
C.
Thermogravimetricanalysesoftheprecursors(Fig.1)showed threemainweightlossesat150–300,400–550and700–900◦C.
Thefirstweightloss,upto300◦C,isprobablyrelatedtotheloss
ofwater,partialdecompositionoftheprecursorsandevaporation ofexcessglycerol.Infact,pureglycerolshowsa100%weightloss between140and240◦Cduetoitsevaporation(Fig.1).The
sec-ondweightloss,relatedtoprecursordecomposition,variesfrom 3up to10%.Above 700◦C, theweight lossis likely duetothe
decompositionofmorestablecompounds,mostlikelyinorganics. Ingeneral,astheNacontentintheprecursorincreased,allthe lossesdecreased whereastheremainedsolidfractionpresentin thesamplesat900◦Cincreased.Basedontheseresults,the
ther-maldecompositionofthesubstrateswasinvestigatedat400,550 and900◦C.
Decompositionofthedifferentprecursorscarriedoutat400◦C
producedthreefractions:solid,liquidandgas.Theobtainedmass balancesforthesefractionsareshowninFig.2.
Forallprecursors,thesolidproductrepresentedca.70–90%.In thisfraction17–39%isrelatedtothepresenceofNa+,likelyinthe
formofoxidesandcarbonatesalts.Theliquidfractionwasonly significantforGlyc:1NaandGlyc:2Na.Itcanbeobservedthatthe gasfractionslightlyincreasedfrom12to18%astheNacontent increasedintheprecursor.
Fig.3.SEMimagesandEDSforrawsolidobtainedbydecompositionofthe precur-sorsat400◦
C.
Fig.4. SEMandTEMimagesandEDSforthewashedsolidobtainedby decomposi-tionoftheprecursorsat400◦
C.
treatment)showedfibrous-needle-shapedstructures,commonfor carbonatesalts[26,27].Infact,EDSanalysisconfirmedthepresence ofsodium,carbonandoxygenatoms(Fig.3).
After acid wash, a completely different morphology was observedbySEMandtheEDSspectrumsuggeststhatthesodium wasremovedand onlycarbon waspresent.TEMimagesofthe obtainedblacksolid afterwashing suggestedfor mostparticles agraphite-likestructureandinsomecasesfewlayersgraphene structure(Fig.4).
TheRamanspectrafor theraw solid presented a bandnear 1188cm−1,relatedtoacarbonate(CO
3−)COstretching[28],and
twobandsduetothepresenceofcarbon,i.e.Dbandrelatedto lessorganizedcarbonstructuresandGbandrelatedtoorganized graphenestructure[29](Fig.5).AfterwashingwithHCltheseDand Gbandbecamemorepronounced(seedetailofFig.5)
Thediffractogramfortherawsolidsampleswereinperfect cor-relationwiththepatternofNa2CO3,accordingtoJCDPS37-457(see
SupplementaryMaterial).
TGanalysesof theraw solidobtainedat400◦C inair
atmo-sphereshowedanexothermicweightlossbetween300and500◦C
(seeDTAinSupplementaryMaterial)likelyrelatedtooxidationof thecarbonaceousproducts(Fig.6).Therelativelylow oxidation temperaturessuggestthepresenceofamoreamorphousreactive
Fig.5.Ramanspectrumforthecrudesolidandforthewashedsolid(detail)obtained bydecompositionoftheprecursorsat400◦
C.
Fig.6.TGanalysesfortherawsolidobtainedbydecompositionoftheprecursors at400◦C.
Table1
Compositionofthesolidfractionobtainedbythedecompositionofthedifferent precursorsat400◦
C.
Substrates %Ca %Na
2CO3b %Na2Oc
Glyc:1Na 11 85 4
Glyc:2Na 10 89 1
Glyc:3Na 4 85 11
Glyc:5Na 2 80 18
aDeterminedbyTG. bDeterminedbyTC. c Calculatedbydifference.
carbon[30].Itcanalsobeobservedaweightlossnear850◦Clikely
relatedtothesodiumcarbonatedecomposition.
Therawsolidwassolubilizedinwaterandthenfiltered.The amountofcarbonatewasestimatedfromtheremainingsolutionby TC(TotalCarbonanalysis).Thus,thecompositionofthesolid frac-tionwasdeterminedusingtheamountofcarbonestimatedfrom TG,carbonateestimatedfromTCmeasurementsandNa2Ofromthe
difference(Table1).
Asitcanbeobserved,carbonatesaltrepresentsthemain com-ponentofthesolidfraction,morethan80%.Also,asexpected,the amountofNa2OincreasesfortheprecursorswithhigherNa
con-tent,whiletheoppositeoccursforcarbon.
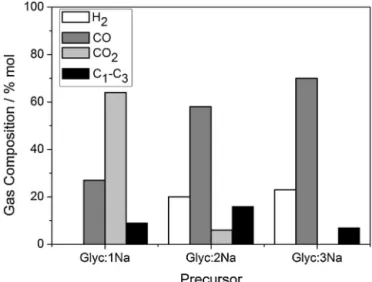
Fig.7. Gascompositionobtainedforthedifferentprecursorsdecomposedat400◦ C.
Glyc:2Naat400◦C,i.e.ca.20%.Inthesesamples,GC–MS
analy-sisshowedthat non-reactedglycerolwasthemain component andtracesof1,2-propanediolwereidentified.Theothersubstrates Glyc:3NaandGlyc:5Nashowedonlytracesofliquidderivatives. Thegasproductsformedupto400◦C,collectedandidentifiedby
GC,werecomposedmainlybyH2,verysmallamountsofCO,CO2,
CH4 and tracesof hydrocarbonsC2 andC3.Asshown in Fig.7,
theselectivityforH2 increasedforthesubstrateswithexcessof
NaOHinitspreparation,reaching99%forGlyc:5Na.Onwudiliand Williams[31]studiedtheroleofNaOHinhydrothermalreactions withseveralbiomasssamplessuchascellulose,glucose,starchand others.ItwasobservedthatthepresenceofNaOHfavouredthe gasificationreactionsandtheproductionofH2.Somemechanistic
studies[32,33]suggestedthattheNaOHreactswithCO2toform
Na2CO3orNaHCO3favouringtheWaterGasShiftreaction.
FortheAlkoxycleinvestigated inthis work,onlyin thefirst decompositionstep,thecalculatedH2productionwas4.7molofa
theoreticalmaximumof5.5molwhichcorrespondstoa85%yield forGlyc:3Na,consideringallhydrogenatomsfromglyceroland NaOH.
Thesecondstageofthermaldecomposition(observedintheTG curvesnear500◦C)wasalsoinvestigatedbycollectingthegases
onlyintheseconddecomposition,between400and550◦C. GC
analysesshowedthepresenceofmainlyCOandsmallamountsof CO2 andH2 (Fig.8).TheprecursorGly:5Nadidnotproduce
sig-nificantamountofgasin thetemperaturerange 400–550◦C as
observedbyTGinFig.1.
Thegasesformedinthethirdstageofthethermal decomposi-tion(observedintheTGcurvesafter700◦C)wasalsoinvestigated.
Thegaseswerecollectedbetween550and900◦Candanalyzedby
GC.ItcanbeobservedthatthemaingasesareCOandCO2,especially
fortheGlyc:2Na,Glyc:3NaandGlyc:5Naprecursors(Fig.9). ThehighamountofCOandCO2 atthistemperatureislikely
relatedtotworeactions,theNa2CO3decomposition(Eq.(5))and
alsothe reverse Boudouard reaction (Eq.(6))which shouldbe favouredat900◦C[34,35].
Na2CO3(s)→ CO2(g)+ Na2O(s) (5)
C(s)+ CO2(g)→ 2CO(g) (6)
Afterthermaldecomposition(900◦C),theremainingwhitesolid
wascompletelysolubleinwaterandstronglyalkaline.Moreover, TCanalysisofthesolutionalsoshowedtheabsenceofcarbonates, suggestingthepresenceofonlyNa2O.Basedontheresultsobtained
inthisworksomeconsiderationscanbemadeontheAlkoxycle.
Fig.8.Gascompositionintherangeof400–550◦ C.
Fig.9.Gascompositionintherangeof550–900◦C.
WhenlowNacontentsareused,i.e.Glyc:1NaandGlyc:2Na,aliquid productisformed.Thisfractionisaresultofglycerolevaporation combinedwithotherprocess,e.g.alkoxidedecompositiontoform glycerolderivativesandotherreactionspromotedbythestrong alkalinemedium.Thisliquidis,therefore,acomplexmixtureof differentcompoundswithnodirectapplication.Whenmolarratios NaOH:glycerolhigherthan3:1areused,nosignificantamountof liquidproductisformed.Theseresultssuggestthatthe combina-tionof1Naatomfor1Oatomofglycerolinducesthecomplete collapseoftheglyceroltoformonlysmallgasmolecules. Appar-ently,inthefirstthermaldecompositionstepmostoftheoxygen andcarbonatomswillremaininthesolidphaseascarbonate.Some oftheCatomsaromatizetoformasolidcarboncontaining rela-tivelyhighconcentrationofoxygenfunctionalitiesandproduceH2
(Eq.(7)).
C3H5(O−Na+)3→ Na2CO3/Na2O/Carbon(OxHy)+ H2 (7) Althoughtheprocesstakingplaceintheseconddecomposition stageisnotclear,onepossibilityisthedecompositionofthehigh functionalizedcarbontolosesmallamountsofH2andoxygenas
COandCO2(Eq.(8)).
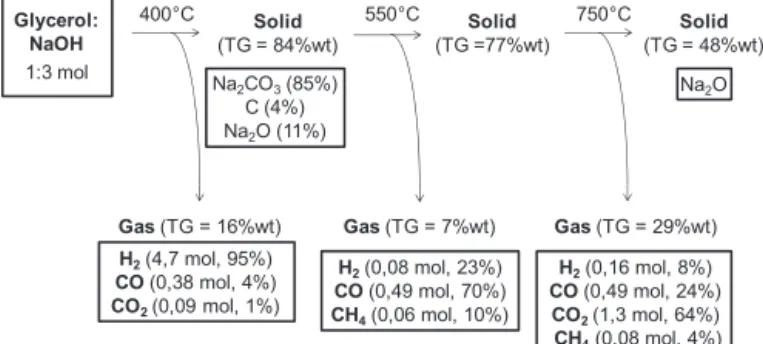
Na2CO3(85%)
C (4%) Na2O (11%)
Glycerol: NaOH
1:3 mol
400°C Solid
(TG = 84%wt)
Gas(TG = 16%wt) Gas(TG = 7%wt) Gas(TG = 29%wt) 550°C Solid
(TG =77%wt)
Solid
(TG = 48%wt)
Na2O
750°C
H2(4,7 mol, 95%)
CO(0,38 mol, 4%)
CO2(0,09 mol, 1%)
H2(0,08 mol, 23%)
CO(0,49 mol, 70%)
CH4(0,06 mol, 10%)
H2(0,16 mol, 8%)
CO(0,49 mol, 24%)
CO2(1,3 mol, 64%)
CH4(0,08 mol, 4%)
Fig.10.SummaryofcompletethermaldecompositionoftheprecursorGlyc:3Na.
Fig.11. Representationofthe“Alkoxycle”fortheproductionofH2andCO/CO2from
glycerolandNaOH.
Inthethirdstageattemperatureshigherthan700◦C,
appar-entlythesodiumcarbonatedecomposestoproduceCO2and,bya
reactionwiththecarbon,producesCO.
Fig.10representsasummaryofallthermaldecompositionsteps fortheprecursorGlyc:3Na.At400◦C,H
2wasproducedwithhigh
selectivity(95%)andyieldof4.7ofanominalmaximumof5.5mol (85%),consideringallhydrogenatomsfromglycerolandsodium hydroxide.Theyieldwascalculatedusingtheresultsfrom thermo-gravimetricanalysisandgaschromatography.Inthesecondand thirddecompositionstages,COandCO2werethemainproducts,
70and64%,respectively.Theseresultssuggestthatispossibleto produceH2withhighselectivityinadifferentstep,separatedfrom
theothergases,mainlyCOx.
Fig. 11 represents the complete Alcoxycle, where basically hydrogen is produced at 400◦C and a mixture of CO/CO
2 was
obtainedat900◦C.Furthermore,onlyNa
2Owasobservedatthe
endoftheprocess,showingapossibilityofrecoveringtheNaOH. Animportantaspectofthisprocessistheenergyconsumption. Detailedstudyonthethermodynamicsandenergybalanceis cur-rentlyinprogress.However,onecanenvisagethatcomparedtothe otherglycerolconversionprocesses,i.e.steam,dryandautothermal reform,theenergyconsumptionshouldbesimilar.
4. Conclusion
ThermaldecompositionofNa+alkoxidesfromglycerolat400◦C
producesmainlyH2withselectivityof95mol%and85%yieldfor
Glyc:3Na.ThesolidfractioniscomposedmainlybyNa2CO3,but
alsocontainedNa2Oandcarbon.Furthertreatmentupto900◦C
produced mainlyCO and CO2. Thiscyclic process showedvery
promisingresultstoconvertglyceroltosyngaswiththeadvantages ofproducingH2andCOxinseparateanddifferentstagesaswell
asrecoveringtheNaOHpresentintheglycerolfromthebiodiesel process.
Acknowledgments
Theauthorsaregratefulforthefinancialsupportforthiswork byPetrobras,FAPEMIG,CAPESandCNPq.Theauthorswouldliketo acknowledgetheCenterofMicroscopyattheUniversidadeFederal deMinasGerais(http://www.microscopia.ufmg.br)forproviding theequipmentandtechnicalsupportfor experimentsinvolving electronmicroscopy.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound, intheonlineversion,athttp://dx.doi.org/10.1016/j.cattod.2016.06. 039.
References
[1]M.Zhang,H.Wu,Effectofmajorimpuritiesincrudeglycerolonsolubilityand propertiesofglycerol/methanol/bio-oilblends,Fuel159(2015)118–127. [2]M.Ayoub,A.Z.Abdullah,Criticalreviewonthecurrentscenarioand
significanceofcrudeglycerolresultingfrombiodieselindustrytowardsmore sustainablerenewableenergyindustry,Renew.Sustain.EnergyRev.16 (2012)2671–2686.
[3]A.B.Leoneti,V.Aragão-Leoneti,S.V.W.B.deOliveira,Glycerolasaby-product ofbiodieselproductioninBrazil:alternativesfortheuseofunrefined glycerol,Renew.Energy45(2012)138–145.
[4]C.J.A.Mota,Gliceroquímica:novosprodutoseprocessosapartirdaglicerina deproduc¸ãodebiodiesel,SociedadeBrasileiradeQuímica,2009.
[5]C.A.G.Quispe,C.J.R.Coronado,J.A.CarvalhoJr.,GlycerolProduction, consumption,prices,characterizationandnewtrendsincombustion,Renew. Sustain.EnergyRev.27(2013)475–493.
[6]H.W.Tan,A.R.AbdulAziz,M.K.Aroua,Glycerolproductionanditsapplications asarawmaterial:areview,Renew.Sustain.EnergyRev.27(2013)118–127. [7]G.P.daSilva,M.Mack,J.Contiero,Glycerol:apromisingandabundantcarbon
sourceforindustrialmicrobiology,Biotechnol.Adv.27(2009)30–39. [8]A.Corma,G.W.Huber,L.Sauvanaud,P.O’Connor,Biomasstochemicals:
catalyticconversionofglycerol/watermixturesintoacroleinreaction network,J.Catal.257(2008)163–171.
[9]B.Katryniok,S.Paul,V.Belliere-Baca,P.Rey,F.Dumeignil,Glycerol dehydrationtoacroleininthecontextofnewusesofglycerol,GreenChem. 12(2010)2079–2098.
[10]L.Ott,M.Bicker,H.Vogel,Catalyticdehydrationofglycerolinsub-and supercriticalwater:anewchemicalprocessforacroleinproduction,Green Chem.8(2006)214–220.
[11]N.Rahmat,A.Z.Abdullah,A.R.Mohamed,Recentprogressoninnovativeand potentialtechnologiesforglyceroltransformationintofueladditives:a criticalreview,Renew.Sustain.EnergyRev.14(2010)987–1000.
[12]M.deAraújoMedeiros,M.T.C.Sansiviero,M.H.Araújo,R.M.Lago,Modification ofvermiculitebypolymerizationandcarbonizationofglyceroltoproduce highlyefficientmaterialsforoilremoval,Appl.ClaySci.45(2009)213–219. [13]M.A.Medeiros,C.M.M.Leite,R.M.Lago,Useofglycerolby-productofbiodiesel
toproduceanefficientdustsuppressant,Chem.Eng.J.180(2012)364–369. [14]S.Adhikari,S.D.Fernando,A.Haryanto,Hydrogenproductionfromglycerol:
anupdate,EnergyConvers.Manage.50(2009)2600–2604.
[15]P.D.Vaidya,A.E.Rodrigues,Glycerolreformingforhydrogenproduction:a review,Chem.Eng.Technol.32(2009)1463–1469.
[16]S.Adhikari,S.Fernando,A.Haryanto,Productionofhydrogenbysteam reformingofglycerinoveralumina-supportedmetalcatalysts,Catal.Today 129(2007)355–364.
[17]T.Hirai,N.-o.Ikenaga,T.Miyake,T.Suzuki,Productionofhydrogenbysteam reformingofglycerinonrutheniumcatalyst,EnergyFuels19(2005) 1761–1762.
[18]E.L.Kunkes,R.R.Soares,D.A.Simonetti,J.A.Dumesic,Anintegratedcatalytic approachfortheproductionofhydrogenbyglycerolreformingcoupledwith water-gasshift,Appl.Catal.B:Environ.90(2009)693–698.
[19]H.C.Lee,K.W.Siew,J.Gimbun,C.K.Cheng,Synthesisandcharacterisationof cementclinker-supportednickelcatalystforglyceroldryreforming,Chem. Eng.J.255(2014)245–256.
[20]R.D.Cortright,R.R.Davda,J.A.Dumesic,Hydrogenfromcatalyticreformingof biomass-derivedhydrocarbonsinliquidwater,Nature418(2002)964–967. [21]E.Markoˇciˇc,B.Kramberger,J.G.vanBennekom,H.JanHeeres,J.Vos, ˇZ.Knez, Glycerolreforminginsupercriticalwater;ashortreview,Renew.Sustain. EnergyRev.23(2013)40–48.
[22]G.Nahar,V.Dupont,Recentadvancesinhydrogenproductionvia autothermalreformingprocess(ATR):areviewofpatentsandresearch articles,RecentPat.Chem.Eng.6(2013)8–42.
productionbyliquidphasereformingofglycerol:catalyticpropertiesand performance,anddeactivationstudies,Top.Catal.57(2014)1066–1077. [25]H.S.Fry,E.L.Schulze,Theliberationofhydrogenfromcarbonccompunds.IV.
theinteractionofglycolandglycerolwithfusedcausticalkalies,J.Am.Chem. Soc.50(1928)1131–1138.
[26]J.Jiang,M.-R.Gao,Y.-H.Qiu,G.-S.Wang,L.Liu,G.-B.Cai,S.-H.Yu,Confined crystallizationofpolycrystallinehigh-magnesiumcalcitefromcompact Mg-ACCprecursortabletsanditsbiologicalimplications,CrystEngComm13 (2011)952–956.
[27]M.Wang,H.K.Zou,L.Shao,J.F.Chen,Controllingfactorsandmechanismof preparingneedlelikeCaCO3underhigh-gravityenvironment,Powder Technol.142(2004)166–174.
[28]S.Gunasekaran,G.Anbalagan,S.Pandi,Ramanandinfraredspectraof carbonatesofcalcitestructure,J.RamanSpectrosc.37(2006)892–899. [29]A.C.Ferrari,J.Robertson,InterpretationofRamanspectraofdisorderedand
amorphouscarbon,Phys.Rev.B61(2000)14095–14107.
[30]J.P.Trigueiro,G.G.Silva,R.L.Lavall,C.A.Furtado,S.Oliveira,A.S.Ferlauto,R.G. Lacerda,L.O.Ladeira,J.W.Liu,R.L.Frost,G.A.George,Purityevaluationof carbonnanotubematerialsbythermogravimetric,TEM,andSEMmethods,J. Nanosci.Nanotechnol.7(2007)3477–3486.
hydrogengasfromthehydrothermalgasificationofbiomass,Int.J.Hydrogen Energy34(2009)5645–5656.
[32]J.A.Onwudili,P.T.Williams,Reactionofdifferentcarbonaceousmaterialsin alkalinehydrothermalmediaforhydrogengasproduction,GreenChem.13 (2011)2837–2843.
[33]J.A.Onwudili,P.T.Williams,Hydrothermalreactionsofsodiumformateand sodiumacetateasmodelintermediateproductsofthesodium
hydroxide-promotedhydrothermalgasificationofbiomass,GreenChem.12 (2010)2214–2224.
[34]Y.Jiao,W.Tian,H.Chen,H.Shi,B.Yang,C.Li,Z.Shao,Z.Zhu,S.-D.Li,Insitu catalyzedBoudouardreactionofcoalcharforsolidoxide-basedcarbonfuel cellswithimprovedperformance,Appl.Energy141(2015)200–208. [35]P.Lahijani,Z.A.Zainal,M.Mohammadi,A.R.Mohamed,Conversionofthe