online | memorias.ioc.fiocruz.br The hepatitis C virus (HCV) is one of the main causes
of chronic hepatitis worldwide and is responsible for a large percentage of cases of cirrhosis and hepatocellular carcinoma as well as referrals for liver transplant (Kim 2002, Thomas & Seeff 2005, Ghany et al. 2009).
Starting in the 1990s, the treatment of chronic hepa-titis C with medication was able to change the history of the disease, thus avoiding its complications (Ghany et al. 2009). The first medication used was [conventional interferon-α (IFN-α) (IFNc)], which was successful in fewer than 20% of cases (Davis et al. 1989). Later, the combination of IFNc with ribavirin (RBV) increased the chances of a sustained virological response (SVR), which denotes the absence of detectable HCV RNA in the plasma for at least six months after treatment is con-cluded in approximately 35-40% of cases (Poynard et al. 1998). In early 2000, the pegylation process allowed an increase in the half-life of IFN-α 2a and 2b (PEG-IFN) (Manns et al. 2001, Fried et al. 2002). Currently, close to half of all cases reach SVR when PEG is combined with RBV (Hadziyannis et al. 2004).
In North America and Europe, HCV is treated with PEG-IFN and RBV for six months (for genotypes 2 and 3) or for 12 months (for genotype 1). In Brazil, the Minis -try of Health [through the national public health system (SUS)] provides these medications free of charge, but limits the prescription of PEG-IFN to genotype 1, sup -plying IFNc for the treatment of other genotypes (pursu -ant to Administrative Rule 34 of 28 September 2007). This practice is based on a multicentre clinical trial of one of the pegylated IFNs on the market (2b), which sug -gested that there was no statistically significant differ-ence between PEG-IFN and IFNc combined with RBV for genotypes 2 and 3 (Manns et al. 2001).
It is possible to attain SVR using PEG-IFN and RBV in patients in whom previous therapy with IFNc and RBV for chronic hepatitis had failed. Sherman et al. (2006) demonstrated SVR in 43% of relapsing patients in whom RNA-HCV suppression was achieved during treatment, but who tested positive again after the medi-cation was discontinued and in 32% of patients who did not respond to IFNc plus RBV.
Despite the evolution of treatment, there are still ob-stacles for its application on a large scale, such as the high cost of the recommended drugs and the high incidence of side effects. This treatment is contraindicated for patients with decompensated cirrhosis, thrombocytopenia or neu-tropenia, serious heart diseases or mental illnesses.
In the past few years, some studies have been pub -lished in the Brazilian medical literature describing the experiences of different healthcare service providers Financial support: CNPq (to FJDS)
+ Corresponding author: fsouto@terra.com.br
Received 3 June 2011 Accepted 24 October 2011
Assessment of the treatment of chronic hepatitis C
in the state of Mato Grosso, central Brazil
Francisco Kennedy Scofoni Faleiros de Azevedo1,
Cassius Clay Scofoni Faleiros de Azevedo2, Francisco José Dutra Souto1/+
1Hospital Universitário Júlio Muller, Universidade Federal de Mato Grosso, Rua Luis Philippe Pereira Leite s/n, 78048-902 Cuiabá, MT, Brasil 2Pronto Socorro Municipal de Várzea Grande, Várzea Grande, MT, Brasil
In Brazil, the treatment of hepatitis C virus (HCV) infection is funded by the national public health system (SUS). To evaluate treatment results in the state of Mato Grosso, central Brazil, we have consulted the files of the office of the State Department of Health responsible for supplying such medications. We obtained information on 232 treat-ments of 201 patients who underwent treatment in or prior to 2008. The study was conducted by reviewing medical records, making telephone calls and interviewing the assistant physicians. Thirty-nine patients (19.4%) had cirrho-sis and HCV genotype 1 predominated (64.3%). Excluding patients with comorbidities or treatment without ribavirin we analysed 175 treatments (sustained virologic response occurred in 32.6% of cases). Twenty-six of these 175 were retreatments and the sustained virological response (SVR) rate among them was 30.8%; the SVR rate was 32.9% among those receiving treatment for the first time. The SVR rate of genotype 1 patients was 27.8%, whereas it was 37.5% in non-1 genotype patients. The adjusted multivariate analysis showed association of SVR with the absence of cirrhosis [odds ratio (OR): 7.7; confidence interval (CI) 95%: 2.5, 33.3], the use of pegylated interferon (OR: 5.8; CI 95%: 1.5, 21.4), non-1 genotype (OR: 5.3; CI 95%: 1.7, 16.7) and uninterrupted treatment (OR: 9.0; CI 95%: 3.3, 45.4). The SVR rates were similar to those found in other Brazilian studies about HCV, but lower than those found in national and international clinical trials. These data suggest that the treatments of chronic hepatitis C that are made available by SUS does not, under normal conditions, work as well as the original controlled studies indicated.
Key words: chronic hepatitis C - treatment - pegylated interferon - ribavirin -
with the treatment of chronic hepatitis C in Brazil (Al -ves et al. 2003, Figueiredo-Mendes et al. 2003, Acras et al. 2004, Brandão et al. 2006, Gonçales et al. 2006, 2010, Parise et al. 2006, Silva et al. 2007, Vigani et al. 2008, Almeida et al. 2009). These reports include experiences in the South and Southeast Regions of the country. The purpose of the present study is to describe and analyse the results of the treatment of chronic hepatitis C in the state of Mato Grosso (MT), as there are no known re -ports of this type for the Central-West Region of Brazil.
PATIENTS, MATERIALS AND METHODS
Upon reviewing the lists of treatment authorised by the Farmácia de Alto Custo (FAC),the government-run pharmacy for the distribution of high-cost drugs of the Mato Grosso State Department of Health (SSEMT), we identified a total of 268 patients treated between 2002-2008. As there is no public health unit specifi -cally designated to manage IFN injections in MT, these medications are delivered directly to the patients, who are then personally responsible for administering the injections. Consequently, there are no official records in FAC files indicating whether these treatments were completed or successful. To gather patient information, we sought the help of the assisting physicians who had ordered the medications. We asked the permission of these physicians to access the medical records of their patients at the respective clinics and healthcare centres; we also obtained permission from the directors of each medical institution.
We sought information regarding the demograph-ics of the patients, the characteristdemograph-ics of the cases, such as the genotype and the kind of IFN-α prescribed and the outcomes of the treatments. Whenever available, the results of the liver biopsies were expressed using the METAVIR score for fibrosis and inflammatory activity (Bedossa & Poynard 1996).
The outcome of interest (the SVR) was defined as non-detection of HCV RNA for at least six months af -ter the conclusion of treatment. Patients who stopped the treatment before the recommended schedule due to side effects, intolerance or problems caused by periods of ir-regularity of drug delivery from the public health service were classified as “having interrupted the treatment”.
The data obtained were stored using the software EpiInfo 6.04d (Centers for Disease Control and Preven -tion, Atlanta, USA). Appropriate statistical tests were
performed to compare continuous and categorical vari-ables, with respective dispersion and a confidence in-terval (CI) of 95%, using the same software. A logistic regression model was created to analyse the association of SVR with the variables related to it according to uni -variate analysis. Classical variables were also included in the model, even if they were not associated with SVR in univariate analysis. For this analysis, a stepwise meth -od was used in Stata 8.2 (Stata Corporation, College Sta-tion, Texas, USA, 2005).
The ethical aspects of the project were analysed and approved on 15 April 2009, by the Research Ethical Com -mittee of the University Hospital Julio Muller of the Fed -eral University of Mato Grosso under protocol 599/08.
RESULTS
Of the 268 individuals registered at the FAC who re -quested medication for the treatment of chronic hepatitis C from 2002-2008 we obtained information on 201 pa-tients corresponding to 232 initial treatments and 31 re-treatments. We were only able to recover the outcomes of 197 treatments. The majority of these treatments (52.6%) took place in public health institutions.
Of the 201 individuals, 145 (72.1%) were male (Table I). The average age of the 201 patients at the beginning of treatment was 48 years old (46 for male patients and 50 for female patients). The average age of the 31 patients who underwent retreatment was 51. The majority of the patients were Caucasians, but there were a large propor-tion of individuals with a mix of European, African and Native South American ancestries. The difficulty of classifying the ethnicities of participants prevented any outcomes analysis that included ethnicity as a variable.
We were able to recover information on the HCV genotypes of 157 patients (77.6%). Genotype 1 was re -sponsible for the largest number of cases (101, 64.3%), followed by genotype 3 (46 cases, 29.3%). It was not pos -sible to recover information on the dosage of RBV used in a large number of the patients. Most of the patients re-ceived 1 g daily, but data on dosage reduction was scarce. Therefore, we were unable to analyse the effects of this important variable on the final result of the treatment.
Of the 197 treatments with recovered results, SVR was present in 30.9% of them (CI 95% = 24.6-38%). Be
-TABlE I
Characteristics of the 201 patients who underwent treatment
for chronic hepatitis C in the state of Mato Grosso, Brazil,
from 2002-2008, with medication supplied by public health
Total n (%)
Total 201
Gender
Male 145 (72.1)
Female 56 (27.9)
Age (average, extremes) 48 (16-68)
Initial treatments 201 (86.6)
Retreatments 31 (13.4)
Know genotype 157 (78.1)
1 101 (64.3)
2 9 (5.7)
3 46 (29.3)
5 1 (0.7)
Unknown 44 (21.9)
Cirrhosis 39 (19.4)
Fibrosis stagea 71 (35.3)
0-2 24 (50.7)
3-4 47 (49.3)
cause some of these patients had not received a thera-peutic scheme with RBV due to contraindication (renal failure and dialysis treatment) and because some of the patients were co-infected with HIV, we did not include these patients to better compare the results with those of other reports and trials. Therefore, 149 patients (26 of whom were retreatments) who received 175 treatments with IFN-α (IFNc or PEG-IFN) and RBV were included in the study. The global SVR in this group was 32.6%. For patients who received PEG-IFN the SVR was 35.3% and for patients who received IFNc, the SVR was 27.1% (p = 0.353). The SVR rates were slightly higher for the patients undergoing initial treatments (32.9%) than for those undergoing retreatment (30.8%, p = 0.831) and they were also higher in patients infected by a non-1 genotype (37.5%) than in those infected by genotype 1 (27.8%, p = 0.295). Regarding only the patients infected by non-1 genotypes, there was a better response to the use of the combination of PEG-IFN and RBV compared to the com
-bination of IFNc and RBV (52.9% vs. 20.9%, p = 0.034) (Table II). Eighty-seven (75%) of 116 treatments with PEG-IFN were performed with PEG-IFN-α 2a. Because there were few treatments (25%) with PEG-IFN-α 2b, comparison between the products was not possible.
Thirty-four (23.8%) of 143 patients had cirrhosis (data unavailable for 6 patients) at the beginning of the treatment and they were diagnosed through histo-pathology or through clinic and laboratory data. They corresponded to 46 treatments and retreatments and 15.2% had SVRs.
Multivariate analysis showed that infection by non-1 genotypes, the use of therapeutic schedules employing PEG-IFN, non-interruption of treatment and the absence of cirrhosis were independent factors associated with SVR (Table III). The outcome was not influenced by the patient’s gender or age, whether the patient was treated at public or private healthcare facilities, or whether the patient was undergoing first treatment or retreatment.
TABlE II
Results of the 175 treatments with ribavirin (RBV) carried out in patients without human immunodeficiency virus infection
or chronic renal failure, regarding type of interferon used, whether it was the initial treatment or retreatment, and genotype
Total n
SVR
n (%) p value
Total 175 57 (32.6)
Initial treatments 149 49 (32.9)
Retreatments 26 8 (30.8) 0.831
Genotype 1 90 25 (27.8)
Genotype other than 1 56 21 (37.5) 0.218
Unidentified genotype 29 11 (37.9) 0.300
All treatments of cirrhotic patients 46 7 (15.2)
All treatments of non-cirrhotic patients 123 49 (39.8) 0.004
Therapy regimen with PEG-IFN and RBV 116 41 (35.3)
Therapy regimen with IFNc and RBV 59 16 (27.1) 0.272
Initial treatments with PEG-IFN and RBV 93 33 (35.5)
Initial treatments with IFNc and RBV 56 16 (28.6) 0.384
Retreatments with PEG-IFN and RBV 23 8 (34.8)
Retreatments with IFNc and RBV 3 0 (0) 0.219a
Genotype 1 (n = 90)
Treatments with PEG-IFN and RBV 78 23 (29.4)
Treatments with IFNc and RBV 12 2 (16.7) 0.355
Initial treatments 77 22 (28.6)
Using PEG-IFN and RBV 66 20 (30.3)
Using IFNc and RBV 11 2 (22.2) 0.497a
Retreatments 13 3 (23.1)
Using PEG-IFN and RBV 12 3 (25)
-Non-1 genotype (n = 56)
Treatments with PEG-IFN and RBV 17 9 (52.9)
-Treatments with IFNc and RBV 39 8 (20.9) 0.034
Initial treatments 44 17 (38.6)
Using PEG-IFN and RBV 7 5 (71.4)
Using IFNc and RBV 37 12 (32.4) 0.089a
Retreatments 12 4 (33.3)
Using PEG-IFN and RBV 10 4 (40)
DISCUSSION
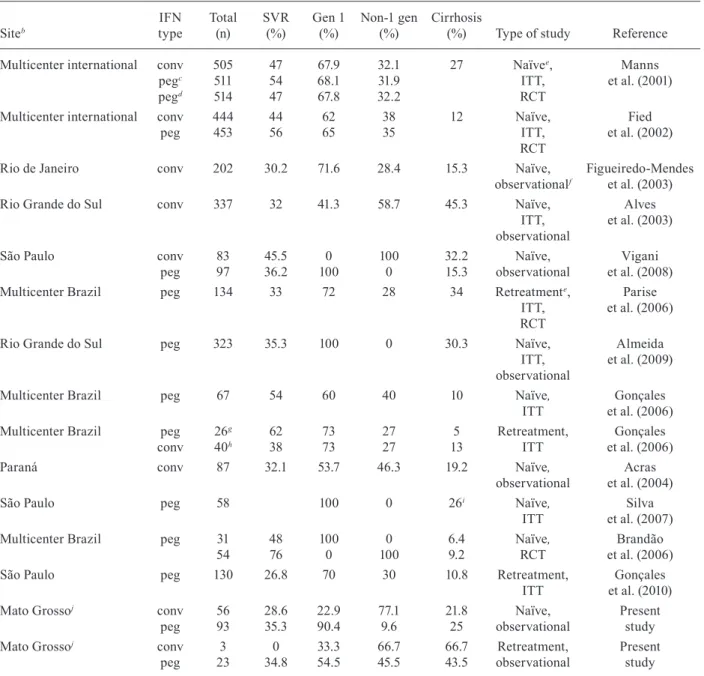
This is the first study that analyses the efficacy of the treatment of chronic hepatitis C in MT. Similar studies have been conducted in the country’s South and South-east Regions, but this is the first one conducted in the Central-West Region. The results of these studies are shown in Table IV. Also included in Table IV are results from Phase 3 approval studies of PEG-INF to charac -terise the differences between experimental and post-market studies.
Because the drugs that treat hepatitis C are very expensive, almost all patients, even those who receive treatment at private hospitals, access these drugs through the SUS. Therefore, we assume that the data presented in this study are representative of hepatitis C therapy in MT. In the present study, treatments administered in private hospitals and clinics did not achieve better SVR than those administered in public health institutions.
It was not possible to obtain information on 67 (25%) of the 268 patients listed at the FAC-SSEMT as receiv -ing these medications between 2002-2008, despite our at-tempts to contact via telephone the institutions in which they were treated, their physicians or even the patients themselves. In many cases, the information on these cases was lost due to changes of address or phone numbers, but it also demonstrates the unreliability of the medical re-cords kept in healthcare institutions and hospitals in MT.
Some of the characteristics of the sample, such as age and the preponderance of both male subjects and genotype 1, demonstrate that the present sample is similar to those
from other series of clinical cases, which corroborates the results found and reduces the chance of a selection bias resulting from the loss of data (Zeuzem et al. 2000, Alves et al. 2003, Figueiredo-Mendes et al. 2003, Gonçales et al. 2006, Parise et al. 2006, Silva et al. 2007, Vigani et al. 2008, Almeida et al. 2009, McHutchison et al. 2009). Regarding genotype in particular, previous studies had already demonstrated that genotype 1 is predominant in MT, as it is in most of Brazil (Campiotto et al. 2005).
For the analysis of SVR rates, we considered 175 treatments that included RBV in the therapeutic regi -men, excluding patients with HIV or renal failure, to make the population studied resemble those in similar studies. The SVR rate of 32.6% was lower than that ob -served in prospective studies, which report an SVR of approximately 50% (Manns et al. 2001, Fried et al. 2002, Brandão et al. 2006, Gonçales et al. 2006, Silva et al. 2007), but similar to that observed in several retrospec -tive studies in the country (Alves et al. 2003, Figueiredo-Mendes et al. 2003, Acras et al. 2004, Vigani et al. 2008, Almeida et al. 2009). These numbers suggest that in real-life conditions, the results obtained by the Brazilian healthcare services are not comparable to the results ob-tained in controlled trials, a fact that should be addressed by the country’s public health authorities.
Cirrhosis was present in 19.4% of the 201 patients. Other national and international studies describe an in -cidence of cirrhosis ranging from 12-27% (Manns et al. 2001, Fried et al. 2002, Alves et al. 2003, Figueiredo-Mendes et al. 2003, Gonçales et al. 2006, Parise et al.
TABlE III
Multivariate analysis of the influencing factors on the sustained virological response (SVR) in the treatment of hepatitis C patients in the state of Mato Grosso, Brazil, considering only therapy regimens using ribavirin (RBV) and patients not infected with human immunodeficiency virus (HIV) or with chronic renal failure
Variablesa
(% of SVR) Crude OR(p value)
Adjusted
OR 95% CI p value
Treatment
Conventional IFN (27.1) 1.0 1.0 -
Pegylated IFN (35.3) 1.3 (0.353) 5.8 1.5-21.4 0.008
Interruption
Yes (6.7) 1.0 1.0 -
No (39.0) 9.0 (0.000) 14.3 3.3-45.4 0.000
Cirrhosis
Yes (15.2) 1.0 1.0 -
No (39.8) 3.7 (0.004) 7.7 2.5-33.3 0.002
Genotype
1 (27.8) 1.0 1.0 -
Non-1 (37.5) 2.5 (0.295) 5.3 1.7-16.7 0.020
a: logistic regression model using stepwise backward technique Stata 8.2 (Stata Corporation, College Station, Texas, USA, 2005),
with 142 of the 175 treatments. Fifty-five treatments were excluded from the model due to patient infected with HIV, chronic re
-nal failure, treatment schedules without RBV or lack of information on the genotype, age or cirrhosis. Other non-associated vari
-ables included in the model were gender, age, nature of the health institution where the treatment took place (public or private),
other comorbidities (diabetes mellitus, porphyria, hyperthyroidism, psoriasis, psychosis) and whether it was the initial treatment
2006, Silva et al. 2007, Vigani et al. 2008, Almeida et al. 2009). Cirrhosis was one of the most important factors associated with treatment failure in our study, with only 15.2% of cirrhotic patients achieving an SVR (Table II). The other variables associated with SVR, such as genotype 1, interruption of treatment and use of PEG-IFN, were also as expected. Another fundamental fac -tor, which unfortunately could not be analysed in this
study due to incomplete treatment information, was the RBV dosage. In the past few years, it has become increasingly clear that a larger dose of RBV is linked to therapeutic success (Davis et al. 1998, Zoulin et al. 1998, Mangia et al. 2010).
The SVR rates in therapy regimens that included PEG-IFN and RBV were similar among initial treat -ments (35.5%) and retreat-ments (34.8%). The good
out-TABlE IV
Comparison of sustained virologic response (SVR) rates in other studiesa with those found in the present study
Siteb IFN type Total (n) SVR (%) Gen 1 (%) Non-1 gen (%) Cirrhosis
(%) Type of study Reference
Multicenter international conv pegc pegd 505 511 514 47 54 47 67.9 68.1 67.8 32.1 31.9 32.2
27 Naïvee,
ITT, RCT
Manns et al. (2001)
Multicenter international conv peg 444 453 44 56 62 65 38 35 12 Naïve, ITT, RCT Fied
et al. (2002)
Rio de Janeiro conv 202 30.2 71.6 28.4 15.3 Naïve, observationalf
Figueiredo-Mendes
et al. (2003)
Rio Grande do Sul conv 337 32 41.3 58.7 45.3 Naïve,
ITT,
observational
Alves
et al. (2003)
São Paulo conv
peg 83
97 45.536.2 0 100 100 0 32.2 15.3 Naïve, observational Vigani et al. (2008)
Multicenter Brazil peg 134 33 72 28 34 Retreatmente,
ITT, RCT
Parise et al. (2006)
Rio Grande do Sul peg 323 35.3 100 0 30.3 Naïve,
ITT,
observational
Almeida
et al. (2009)
Multicenter Brazil peg 67 54 60 40 10 Naïve,
ITT et al. (2006)Gonçales
Multicenter Brazil peg conv 26g 40h 62 38 73
73 2727 135
Retreatment,
ITT et al. (2006)Gonçales
Paraná conv 87 32.1 53.7 46.3 19.2 Naïve,
observational
Acras
et al. (2004)
São Paulo peg 58 100 0 26i Naïve,
ITT et al. (2007)Silva Multicenter Brazil peg 31
54
48
76 1000
0 100
6.4 9.2
Naïve,
RCT et al. (2006)Brandão
São Paulo peg 130 26.8 70 30 10.8 Retreatment,
ITT et al. (2010)Gonçales
Mato Grossoj conv
peg 56 93 28.6 35.3 22.9 90.4 77.1 9.6 21.8 25 Naïve, observational Present study
Mato Grossoj conv
peg 3 23 0 34.8 33.3 54.5 66.7 45.5 66.7 43.5 Retreatment, observational Present study
a: only studies which included treatment with RBV were considered. Studies performed on specific populations were excluded; b: named localities are Brazilian states; c: pegylated (peg) interferon (IFN) (1.5 mcg/Kg/week) for four weeks, afterwards 0.5
mcg/Kg/week; d: pegylated IFN (1.5 mcg/Kg/week) for the duration of the treatment; e: naïve patients; f: Phase 4 (observa -tional); g: relapser patients; h: non-responder patients; i: 26% of patients with F3 or F4; j: excluded patients co-infected with
human immunodeficiency virus or with chronic renal failure; conv: conventional; ITT: intention to treat; RCT: randomized
comes of retreatments compared with initial treatments was likely because 23 (88.4%) of the 26 retreatments were performed with PEG-IFN, most of them in patients who had received IFNc in their initial treatments.
However, when we analysed only the initial treat-ments of the non-1 genotype patients, 84% of whom were treated with IFNc, the SVR rate was 38.6%. A multicentre study of retreatment in Brazil, conducted by Parise et al. (2006), had demonstrated good results. The SVR rate reached in the initial treatment of non-1 genotype with PEG-IFN (71.4%) was higher than the SVR rate found in treatments with IFNc (32.4%), although the differ -ence was not statistically significant. Some of the initial treatments were performed with PEG-IFN, contrary to Brazilian Federal Administrative Rule 34/2007, probably because the medication was provided under a judicial in -junction or because these were cirrhotic patients. These data support the use of pegylated IFN for all genotypes from the start of the treatment, as is performed in other countries (Ghany et al. 2009, Brook et al. 2010), thus sparing patients from the greater risk of therapy failure and the need for a new course of treatments.
In short, this study analyses aspects of the treatment of chronic hepatitis C in MT in the first decade of the 21st century, showing SVR rates of approximately 30%. Numbers similar to these were found in studies carried out in other regions of the country in real-life situations. The sum of these results indicates the need to re-evaluate the protocol of the Brazilian Ministry of Health for the purpose of improving the effectiveness of the program to control hepatitis C in the country.
ACKNOWLEDGEMENTS
To the sanitary authorities of the state of Mato Grosso,
who authorized the access to the official files of the Farmácia de Alto Custo, and to the practitioners and health unities direc -tors that allowed the access to the patient’s medical files.
REFERENCES
Acras RN, Pedroso Ml, Caum lC, Pisani JC, Amarante HM, Carmes ER 2004. The sustained response rates for chronic hepatitis C
patients undergoing therapy with the several interferons and
riba-varins supplied by Brazilian Health Ministry is comparable to
those reported in the literature. Arq Gastroenterol 41: 3-9.
Almeida PR, Mattos AA, Amaral KM, Feltrin AA, Zamin P, Tovo
CV, Picon PD 2009. Treatment of hepatitis C with peginterferon and ribavirin in a public health program. Hepatogastroenterol-ogy 56: 223-226.
Alves AV, Azevedo AP, Perin C, Ramos GZ, Brandão AB, Mattos AA, Almeida PR 2003. Tratamento de pacientes com hepatite crônica pelo vírus C com interferon alfa e ribavirina: a experiên
-cia da Secretaria de Saúde do Rio Grande do Sul. Arq Gastroen-terol 40: 227-232.
Bedossa P, Poynard T 1996. An algorithm for the grading of activity
in chronic hepatitis C. The Metavir Cooperative Study Group.
Hepatology 24: 289-293.
Brandão C, Barone A, Carrilho F, Silva A, Patelli M, Caramori C, Focaccia R, Pereira l, Pedroso M, Tatsch F, Pessoa M, Pegasys Brazilian Study Group 2006. The results of a randomized trial looking at 24 weeks vs. 48 weeks of treatment with peginterferon alpha-2a (40 kDa) and ribavirin combination therapy in patients
with chronic hepatitis C genotype 1. J Viral Hepat 13: 552-559.
Brook G, Soriano V, Bergin C 2010. European guideline for the management of hepatitis B and C virus infections. Int J STD AIDS 21: 669-678.
Campiotto S, Pinho JR, Carrilho FJ, Silva lC, Souto FJ, Spinelli V, Pereira lM, Coelho HS, Silva AO, Fonseca JC, Rosa H, lacet CM, Bernardini AP 2005. Geographic distribution of hepatitis C virus genotypes in Brazil. Braz J Med Biol Res 38: 41-49.
Davis Gl, Balart lA, Schiff ER, lindsay K, Bodenheimer Jr HC, Per
-rillo RP, Carey W, Jacobson IM, Payne J, Dienstag Jl, David H, Van Thiel V, Tamburro C, lefkowitch J, Albrecht J, Meschiev
-itz C, Ortego TJ, Gibas A, The Hepatitis Interventional Therapy
Group 1989. Treatment of chronic hepatitis C with recombinant
interferon alfa. A multicenter, randomized, controlled trial. Hepa
-titis Interventional Therapy Group. N Engl J Med 321: 1501-1506.
Davis Gl, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trépo C, Shiffman Ml, Zeuzem S, Craxi A, ling MH, Albrecht J 1998. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepati
-tis Interventional Therapy Group. N Engl J Med 339: 1493-1499.
Figueiredo-Mendes C, Cardoso C, Brandão-Mello CE, Pinto PT, Castro EJ, Pernambuco CD, Cardoso AC, Rosales Fl, Milagre DR, Reis DM 2003. Eficácia do tratamento da hepatite crônica C com inter -feron alfa-2a ou 2b e ribavirina entre pacientes de hospitais públicos
da cidade do Rio de Janeiro. Gastroenterol Endosc Digest 22: 7.
Fried MW, Shiffman Ml, Reddy KR, Smith C, Marinos G, Gonçales Jr Fl, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, lin A, Hoffman J, Yu J 2002. Peginterferon alfa-2a plus ribavirin for
chronic hepatitis C virus infection. N Engl J Med 347: 975-982.
Ghany MG, Strader DB, Thomas Dl, Seeff lB, American Association
for the Study of Liver Diseases 2009. Diagnosis, management and
treatment of hepatitis C: an update. Hepatology 49: 1335-1374.
Gonçales Jr Fl, Moma CA, Vigani AG, Angerami AF, Gonçales ES, Tozzo R, Pavan MH, Gonçales NS 2010. Retreatment of hepatitis
C patients with pegylated interferon combined with ribavirin in
non-responders to interferon plus ribavirin. Is it different in real
life? BMC Infect Dis 10: 212.
Gonçales Jr Fl, Vigani A, Gonçales N, Barone AA, Araújo E, Focac
-cia R, Oliveira U, Coelho HS, Paixao J, Perez R, lobato C, Weir
-ich J, Rosa H, Borges A, Vila R, Corrêa-Giannella Ml, Ferraz
ML 2006. Weight-based combination therapy with
peginterfer-on-2b and ribavirin for naïve, relapser and non-responder patients
with chronic hepatitis C. Braz J Infect Dis 10: 311-316.
Hadziyannis SJ, Sette Jr H , Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer Jr H, Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, lin A, Ackrill AM, PEGASYS International Study
Group 2004. Peginterferon-alpha2a and ribavirin combination
therapy in chronic hepatitis C: a randomized study of treatment
duration and ribavirin dose. Ann Intern Med 140: 346-355.
Kim WR 2002. The burden of hepatitis C in the United States. Hepa-tology 36: 30-34.
Mangia A, Dalgard O, Minerva N, Verbaan H, Bacca D, Ring-larsen H, Copetti M, Carretta V, Piazzolla V, Cozzolongo R, Mottola l, Andriulli A 2010. Ribavirin dosage in patients with HCV geno -types 2 and 3 who completed short therapy with peg-interferon alpha 2b and ribavirin. Aliment Pharmacol Ther 31: 1346-1353.
Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, ling M, Albrecht JK 2001.
Peginterferon alfa-2b plus ribavirin compared with interferon
al-fa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet 358: 958-965.
McHutchison JG, lawitz EJ, Shiffman Ml, Muir AJ, Galler GW, Mc
Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone lD, Brass CA, Albrecht JK, Sulkowski MS, IDEAl
Study Team 2009. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 361: 580-593.
Parise E, Cheinquer H, Crespo D, Meirelles A, Martinelli A, Sette H, Brazilian Pegasys Cooperative Study Group 2006. Peginter -feron alfa-2a plus ribavirin in retreatment of chronic hepatitis C patients, nonresponders and relapsers to previous conventional interferon plus ribavirin therapy. Braz J Infect Dis 1: 11-16.
Poynard T, Marcellin P, lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J 1998. Randomized trial of interpheron alpha 2b plus ribavirin for 48 weeks or for 24 weeks versus interpheron alpha 2b plus placebo for 48 weeks
treatment of chronic infection with hepatitis C virus. Hepatitis
Interventional Therapy Group. Lancet 352: 1426-1432.
Sherman M, Yoshida EM, Deschenes M, Krajden M, Bain VG, Peltekian K, Anderson F, Kaita K, Simonyi S, Balshaw R, lee
SS, Canadian Pegasys Study Group 2006. Peginterferon alfa-2a (40 KD) plus ribavirin in chronic hepatitis C patients who failed previous interferon therapy. Gut 352: 1631-1638.
Silva GF, Rodrigo RJ, Pardini MI, Corvino SM, Henriques RM, Peres MN, Silveira lV, Coelho KI 2007. Using pegylated interferon
alfa-2b and ribavirin to treat chronic hepatitis patients infected
with hepatitis C virus genotype 1: are nonresponders and relaps -ers different populations? Braz J Infec Dis 11: 554-560.
Thomas Dl, Seeff lB 2005. Natural history of hepatitis C. Clin. Liver Dis 9: 383-398.
Vigani AG, Pavan MH, Tozzo R, Gonçales ES, Feltrin A, Fais VC, lazarini MS, Gonçales NS, Gonçales Jr Fl 2008. Comparative
study of patients with chronic hepatitis C virus infection due to
genotypes 1 and 3 referred for treatment in Southeast Brazil.
BMC Infect Dis 8: 164.
Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, lai MY, Gane E, O’Grady J, Reichen J, Diago M, lin A, Hoffman J, Brunda MJ
2000. Peginterferon alpha-2a in patients with chronic hepatitis C.
N Engl J Med 343: 1666-1672.
Zoulin F, Haem J, Ahmed SS, Chossegros P, Habersetzer F, Cheval
-lier M, Bailly F, Trépo C 1998. Ribavirin monotherapy in patients with chronic hepatitis C: a retrospective study of 95 patients.