INTRODUCTION
Porcelain stoneware tile is a ceramic product characterised by low water absorption (≤ 0.5% according to the standard ISO 13006 [1]), making it a high-performance material [2]. Porcelain tile is typically used in looring, wall cladding, and ventilated façades [3]. In recent years, porcelain tile production and sales have grown, compared withproduction and sales rates of other ceramic construction materials, as a result of its
high technological properties, particularly in regard to water absorption and frost resistance, and mechanical properties, such as modulus of ruptureand abrasionresistance [2-5].
A typical porcelain tile composition consists of 40-50% illitic-kaolinitic clay, 10-15% quartz, and 35%-45% feldspar (all percentages by weight) [6, 7]. Feldspar is a high-cost raw material and replacement would represent a signiicant reduction in porcelain tile production costs [8, 9]. Feldspar is a minerallux commonly used in porcelain tile bodies.
Valorization of rice straw waste: production of porcelain tiles
(Valorização de resíduos de palha de arroz: produção de porcelanatos)
Álvaro Guzmán A1*, Silvio Delvasto A1, Maria Francisca Quereda V2, Enrique Sánchez V2
1Composite Materials Group (Grupo de Materiales Compuestos), GMC. Escuela de Ingeniería de Materiales,
Facultad de Ingeniería, Universidad del Valle, Cali, Colombia. Calle 13 # 100 – 00, Edif. 349
2Instituto de Tecnología Cerámica, ITC. Universidad Jaume I, Castellón, Spain. Campus Universitario Riu Sec
[James I University, Castellón, Spain. Campus Riu Sec], Av. Vicent Sos Baynat s/n, 12006 Castellón. *alvaro8308@hotmail.com.co, silvio.delvasto@correounivalle.edu.co, paqui.quereda@itc.uji.es,
enrique.sanchez@itc.uji.es
Abstract
The rice industry generates huge amounts of rice straw ashes (RSA). This paper presents the results of an experimental research work about the incorporation of RSA waste as a new alternative raw material for production of porcelain tiles. The RSA replaces, partially or completely, the non-plastic raw materials (quartz (feldspathic sand in this research) and feldspar), that together with the clays, constitute the major constituents of formulations of porcelain tiles. A standard industrial composition (0% RSA) and two more compositions in which feldspar and feldspathic sand were replaced with two percentages of RSA (12.5% RSA and 60% RSA) were formulated, keeping the clay content constant. The mixtures were processed, reproducing industrial porcelain tile manufacturing conditions by the dry route and ired at peak temperatures varying from 1140-1260 °C. The results showed that additions of 12.5% RSA in replacement of feldspar and feldspathic sand allowed producing porcelain tiles that did not display marked changes in processing behaviour, in addition to obtain a microstructure and the typical mineralogical phases of porcelain tile. Thus, an alternative use of an agricultural waste material is proposed, which can be translated into economic and environmental beneits.
Keywords: porcelain tile, rice straw ash, feldspar, feldspathic sand.
Resumo
A indústria do arroz gera enormes quantidades de cinzas de palha de arroz (RSA). Este artigo apresenta os resultados de um trabalho de pesquisa experimental sobre a incorporação de resíduos RSA como uma nova matéria-prima alternativa para a produção de porcelanato. A RSA substitui, total ou parcialmente, as matérias-primas não-plásticas (quartzo (areia feldspática nesta pesquisa) e feldspato), que, juntamente com as argilas, constituem os principais componentes de formulações de porcelanato. A composição padrão industrial (0% RSA) e mais duas composições em que feldspato e areia feldspática foram substituídos por duas porcentagens de RSA (12,5% e 60% RSA RSA) foram formuladas, mantendo a argila constante. As misturas foram processadas, reproduzindo porcelanato nas condições de
produção industrial por via seca e queimas em temperaturas máximas variando entre 1140-1260 °C. Os resultados
mostraram que adições de 12,5% RSA em substituição da areia feldspato e feldspático permitiram produzir porcelanato que não mostraram mudanças marcantes no comportamento do processamento, além de obter uma microestrutura e as fases mineralógicas típicas de porcelanato. Assim, é proposta uma alternativa de uso de um material de resíduos agrícolas, o que pode ser traduzido em benefícios econômicos e ambientais.
However, high-grade feldspathic minerals resources have recently begun to become scarce, thus making it necessary to consider alternative sources of luxing materials that can form glassy phase at temperatures equal toor lower than those of the feldspars used at present [10]. It should be added that deposits of good quality quartz sand in Colombia are limited. As a result, various alternative luxes (soda-lime glasses, glass scrap (TV/PC cathode ray tubes and screens), blast furnace slag, metallurgical slags, zeolites, rice straw ash (RSA), etc.) and non-plastic materials (rice husk ash(RHA), silica fume (SF), ly ash (FA)) have been incorporated intoporcelain tile body compositions, with a view to studying their effecton product iring behaviour and end-product technical properties [10-24]. However, the use of secondary raw materials is considered feasible only if the industrial process remains essentially unaltered and product quality and properties are not impaired [13, 19].
As feldspars generally come from just a few regions, e.g. Germany, Turkey, and France, in addition to the limited
deposits of good quality quartz sands in Colombia, their possible replacement with rice straw ash (RSA) is an attractive option, taking into account the results of Guzmán et al. [24] and the K2O and SiO2 contained in RSA, which is of the order
of 11.30–12.30% and 74.31-74.67% by weight of the ash, respectively [25, 26].
This study was undertaken to examine the feasibility of using rice straw ash (RSA) in ceramic mixtures topartially replacenon-plastic materials (sodium feldspar and feldspathic sand) used in manufacturing porcelain tilebodies.The effects due to the use of RSA were investigated in laboratory experiments and discussed in terms of iring behaviour and physical–mechanical properties.
EXPERIMENTAL PROCEDURE
Materials
The raw materials used in preparing triaxial ceramic
Element and/or compound
Concentration by weight (%)
Illitic-kaolinitic clay
(A) Sodium feldspar (F) Feldspathic sand (AF) RSA (C)
SiO2 59.00 70.00 91.00 79.62
Al2O3 27.30 18.00 5.00 0.27
Fe2O3 0.93 0.08 0.12 0.26
CaO 0.27 0.50 0.10 2.80
MgO 0.59 0.10 0.01 0.89
Na2O 0.52 9.70 0.10 0.35
K2O 2.45 0.35 2.50 10.53
TiO2 1.45 0.11 0.08
-MnO <0.01 - - 0.71
P2O5 0.05 - - 1.61
Cl - - - 0.59
S - - - 1.74
Zn - - - 0.01
Rb - - - 0.01
Sr - - - 0.01
Cu - - - 0.01
LOI 7.29 0.50 1.10 0.59
Mineralogical phases
Kaolinite (ICSD
87771) Albite (ICSD 90142) Quartz (ICSD 83849) Cristobalite a (ICSD 74530)
Muscovite (ICSD
202263) Muscovite (ICSD 25803) Kaolinite (ICSD 87771) Tridymite a(ICSD 1109)
Quartz (ICSD 90145) Quartz (ICSD 34636) Orthoclase (ICSD 159347)
Anatase (ICSD 96946)
Table I - Chemical and mineralogical compositions of the raw materials.
bodies were feldspathic sand, sodium feldspar, and illitic-kaolinitic clay. To obtainthe RSA, amethodology was usedfor the obtainment of RSA by a calcination process of the materialat 800 ºC to eliminate chlorine and sulphuras far as possiblewith a moderate content of potassium in the ash [24]. After the RSA had been obtained, it was subjected to milling for one hour in a laboratory ball mill. The chemical and mineralogical compositions of the raw materials were determined by X-ray luorescence and X-ray diffraction, respectively (see Table I). The mineralogical phases are reported with the Inorganic Crystal Structure Database (ICSD) patterns corresponding to the identiied minerals.
The chemical composition, determined by XRF, of the RSA obtained by calcination at 800 °C showed that the main constituents were SiO2 and K2O (see Table I), corroborating
the results reported by Jenkins et al. [25] and Thy et al. [26]. With relation to the clay, this consisted mainly of SiO2 and
Al2O3. In addition, it displayed low contents of K2O and TiO2
(2.5% and 1.5%, respectively), in which the K2O could act as
a lux, while TiO2 was a chromophore oxide that provided a
yellowish hue [27]. The feldspar, in turn, consisted mainly of SiO2 and Al2O3, as well as 9.7% Na2O, indicating that it was
a sodium feldspar. On the other hand, the feldspathic sand mainly contained SiO2 and Al2O3, in addition to 2.5% K2O,
which could act as a lux.
Ceramics formulation and testing
A standard industrial composition labelled 0% RSA, and two more compositions labelled 12.5% RSA and 60% RSA in which feldspar and feldspathic sand were replaced with two percentages of RSA (12.5 wt.% and 60 wt.%, respectively) were formulated, keeping the clay content constant in all formulations. The chemical compositions of the corresponding porcelain are reported in Table II.
The replacement of feldspar and feldspathic sand with RSA in the mixture, in general, led to an increase in the SiO2,
K2O, MgO, CaO, Fe2O3 and Cl contents, in addition to a reduction in the Al2O3 and Na2O contents (Table II). Thus,
the expected behaviour of compositions is, in some extent, unpredictable due to these compositional changes.
In order to obtain the typical particle size of porcelain tile body compositions, one kg of each ceramic formulation wasball-milled to a residue (R) of1.5–2.0% on a 40 µm sieve. Table III details the dry milling times required to prepare the formulations to a residue of 1.5–2.0% on a40 µm sieve. As observed, the higher the RSA content the shorter is the milling time.
In order to determine the behaviour of the ceramic bodies in pressing and iring, cylindrical test pieces about 7 mm thick and 40 mm in diameter, in addition toprism-shaped test pieces measuring 80x20x6 mm,were prepared to determine the mechanical properties. The test pieces were formed at a moisture contentof 5.5 wt.% (on a dry basis) by uniaxial pressing at a pressure of 400 kg/cm2. After they had been
pressed, the test pieces were dried at 110 ± 5°C in an electric laboratory oven. They were then sintered in a Pirometrol R
electric laboratory kiln with a heating ramp of 70 °C/min between 25 °C and 500 °C, and 25 °C/min from 500 °C to the respective peak iring temperature. The residence time at peak iring temperature was 6 min, and the ired test pieces were cooled inside the kiln in order to avoid macroscopic residual stresses. The peak iring temperatures encompassed the range 1140-1260 °C, at intervals of 20°C, depending on each composition. The measurements of dried and ired dimensions of the test pieces were made using a digital calliper.
The maximum densiication temperature was determined for each mixture from the vitriication curve, constructed after determining the bulk density, using the experimental conditions described previously. Owing to the scarce variation of water absorption with temperature at values below 1%, it was preferable to determine the maximum densiication
Element and/or compound
Concentration by weight (%)
0% RSA 12.5% RSA 60% RSA
SiO2 67.70 67.85 71.37
Al2O3 20.42 18.85 11.08
Fe2O3 0.42 0.44 0.53
CaO 0.37 0.68 1.79
MgO 0.29 0.39 0.77
Na2O 5.07 4.38 0.42
K2O 1.41 2.57 7.30
TiO2 0.64 0.63 0.58
MnO 0.00 0.09 0.43
P2O5 0.02 0.22 0.99
Cl 0.00 0.07 0.35
S 0.00 0.22 1.04
Zn 0.00 0.00 0.01
Rb 0.00 0.00 0.01
Sr 0.00 0.00 0.01
Cu 0.00 0.00 0.01
Table II - Chemical composition (wt.%) of porcelain samples as formulated.
[Tabela II - Composição química (peso%) das amostras de porcelana, como formulado.]
Mixture t (min) % R (40 µm)
0% RSA (10AF;50F;0C;40A) 50 2,2
12.5% RSA (5AF;42.5F;12.5C;40A) 25 2,0
60% RSA (0AF;0F;60C;40A) 17 1,6
Table III - Milling time required to reach a residue of 1.5-2.0% on a 40 µm sieve.
temperature as the characteristic temperature for industrial iring temperatures instead of the temperature at which the water absorption was less than 0.5%.
The technological properties of the ired test pieces were evaluated by performing the following tests: linear shrinkage, apparent porosity, water absorption, and bulk density. The linear shrinkage, LS (%), of ired samples was determined according to the Standard Test Method for Drying and Firing Shrinkages of Ceramic Whiteware Clays [28] by means of the following equation:
LS=Ld - Lf x100 Ld
(A)
being Ld and Lf the diameter (mm) of dried and ired samples,
respectively.
The bulk densities, BD (g/cm3), ofdried (BD
d) and ired
(BDf) samples were determined by the mercury displacement
method measured by means of the following equation:
BD = mx x - rHg x100
mHg (B)
being mx the mass of dried (md) or ired sample (mf), rHgthe density of mercury (13.53 g/cm3) and m
Hg the mass of each
sample submerged in mercury [29].
The water absorption, WA (%),was measured according to the Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Speciic Gravity of Fired Whiteware Products [30], which involves drying the test specimens to constant mass (D), boiling in distilled water for 5 h and soak for an additional 24 h at room temperature. After impregnation, the saturated mass (M) of each specimen is determined. WA expresses the relationship of the mass of water absorbed to the mass of the dry specimen as follows:
WA = M - D x100
D (C)
The apparent porosity, εα (%), expresses the relationship of the volume of open pores to the exterior volume of the specimen and is calculated as follows:
ea = BDf x WA (D)
The modulus of rupture was determined analogously to the Standard Test Methods for Flexural Properties of Ceramic Whiteware Materials [31] by a three-point bending test assembly, the span between supports being 62.2 mm and the load application rate being 5 mm/min. An average of ten measurements was taken for this purpose. In the STD mixture (0% RSA) and the mixture with RSA in replacement of feldspar that exhibited the best modulus of rupture (12.5% RSA), the main crystalline phases were identiied by XRD using a PANalytical X’Pert PRO X-ray diffractometer. The microstructural characteristics were observed by scanning
electron microscopy, for which the fracture surface of each sample was polished and attacked with a hydroluoric acid (HF) solution at 5% for 3 min, washed with distilled water and ethyl alcohol, and subsequently dried and coated with carbon.
RESULTS AND DISCUSSION
Feasibilityof using RSA as a non-plastic material in porcelain tile compositions
The vitriication curves of the mixtures 0% RSA, 12.5% RSA and 60% RSA are plotted in Fig. 1. Regarding optimum iring range (interval of temperatures where water absorption is lower than 0.5% and bulk density is maximized), it can be observed that 12.5% RSA composition showed a similar temperature range than 0% RSA. Moreover, the 60% RSA composition showed a shorter temperature range than 0% RSA; this indicates that more careful control is required during the iring process of this porcelain. From the vitriication curves, the optimum iring temperatures (those corresponding to maximum density) for each composition were determined to be 1183 °C, 1183 °C, and 1234 °C, respectively.
The results of the technical properties obtained for each dried as well as ired composition, at its respective optimum iring temperature, are detailed in Table IV.
The increase in the quantity of RSA in the mixture decreased the compactness of the test piece, evidenced by a reduction in dry bulk density. This behaviour could stem from the fact that it adversely affected the balance between the non-plasticand the colloidal plastic particles, raising the linear shrinkage of the ired test pieces. Compositions 12.5% RSA and 60% RSA displayed this behaviour, with an increase in linear shrinkage in comparison with standard mixture 0% RSA (8.1%). The optimum vitriication range is achieved when apparent porosity reaches a minimum value, tending to be nearly zero and simultaneously bulk density and linear shrinkage are maxims. Firing above the vitriication range has a drastic adverse effect on the physical properties owing to an increase in the pressure of the gases trapped in the pores, producing swelling or bloating of the piece [4]. The maximum bulk densityof each composition decreased as the replacementof feldspar and feldspathic sandwith RSA increased, in comparison with that of standard mixture 0% RSA, as a result of the lowerdry bulk density of the compositions with RSA. Mixture 12.5% RSA exhibited the highest bulk density (2.36 g/cm3) with respect to the
other mixture with RSA. Thus, mixture 60% RSA displayed a reduction in bulk density (2.12 g/cm3) to the extent of being
lower than the minimum value of 2.30 g/cm3 speciied by the
European standard UNI EN 87 [32].
the bloating caused by the greater content ingas-generating substances (Fe2O3, Cl, andLOI) in the RSA, in addition to the
increase in the quantity ofSiO2, which reduced the sintering
capacity, and the increase in the quantity of liquid phase of potassium origin, increasing its viscosity in comparison with that of the liquid phase of sodium originin agreement with the indings previously reported [7, 33]. These phenomena were corroborated by the decrease in shrinkage and increase in porosity displayed by mixture 60% RSA (see Table IV), typical behaviourof the bloating phenomenon during liquid-phase sintering of traditional ceramics [4].
The modulus of ruptureof the ired test pieces (see Table IV) evidences a similar trend to that displayed by ired bulk density. The previous phenomena are consistent with reported indings [8], who noted that generally, at greater bulk density, the modulus of rupture increased as a consequence of reduction of porosity. According to the criteria of standard IS0 13006 for dry-pressed ceramic tiles, ceramic tile is deined as porcelain stoneware tile belonging to group BIa, when it exhibits a modulus of rupture≤35 MPa and water
absorption ≤0.5%. In view of the results of the modulus of ruptureand water absorption (see Table IV), compositions
Technical properties RSA0% 12.5% RSA RSA60%
Dry bulk density (g/cm3) 1.91 1.83 1.65
Optimum iring temperature (°C) 1,183 1,183 1,234
Linear shrinkage (%) 8.1 8.7 8.6 Fired bulk density (g/cm3) 2.43 2,36 2.12
Water absorption (%) <0.1 <0.1 0.2 Apparent porosity (%) <0.1 <0.1 0.5 Modulus of rupture (MPa) 69 67 48
Figure 1: Linear shrinkage, water absorption and bulk density in the porcelain tile samples (0% RSA, 12.5% RSA and 60% RSA) as a function of iring temperature. 0% RSA appears in all the plots as a standard composition.
[Figura 1: Retração linear, absorção de água e densidade aparente das amostras de azulejos de porcelana (0% de RSA, 12,5% e 60% de RSA RSA) como uma função da temperatura de queima. 0% RSA aparece em todas as parcelas, como uma composição padrão.]
10 10 2.5 2.6 2.5 2.4 2.4 2.3 2.3 2.1 2.2 2.2 1.8 1.9 2.0 2.1 1.6 1.7 6 6 6 6 16 16 8 8 8 8 20 4 4 4 12 4 12 9 9 5 5 5 15 5 14 7 7 7 18 21 7 3 3 3 9 3 10 1 3 1 6 2 1120 1120 1120 1120 Temperature (ºC)
Temperature (ºC) Temperature (ºC)
Temperatue (ºC) W a te r a b s o rp ti o n (% ) W a te r a b s o rp ti o n (% ) W a te r a b s o rp ti o n (% ) W a te r a b s o rp ti o n (% ) L in e a r Sh ri n k a g e (% ) L in e a r Sh ri n k a g e (% ) B u lk D e n s ity (g /c m 3) B u lk D e n s ity (g /c m 3) 1200 1200 1200 1200 1160 1160 1160 1160 1240 1240 1240 1240 1140 1140 1140 1140 1220 1220 1220 1220 1180 1180 1180 1180 1260
12601280 12601280
1260 2 6 2 8 4 2 0 0 0 0
Table IV - Physical and mechanical properties of the dried and ired test pieces obtained at their optimum iring temperature.
0% RSA, 12.5% RSA and 60% RSA could all be deemedas porcelain stoneware tile belonging to group BIa. However, in composition 60% RSA, there must also bean increase in the optimum iring temperature. In addition, the optimum iring range was very narrow (see Fig. 1), reducing the feasibility of using these mixture on an industrial scale.
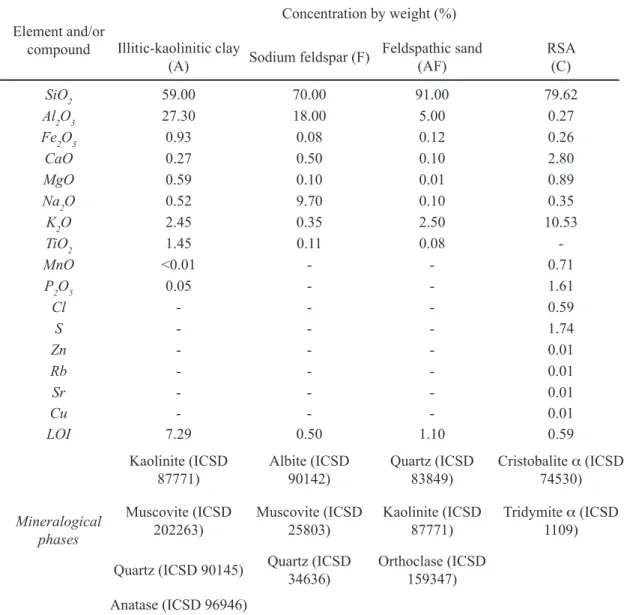
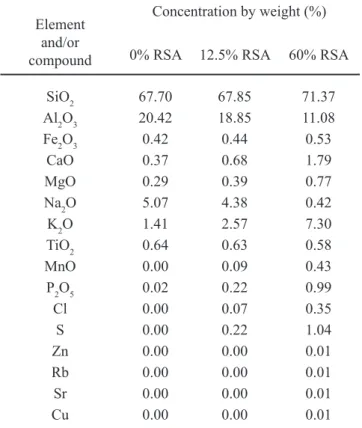
The SEM micrographs at 100X in backscattered electron mode of the polished surfaces (see Figs. 2a and 2b) showed that the surface of the test piece ired under maximum densiication conditions of composition 12.5% RSA displayed a greater quantity of closed pores than the test piece of composition 0% RSA; which was consistent with the density and porosity values noted previously.
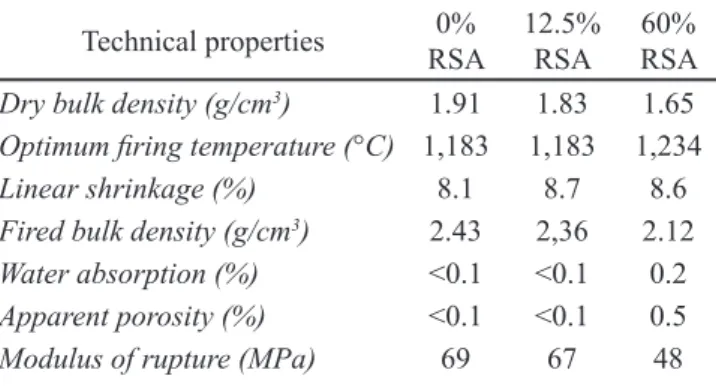
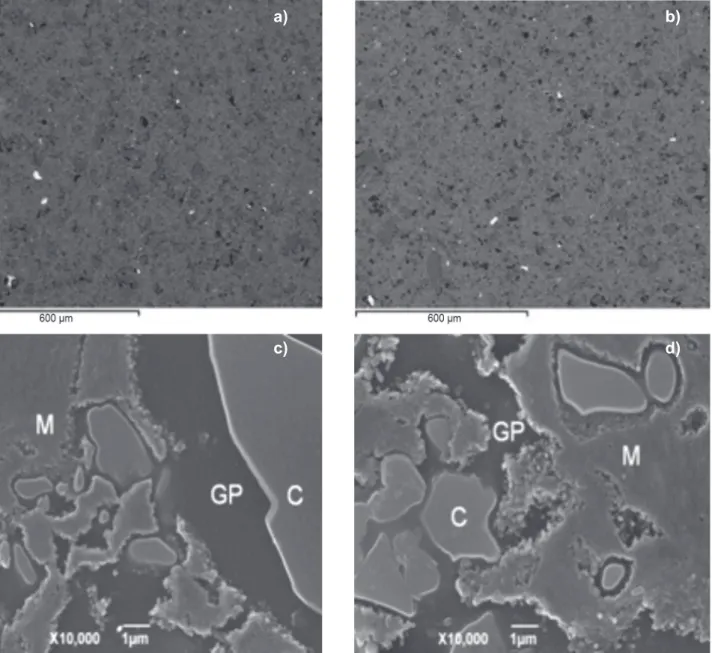
The SEM micrographs at 10000X in secondary electron
imaging (SEI) mode (see Figs. 2c and 2d) showed the presence of primary mullite crystals (M) and quartz crystals (C) embedded in a glassy phase (GP) in the microstructure of ired test pieces of compositions 0% RSA and 12.5% RSA. The SEM results were corroborated by XRD tests of ired test pieces of compositions 0% RSA and 12.5% RSA (see Fig. 3), highlighting the presence of a-quartz (2q= 20.86° and 26.64º) (ICSD 83849), albite (2q= 22.03° and 27.91º) (ICSD 240519),and mullite (2q= 16.43º; 33.21º; 35.26º; 39.24°, and 40.87º) (ICSD 99328), this last phase being partially responsible for porcelain mechanical strength. In addition, in 12.5% RSA the presence of a-cristobalite was evidenced (2q= 21.76º) (ICSD 74530), basically stemming from the RSA (see Table I) in agreement with reported indings [34]. Figure 2: SEM micrographs in BSE (backscattered electron) mode of polished surfaces of ired test pieces 0% RSA (a) and 12.5% RSA (b) (100X); and SEM micrographs in SEI (secondary electron imaging) mode of polished surfaces of ired test pieces 0% RSA (c) and 12.5% RSA (d) (10000X).
[Figura 2: Micrograias obtidas em mivroscópio eletrônico de varredura em BSE (elétrons retroespalhados) de superfícies polidas das peças queimadas com 0% RSA (a) e 12,5% RSA (b) (100X); e micrograias em SEI (imagem de elétron secundária) de superfícies polidas das peças queimadas com 0% RSA (c) e 12,5% RSA (d) (10000x).]
a)
c)
b)
CONCLUSIONS
Rice straw ashes (RSA) displayed anon-plastic character, allowing them to be used as partial replacement of feldspar and quartz in porcelain tile compositions. No pronounced change took place in the technological process when RSA was added to the composition in a quantity of 12.5%, in replacement of feldspar and feldspathic sand, allowing the obtaining of mineralogical phases and microstructure of typical porcelain tiles.The ired test pieces of all the compositions studied with a RSA addition (12.5% RSA and 60% RSA) exhibited the characteristics required for porcelain stoneware tile, BIa group (modulus of rupture ≥ 35 MPa and water absorption ≤ 0.5%) according to standard ISO 13006. However, in the composition in which RSA completely replaced feldspathic sand and feldspar, 60% RSA, the optimum iring range was narrower, in addition to requiring higher iring temperatures. This reduces the feasibilityof using this last mixtureon an industrial scale.
ACKNOwLEDGEMENTS
The authors would like to thank Universidad del Valle (Colombia), Instituto de Tecnología Cerámica (ITC) (España) and COLCIENCIAS (Colombia) for their support provided to conduct this study. In particular, this paper presents the partial results from the research project “Triaxial ceramics based on rice straw ash”, code 110652128358, supported by COLCIENCIAS, Oficial Call 521 of 2010, contract RC. No. 325-2011.
REFERENCES
[1] ISO 13006:1998,“Ceramic tiles - Deinitions, classiication, characteristics and marking. International
Organization for Standardization” (1998).
[2] E. Sánchez, M.J. Ibáñez, J. García-Ten, M.F. Quereda, I.M. Hutchings, Y.M. Xu, “Porcelain tile microstructure: Implications for polished tile properties”, J. Eur. Ceram. Soc. 26 (2006) 2533-2540.
[3] C. Zanelli, M. Raimondo, G. Guarini, M. Dondi, “The vitreous phase of porcelain stoneware: Composition, evolution during sintering and physical properties”, J. Non-Cryst. Solid. 357 (2011) 3251-3260.
[4] J.M. Márquez, J.M. Rincón, M. Romero, “Effect of iring temperature on sintering of porcelain stoneware tiles”, Ceram. Int. 34 (2008) 1867-1873.
[5] A. De Noni Jr, D. Hotza, V. Cantavella, E. Sánchez, “Analysis of the development of microscopic residual stresses on quartz particles in porcelain tile”,J. Eur. Ceram. Soc. 28 (2008) 2629-2637.
[6] F. Andreola, L. Barbieri, A. Corradi, I. Lancellotti, T. Manfredini, “Utilisation of municipal incinerator grate slag for manufacturing porcelainized stoneware tiles manufac-turing”, J. Eur. Ceram. Soc. 22 (2002) 1457–1462.
[7] C. Zanelli, M. Raimondo, M. Dondi, G. Guarini, P.M. Tenorio, “Sintering mechanisms of porcelain stoneware tiles”, In Qualicer 2004, Proc. VIII World Cong. Ceramic Tile Quality, Cámara Oicial de Comercio, Industria y Navegación, Castellón, Spain. papers C (2004) 247-259. (available at: <http://aulavirtual.camaracastellon.com/ qualicerCD/pdf/0413191e.pdf> [consulted on July 20, 2014]).
[8] S.R. Braganca, C.P. Bergmann, “Traditional and glass powder porcelain: technical and microstructure analysis”, J. Eur. Ceram. Soc. 24 (2004)2383-2388.
[9] F. Andreola, L. Barbieri, E. Karamanova, I. Lancellotti, M. Pelino, “Recycling of CRT panel glass as luxing agent in the porcelain stoneware tile production”, Ceram. Int. 34 (2008) 1289-1295.
[10] K. Dana, S.K. Das, “Partial substitution of feldspar by B.F. slag in triaxial porcelain: Phase and microstructural evolution”, J. Eur. Ceram. Soc. 24 (2004) 3833-3839. [11] C.S. Prasad, K.N. Maiti, R. Venugopal, “Effect of rice husk ash in whiteware compositions”, Ceram. Int. 27 (2001) 629-635.
[12] C.S. Prasad, K. N. Maiti, R. Venugopal,“Effect of silica fume addition on the properties of whiteware compositions”, Ceram. Int. 28 (2002) 9-15.
[13] F. Matteucci, M. Dondi, G. Guarini, “Effect of soda-lime glass on sintering and technological properties of por-celain stoneware tiles”, Ceram. Int. 28 (2002) 873-880. [14] R. De Gennaro, P. Cappelletti, G. Cerri, M. De Genna-ro, M. Dondi, G. Guarini, A. Langella, D. Naimo, “Inluence of zeolites on the sintering and technological properties of porcelain stoneware tiles”, J. Eur. Ceram. Soc. 23 (2003) 2237-2245.
[15] K. Dana, S.K. Das, “High strength ceramic loor tile compositions containing Indian metallurgical slags”,J. Ma-ter. Sci. Lett. 22 (2003) 387-389.
[16] K. Dana, S.K. Das, “Evolution of microstructure in ly ash-containing porcelain body on heating at different Figure 3: X-ray diffraction patterns: 0% RSA (a) and 12.5% RSA
(b) (M = mullite, C = quartz, Cr = cristobalite, A = albite).
[Figura 3: Difração de raios X: 0% de RSA (a) e 12,5% de RSA (b) (M = mulita, C = quartzo, Cr = cristobalita, A = albita).]
0
2q (degree)
In
te
n
s
ity
temperatures”, Bull. Mater. Sci. 27 (2004b)183-188. [17] K. Dana, S.K. Das, “Enhanced resistance to thermal cy-cling of slag-containing vitriied porcelain tiles”, Industrial Ceramics 28 (2008) 121-124.
[18] K. Dana, S. Das, S.K. Das, “Effect of substitution of ly ash for quartz in triaxial kaolin-quartz-feldspar system”, J. Eur. Ceram. Soc. 24 (2004) 3169-3175.
[19] A. Tucci, L. Esposito, E. Rastelli, C. Palmonari, E. Rambaldi, “Use of soda-lime scrap-glass as a luxing agent in a porcelain stoneware tile mix”, J. Eur. Ceram. Soc. 24 (2004) 83-92.
[20] K. Dana, J. Dey, S.K. Das, “Synergistic effect of ly ash and blast furnace slag on the mechanical strength of tradi-tional porcelain tiles”, Ceram. Int. 31 (2005) 147-152. [21] R. De Gennaro, M. Dondi, P. Cappelletti, G. Cerri, M. De Gennaro, G. Guarini, A. Langella, L. Parlato, C. Zanelli, “Zeolite-feldspar epiclastic rocks as lux in ceramic tile manufacturing, Microporous”, Mesoporous Mater. 105 (2007) 273-278.
[22] M. Raimondo, C. Zanelli, F. Matteucci, G. Guarini, M. Dondi, J.A. Labrincha, “Effect of waste glass (TV/PC ca-thodic tube and screen) on technological properties and sin-tering behaviour of porcelain stoneware tiles”, Ceram. Int. 33 (2007) 615-623.
[23] A.S. Demirkiran, E. Artir, E. Avci, “Effect of natural zeolite addition on sintering kinetics of porcelain bodies”, J. Mater. Process. Technol. 203 (2008) 465-470.
[24] A. Guzmán, S. Delvasto, E. Sánchez, V. Amigó, “Cenizas del tamo de arroz como substituto del feldespato en la fabricación de cerámica blanca”, Boletín de la Sociedad Española de Cerámica y Vidrio 52 (2013) 25-30.
[25] B.M. Jenkins, L.L. Baxter, T.R. Miles Jr, T.R. Miles, “Combustion properties of biomass”, Fuel. Process. Tech-nol. 54 (1998) 17-46.
[26] P. Thy, B.M. Jenkins, S. Grundvig, R. Shiraki, C.E.
Lesher, “High temperature elemental losses and mineral-ogical changes in common biomass ashes”, Fuel. 85 (2006) 783-795.
[27] A. Barba, V. Beltrán, C. Felíu, J. García, F. Ginés, E. Sánchez, V. Sanz, “Materias primas para la fabricación de soportes de baldosas cerámicas, 2nd Ed., Instituto de
Tecnología Cerámica (ITC)”, Castellón (2002) 272-286. [28] ASTM C326 - 09, Standard Test Method for Drying and Firing Shrinkages of Ceramic Whiteware Clays, American
Society for Testing and Materials, West Conshohocken, PA, USA(2009).
[29] J. García-Ten, Descomposición durante la cocción del carbonato cálcico contenido en el soporte crudo de los azulejos, Ph.D. Thesis, Universidad Jaume I, Castellón,
Valencia, Spain(2005).
[30] ASTM C373 - 88,Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent
Speciic Gravity of Fired Whiteware Products, American Society for Testing and Materials, West Conshohocken, PA, USA (2006).
[31] ASTM C674 - 88, Standard Test Methods for Flexural Properties of Ceramic Whiteware Materials”, American Society for Testing and Materials, West Conshohocken, PA,
USA (2006).
[32] G. Bifi, O gres porcelanato: manual de fabricação e
técnicas de emprego, 3rd Ed., Faenza Ed. do Brasil Ltda., S.
Paulo, Brazil (2002).
[33] S.K. Das, K. Dana, “Differences in densiication behaviour of K- and Na-feldspar-containing porcelain bodies”, Thermochim. Acta. 406 (2003) 199-206.
[34] A. Guzmán, Utilización de ceniza de tamo de arroz como reemplazo del feldespato y del cuarzo en la elabora-ción de gres porcelánico, Ph.D. Thesis, Universidad del
Val-le, Cali, ValVal-le, Colombia (2014).