UNIVERSIDADE DA BEIRA INTERIOR
Ciências da saúde
Avaliação de Marcadores de Morte
Celular e Parâmetros Reprodutivos em
Modelos de Diabetes Mellitus
Tânia Isabel Rodrigues Amaral Dias
Dissertação apresentada à Universidade da Beira Interior para obtenção do
grau de Mestre em Ciências Biomédicas
(2º ciclo de estudos)
Orientador: Prof. Doutor Pedro Fontes Oliveira (CICS-UBI)
Co-orientador: Prof. Doutora Graça Lopes (ICBAS-UP)
ii
Agradecimentos
Embora uma tese seja, pela sua finalidade académica, um trabalho individual, há contributos de natureza diversa que não podem nem devem deixar de ser realçados. Por essa razão, desejo expressar os meus sinceros agradecimentos a todas as pessoas que, direta ou indiretamente, contribuíram para a realização desta Tese de Mestrado:
Ao Professor Doutor Pedro Fontes Oliveira, meu orientador, pela competência científica, acompanhamento e organização do trabalho, pela disponibilidade e generosidade reveladas ao longo deste ano, assim como pelas críticas, correções e sugestões relevantes feitas durante a orientação.
À Professora Doutora Graça Lopes, minha co-orientadora, pela competência científica e orientação dada, bem como pela disponibilidade e simpatia demonstradas e pelas correções e sugestões relevantes para melhorar a minha tese.
Ao Doutor Marco Alves, pela valiosa ajuda, bons concelhos, correções e sugestões que contribuíram para a elaboração desta tese.
Aos meus colegas de laboratório: Sara, Cátia, Daniel, Margarida, Inês, Carina, Ricardo, Vera, Ana, Aline e principalmente ao Luís por toda a ajuda, bons concelhos, pela disponibilidade sempre manifestada, pela amizade e pelo bom ambiente de trabalho.
Às grandes amigas que fiz durante o curso: Vânia Reis, Filipa Pinheiro, Joana Sousa, Vânia Vieira e Sofia Marques, sem vocês a elaboração desta tese não seria possível. Foram vocês que sempre me apoiaram, me deram bons concelhos e me fizeram continuar e ver o lado positivo mesmo quando as coisas não corriam da melhor maneira.
À minha família, principalmente aos meus pais que sempre me encorajaram a trabalhar para atingir os meus objetivos e sempre me apoiaram em todas as minhas decisões.
iii
Resumo
A diabetes mellitus (DM) representa uma das maiores ameaças à saúde global moderna e a sua incidência está a aumentar rapidamente em todo o mundo. Esta doença consiste numa desordem metabólica, caracterizada por hiperglicemia, resultante de uma secreção defeituosa de insulina, resistência à ação da insulina ou ambas. Existem dois tipos de DM, tipo 1 e tipo 2, ambas as condições relacionadas com a infertilidade masculina. A DM tipo 1 está associada com a privação de insulina e embora os efeitos globais in vivo na função reprodutiva sejam bem conhecidos, há uma falta de estudos relativamente ao controlo da insulina sobre as funções fisiológicas das células do sistema reprodutivo. Sobretudo, os estudos in vivo são frequentemente feitos depois de a doença estar completamente estabelecida, mas sabe-se que há um estado pré-diabético, caracterizado por resistência à insulina, que antecede o desenvolvimento da DM, especialmente da DM tipo 2. Com o nosso trabalho, pretendemos investigar mais profundamente a ligação entre a DM e a infertilidade masculina, através da análise de vários marcadores de morte celular e de parâmetros reprodutivos. Para isso, simulámos o estado de DM tipo 1 humano em células de Sertoli de rato e analisámos os níveis de expressão de mRNA e proteína de vários marcadores de morte celular envolvidos na via mitocondrial. Por outro lado, também desenvolvemos um modelo animal de pré-diabetes de modo a reproduzir esta condição patológica e avaliar alterações produzidas nos parâmetros reprodutivos, assim como nos níveis de expressão de mRNA e proteína de marcadores de morte celular envolvidos na via mitocondrial.
Os resultados obtidos levam-nos a sugerir que a insulina interfere com a interação entre proteínas pró- e anti-apoptóticas. Uma vez que esta interação pode decidir o destino celular e exercer um controlo rigoroso sobre a sinalização apoptótica, a insulina terá um papel chave na manutenção da espermatogénese. O modelo de rato utilizado compartilhava muitas das características clínicas e metabólicas do estado pré-diabético observado em humanos, tais como resistência à glucose e a progressão de normoglicemia/normoinsulinemia para hiperglicemia/hiperinsulinemia moderada devido à ingestão de alimentos. Este estado pré-diabético induziu alterações significativas na morfologia dos espermatozoides da cauda do epidídimo, mostrando que esses animais poderão desenvolver problemas de subfertilidade ou de fertilidade. Na sinalização apoptótica na cauda do epidídimo, os animais sujeitos à dieta de alta energia (HED) apresentaram níveis mais baixos de mRNA Bax e da proteína citocromo C, embora a avaliação quantitativa do endpoint apoptótico, a atividade da caspase-3, não tenha evidenciado quaisquer alterações entre a situação HED e controle. Isto sugere que o processo apoptótico pode ser controlado por outros mecanismos que não somente os proteicos pró-apoptóticos mitocondriais, como por exemplo os sistemas anti-apoptóticos celulares.
iv
Os resultados obtidos com estes dois modelos experimentais levam-nos a concluir que os problemas de subfertilidade/infertilidade causados pela DM podem ser mediados pela insulina, que tem um efeito importante na regulação da interação entre proteínas pró e anti-apoptóticas e, por esse motivo, deverá ser dedicada uma atenção especial às disfunções ocorridas no estado pré-diabético, onde observámos alterações cruciais na morfologia de espermatozoides epididimais de ratos. Devido à crescente incidência da DM e às complicações associadas ao nível da infertilidade masculina é fundamental aprofundar o conhecimento nestes dois sistemas, de modo a isolar possíveis mecanismos envolvidos e a avaliar os efeitos globais, como uma estratégia para desenvolver possíveis abordagens terapêuticas.
Palavras-chave
Diabetes mellitus; Infertilidade masculina; Parâmetros reprodutivos; Marcadores de morte celular.
v
Resumo alargado
Nos mamíferos, os testículos são os elementos centrais do sistema reprodutor masculino e estão envolvidos na síntese de esteroides e na produção de espermatozoides. A espermatogénese é o processo de expansão e desenvolvimento das células germinativas que ocorre dentro dos túbulos seminíferos dos testículos determinando a fertilidade masculina. Uma espermatogénese normal envolve um equilíbrio entre a proliferação celular e a apoptose, porque, mesmo durante condições normais, um número de células germinativas morre por apoptose antes de atingir a maturidade. Depois de completarem a espermatogénese, os espermatozoides são células diferenciadas incapazes de nadar ou fertilizar um óvulo. Essa capacidade de fertilização será adquirida durante as diferentes fases que ocorrem até ao encontro com o gâmeta feminino, sendo uma delas a passagem através do epidídimo. Este consiste num túbulo longo e enrolado que cria um microambiente único que ajuda a transformar os espermatozoides imaturos e sem motilidade em células competentes e completamente férteis. Permite também, o armazenamento dos espermatozoides férteis num estado viável dentro da cauda do epidídimo até serem ejaculados.
As células de Sertoli (SCs) desempenham um papel importante no desenvolvimento funcional dos testículos e consequentemente na expressão do fenótipo masculino. Elas fornecem nutrientes e fatores regulatórios para a sustentação das células germinativas. Para além disso, têm uma grande capacidade para produzir lactato que é um elemento importante para as células germinativas devido ao seu efeito anti-apoptótico e ao seu papel como fonte de energia. Nas SCs, a glucose é metabolizada para lactato para depois este ser usado pelas células germinativas como o principal substrato para a produção de ATP. Por isso, a regulação do metabolismo da glucose nas SCs é crucial para uma espermatogénese normal e para a fertilidade. A insulina é uma hormona na regulação do metabolismo celular e a sua disfunção (deficiência ou resistência) tem atraído muita atenção, uma vez que se traduz em patologias como a diabetes mellitus (DM). A DM representa uma das maiores ameaças para a saúde global moderna e a sua incidência está a aumentar rapidamente em todo o mundo. Esta doença poder ser divida em dois tipos, tipo 1 e tipo 2, que podem ser descritos sumariamente como desordens metabólicas caracterizadas por um estado de hiperglicemia (baixos valores de glucose no sangue) resultando de uma secreção defeituosa de insulina, resistência à ação da insulina, ou ambas. A DM tipo 1 desenvolve-se geralmente em idade jovem e é caracterizada por uma destruição autoimune das células beta do pâncreas (produtoras de insulina) em indivíduos geneticamente susceptíveis, resultando na dependência de um tratamento com insulina exógena. A DM tipo 2 ocorre quando a produção de insulina pelas células beta não é suficiente para manter os níveis de glucose no sangue dentro de valores
vi
fisiológicos normais, levando a exaustão funcional das células beta. A DM tipo 2 pode ser prevenida se for detetado precocemente o estado de pré-diabetes que usualmente antecede o aparecimento desta doença. A transição de um estado de pré-diabetes para DM tipo 2 ocorre quando a capacidade secretora das células beta não é capaz de compensar a resistência à insulina.
As alterações hormonais e metabólicas associadas com a DM tipo 1 e tipo 2 comprometem a fertilidade masculina. A influência prejudicial da DM e da obesidade na fertilidade tem recebido maior atenção uma vez que a sua prevalência e incidência têm aumentado em todo o mundo, enquanto a idade do primeiro diagnóstico para ambas as doenças tem diminuído continuamente. Devido a esta situação, cada vez mais, os problemas de fertilidade afetam indivíduos antes e/ou durante os seus anos reprodutivos. Um grande número de casos de infertilidade masculina está associado a uma baixa qualidade dos espermatozoides devido a parâmetros reprodutivos anormais, sendo, por isso, a análise do sémen o teste inicial para a avaliação do fator de infertilidade masculina.
Com este trabalho, pretendeu-se investigar a associação entre a DM e a infertilidade masculina através da análise de vários marcadores de morte celular envolvidos na via mitocondrial e parâmetros reprodutivos dos espermatozoides (motilidade, viabilidade, concentração e morfologia). Um dos objetivos deste trabalho foi reproduzir a doença humana de DM tipo 1 em SCs de rato cultivadas (in vitro) para analisar o seu efeito nas vias apoptóticas de morte celular. Para isso, foram isoladas SCs de ratos Wistar normais e foram sujeitas a uma situação de privação de insulina, de modo a simular a total falta de insulina característica da DM tipo 1. Por outro lado, pretendeu-se também desenvolver um modelo animal de pré-diabetes para analisar o feito deste estado patológico na função reprodutiva masculina. Para isso, administrou-se diariamente uma dieta de alta energia a ratos Wistar e analisaram-se os parâmetros reprodutivos e efeitos ao nível das vias apoptóticas no epidídimo desses animais. Para analisar eventuais alterações nas vias apoptóticas analisámos os níveis de expressão de mRNA e proteína (por RT-PCR e western blot, respetivamente) de alguns dos marcadores de apoptose envolvidos na via mitocondrial, tais como: p53, Bax, caspase-9, caspase-3 e citocromo C. Também foram feitos ensaios enzimáticos com a caspase-3 para estudar a cinética enzimática como medida aproximada dos níveis de apoptose.
Os resultados obtidos levam-nos a sugerir que a insulina interfere com a interação entre proteínas pró- e anti-apoptóticas. Uma vez que esta interação pode decidir o destino celular e exercer um controlo rigoroso sobre a sinalização apoptótica, a insulina terá um papel chave na manutenção da espermatogénese. O modelo de rato utilizado compartilhava muitas das características clínicas e metabólicas do estado pré-diabético observado em humanos, tais como resistência à glucose e a progressão de normoglicemia/normoinsulinemia para hiperglicemia/hiperinsulinemia moderada devido à ingestão de alimentos. Este estado pré-diabético induziu alterações significativas na morfologia dos espermatozoides da cauda do
vii
epidídimo, mostrando que esses animais poderão desenvolver problemas de subfertilidade ou de fertilidade. Na sinalização apoptótica na cauda epidídimo, os animais sujeitos à dieta de alta energia (HED) apresentaram níveis mais baixos de mRNA Bax e da proteína citocromo C, embora a avaliação quantitativa do endpoint apoptótico, a atividade da caspase-3, não tenha evidenciado quaisquer alterações entre a situação HED e controle. Isto sugere que o processo apoptótico possa ser controlado por outros mecanismos que não somente os proteicos pró-apoptóticos mitocondriais, como por exemplo os sistemas anti-pró-apoptóticos celulares.
Os resultados obtidos com estes dois modelos experimentais levam-nos a concluir que os problemas subfertilidade/infertilidade causados pela DM podem ser mediados pela insulina, que tem um efeito importante na regulação da interação entre proteínas pró e anti-apoptóticas e, por esse motivo, deverá ser dedicada uma atenção especial às disfunções ocorridas no estado pré-diabético, onde observámos alterações cruciais na morfologia dos espermatozoides epididimais de rato. Devido à crescente incidência da DM e às complicações associadas ao nível da infertilidade masculina é fundamental aprofundar o conhecimento nestes dois sistemas, de modo a isolar possíveis mecanismos envolvidos e a avaliar os efeitos globais, como uma estratégia para desenvolver possíveis abordagens terapêuticas.
viii
Abstract
Diabetes mellitus (DM) represents one of the greatest threats to modern global health and its incidence is rapidly rising worldwide. It describes a metabolic disorder characterized by hyperglycaemia resulting from defective insulin secretion, resistance to insulin action, or both. There are two types of DM, type-1 DM (T1DM) and type-2 DM (T2DM), both associated with male infertility. T1DM is associated with insulin deprivation and although the overall in
vivo effects in the reproductive function is well known, there is a lack of studies concerning
about the insulin control over the physiological functions of cells from the reproductive system. Importantly, the in vivo studies are often focused after the disease is fully establish, but it is known that a prediabetic state, which is characterized by insulin resistance, precedes the development of DM, especially T2DM. With our work, we aimed to further investigate the association between DM and male infertility by analyzing several apoptotic markers and reproductive parameters. To do so, we simulated type 1 DM in cultured rat Sertoli cells and analyzed the mRNA and protein expression levels of several cellular markers involved in the mitochondrial apoptotic pathway. We also developed an animal model of prediabetes to evaluate the effect of this pathological state in the reproductive parameters as well as in the mitochondrial apoptotic pathway.
Our results lead us to suggest that insulin interferes with the interaction between pro and anti-apoptotic proteins. As the interaction of these proteins decide the cell fate and exert a strict control over the apoptotic signaling, insulin has a key role in the maintenance of the spermatogenesis. Our rat model shared many of the clinical and metabolic characteristics of the prediabetic state observed in humans such as glucose resistance and progression from normoglycaemia/normoinsulinemia to moderate hyperglycaemia/hyperinsulinemia due to food intake. This prediabetic state induced important alterations in cauda epididymis spermatozoa morphology showing that these animals may develop subfertility or fertility problems. The apoptotic signalling in cauda epididymis spermatozoa of the high-energy (HED) fed animals presented lower Bax mRNA levels and lower cytC protein levels although the apoptotic endpoint, caspase-3 activity, was not altered. This suggests that the apoptotic process may be controlled by other mechanisms rather than the mitochondrial pro-apoptotic proteins, such as the anti-apoptotic cellular systems.
Those two experimental models led us to conclude that the subfertility/infertility problems caused by DM may be mediated by insulin, which has an important effect in the regulation of the interaction between pro and anti-apoptotic proteins and special attention must be taken in the prediabetic state where crucial alterations in rat spermatozoa occurred. Due to the rising incidence and associated complications of DM and male infertility it is
ix
crucial to further investigate in these two systems to isolate possible mechanisms and evaluate the overall effects as a strategy to develop possible therapeutics.
Keywords
x
Table of contents
Agradecimentos ... ii Resumo ...iii Resumo alargado ...v Abstract ... viii Table of contents ... xList of Figures ... xii
List of tables ... xiii
List of abbreviations ... xiv
I. Introduction ... 1
1. The Mammalian Testis ... 2
1.1 Spermatogenesis ... 3
1.1.1 Hormonal Regulation of Spermatogenesis ... 5
1.2 Sertoli cells ... 7
1.3 Mammalian Spermatozoon ... 9
2. The Epididymis ... 12
2.1 Functions of the Mammalian Epididymis ... 16
2.1.1 Epididymal Sperm Maturation ... 16
2.1.2 Epididymal Sperm storage ... 17
2.2 Epididymal sperm abnormalities ... 18
3. Diabetes mellitus and male infertility ... 19
4. Apoptotic pathways in the testis ... 21
II. Aims of the present study ... 24
III. Materials and methods ... 26
1 – Chemicals ... 27
2 – Animals ... 27
3 - Glucose tolerance test ... 28
xi
4.1 – Sperm Motility ... 28
4.2 – Sperm Concentration ... 28
4.3 – Sperm Viability ... 29
4.4 – Sperm Morphology ... 29
5 - Sertoli cells isolation procedure ... 30
5.1 Immucytochemestry ... 31 5.2 - Hormonal treatments... 32 6 – RT-PCR ... 32 7 - Western Blot ... 33 8 – Enzymatic assays ... 34 9 - Statistical analysis ... 34 IV. Results ... 35
A – Apoptosis signaling pathways in insulin-deprived rat Sertoli cells ... 36
1. Rat Sertoli cells culture ... 36
2. Insulin deprivation decreases p53 mRNA levels in in vitro cultured rat Sertoli cells .. 36
3. Insulin deprived rat Sertoli cells exhibit decreased caspase-9 mRNA levels ... 37
4. Insulin deprivation does not alter protein levels of Bax and caspase-9 in in vitro cultured rat Sertoli cells ... 38
5. Insulin deprivation does not alter caspase-3 activity in rat Sertoli cells ... 38
B – Apoptotic signaling pathways in whole cauda epididymis ... 40
1. High-energy diet animal model ... 40
2. Glucose tolerance test ... 40
3. Motility is increased in cauda epididymis sperm of the high-energy diet fed animals . 41 4. Morphologically normal sperm decreased in epididymal sperm of the high-energy diet fed animals ... 42
5. High-energy diet decreases Bax mRNA expression levels in cauda epididymis ... 43
6. High-energy diet decreases cytochrome c protein levels in cauda epididymis ... 44
7. High-energy diet does not alter caspase-3 activity in cauda epididymis ... 45
V. Discussion ... 47
A – Apoptosis signaling pathways in insulin-deprived rat Sertoli cells ... 48
B – Apoptosis signaling pathways in whole cauda epididymis ... 50
VI. Conclusions ... 54
xii
List of Figures
Figure 1 - Schematic representation of the mammalian testis and epididymis. ... 3
Figure 2 - The process of spermatogenesis ... 4
Figure 3 - Summary of hormonal control of male reproductive function... 6
Figure 4 - General features of the mammalian spermatozoon ... 10
Figure 5 - Lateral view of the falciform-shaped head of rat spermatozoa ... 11
Figure 6 - Schematic and histological representation of the male reproductive tract and excurrent ducts ... 12
Figure 7 - Segmental structure of the epididymis ... 13
Figure 8 - Schematic view of the main epithelial cell types in the epididymis: narrow, clear, principal and basal cells ... 14
Figure 9 - The two main apoptotic pathways ... 22
Figure 10 - Light microscopy image of a typical rat spermatozoon ... 29
Figure 11 - Microscopic images of a normal rat spermatozoon and various morphological defects ... 30
Figure 12 - Effect of insulin deprivation on p53, Bax, caspase-9 and caspase-3 mRNA levels in rat Sertoli cells ... 37
Figure 13 - Effect of insulin deprivation on Bax and caspase-9 protein levels in rat Sertoli cells... ... 38
Figure 14 - Effect of insulin deprivation on caspase-3 activity in rat Sertoli cells ... 39
Figure 15 - Glucose levels of the high-energy diet (HED) group and the control group measured during the glucose tolerance test ... 41
Figure 16 - Effect of high-energy diet (HED) on p53, Bax, caspase-9 and caspase-3 mRNA levels in rat cauda epididymis ... 44
Figure 17 - Effect of high-energy diet (HED) on cytochrome C protein levels in rat cauda epididymis ... 45
xiii
List of tables
Table 1 - Oligonucleotides and cycling conditions for PCR amplification of Bax, p53,
caspase-3, caspase-9 and 18S. ... 33
Table 2 - Average values of the animals weight and glucose blood levels measured in the
high-energy diet group and control group during all the treatment (n=6). ... 40
Table 3 - Sperm motility, viability and concentration values of both experimental groups
(n=6). ... 42
Table 4 - Average percentage of the morphology alteration evaluated in epididymal sperm of
the high-energy diet group and control group animals (n=6). ... 43
xiv
List of abbreviations
AIF – Apoptosis inducing factor
Apaf-1 – Apoptotic peptidase activating factor 1 Apaf–3 - Apoptotic peptidase activating factor 3 ArKO – Aromatase knockout
BEB – Blood-epididymis barrier BTB – Blood-testis-barrier CytC – Cytochrome C DM – Diabetes mellitus EDs – Efferent ductules ERs – Estrogen receptors Erα – Estrogen receptor α
ErαKO - Estrogen receptor α knockout Erβ – Estrogen receptor β
FSH – Follicle-stimulating hormone GnRH - Gonadotropin releasing hormone HED – High energy diet
IFG – Impaired fasting glucose IGT – Impaired glucose tolerance IR – Insulin resistance
LH – Luteinizing Hormone OS –Oxidative stress PIGs – p53-induced genes ROS – Reactive oxygen species SCs – Sertoli cells
STF - Seminiferous tubular fluid T1DM – Type 1 diabetes mellitus T2DM - Type 2 diabetes mellitus TJ – Tight junctions
1
2
1. The Mammalian Testis
Mammalian testes, the central elements of the male reproductive tract, are paired, whitish, ovoid organs, suspended outside the abdomen in the scrotum, which is an outpouching of the abdominal wall and is internally divided into two sacs, one for each testis (Setchell 1978). The two main functions of the testes are steroid synthesis and spermatozoa production (Jayle et al. 1962). Their structural compartmentalization determines the topographical division of its dual functions: spermatogenesis occurring in the seminiferous tubules (avascular compartment) and steroidogenesis taking place in the interstitium (vascularized area) (Schlatt et al. 1997). The interstitial area is encased by a tough fibrous membrane called tunica albuginea (Figure 1) (Saladin 2003). There is also an outer tissue layer, known as tunica vaginalis, a thin serous sac, derived from the peritoneum during the descent of the testes, which covers the anterior and lateral surfaces of the testes but not their posterior surfaces (Setchell 1978; Kent 2001). Fibrous inward septum, extending from the tunica albuginea, partition the testis in 250 to 300 wedge-shaped testicular lobules, each containing one to three loop-shaped seminiferous tubules with both ends opening to a region called mediastinum (Kent 2001; Rabbani et al. 2010). Each seminiferous tubule is bounded by a basement membrane and has a fluid-filled lumen containing spermatozoa (Vander et al. 2001). The contents of the seminiferous tubules are received in the interconnecting tubules of the mediastinum known as rete testis, which in turn is connected to the efferent ductules (EDs) (Rabbani et al. 2010). EDs are a major site for fluid homeostasis, as they reabsorb more than 95% of the luminal fluid released from the seminiferous epithelium. These small-coiled ductules transport sperm from the rete testis and play a major role in concentrating it prior to his maturation in the epididymis (Picciarelli-Lima et al. 2006; Hahn et al. 2009; Lee et al. 2009).
Seminiferous tubules, which represent about 80% of the testicular mass, are lined by layers of germ cells in various stages of development (spermatogonia, spermatocytes, spermatids, spermatozoa) and mainly supporting Sertoli cells (SCs) (Sharpe 1984; Colborn et
al. 1993; Sikka and Wang 2008). Each seminiferous tubule, considered the functional unit of
the testis, is surrounded by mesenchymal cells. Among these are the peritubular myoid cells whose contractile elements generate peristaltic waves along the tubules (Gaytan et al. 1994; Gaytan et al. 1994). In the other hand, interstitium consists of loose connective tissue, blood and lymphatic vessels and various cell types, including Leydig cells, fibroblasts, macrophages and leukocytes (Sharpe 1984; Colborn et al. 1993).
Leydig (or intersticial) cells are the predominant source of the male sex steroid hormone testosterone (Vander et al. 2001). These cells arise from interstitial mesenchymal tissue between the tubules during the eighth week of human embryonic development and
3
play vital roles in downstream masculinization events and in descent of the testes into the scrotum (Akingbemi 2005; Sikka and Wang 2008).
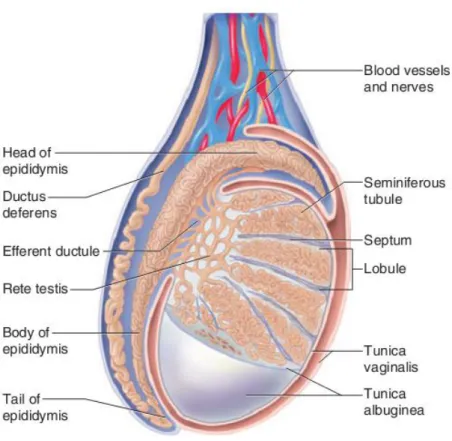
Figure 1 - Schematic representation of the mammalian testis and epididymis. The testis is encased by two tissue layers, from the inside to the outside, tunica albuginea and tunica vaginalis. Various septum extending from the tunica albuginea divides testis in lobules where seminiferous tubules are located. Seminiferous tubules converge to the rete testis that is connected to the efferent ductules (EDs). The head of the epididymis is linked to the testis by various EDs. Adapted from Saladin (2003).
1.1 Spermatogenesis
Spermatogenesis is a multi-step process of germ cell expansion and development which occurs within the seminiferous tubules of the testes determining male fertility (Walker 2010). This process has a duration of approximately 40–50 days in rodents (Sikka and Wang 2008) and 80 days in humans (Sikka and Wang 2008) and refers to the development of mature spermatozoa with half the number of chromosomes (haploid) from the most immature germ cell in the testis, spermatogonia (diploid) (Figure 2) (Han et al. 2009; Bettegowda and Wilkinson 2010). Spermatogenesis is composed of three phases, mitotic, meiotic and
4
spermiogenesis, and occurs during the 14 stages of the seminiferous epithelial cycle of spermatogenesis in rats, 12 stages in mice and 6 stages in men (Cheng et al. 2010). First, spermatogonia multiply by mitosis, producing two types of daughter cells called type A and type B spermatogonia (Saladin 2003). Type A cells remain outside the blood-testis-barrier (BTB) and continue to multiply from puberty until death. Thus men never exhaust their supply of gametes and normally remain fertile throughout old age. Type B spermatogonia migrate closer to the tubule lumen and differentiate into slightly larger cells called primary spermatocytes (Saladin 2003). In the second phase, primary spermatocytes undergo two divisions, meiosis I and meiosis II, to give rise to secondary spermatocytes and haploid round spermatids, respectively (Papaioannou and Nef 2010).
Figure 2 - The process of spermatogenesis. By mitosis spermatogonia produce type A and type B spermatogonium. Type B spermatogonium crosses the blood-testis-barrier (BTB) and differentiates into a primary spermatocyte. After the first meiotic division, secondary spermatocytes are formed, and then divide again resulting in haploid round spematids. The spermatids elongate and differentiate until spermatozoa are formed. Adapted from Saladin (2003).
5
Finally, takes place the spermiogenesis, which comprises the transformation of spermatids into elongated flagellar germ cells and culminates with the release of spermatozoa (spermiation) into the lumen of the seminiferous tubule (Papaioannou and Nef 2010; O'Donnell et al. 2011). During spermiogenesis, spermatids undergo a metamorphosis that does not involve cell division, but a number of morphological changes (acrosome formation, nuclear condensation, development of the flagellum, and cytoplasm reorganization) that eventually result in the generation of the spermatozoa (Papaioannou and Nef 2010).
After completing spermatogenesis and spermiation, spermatozoa are fully differentiated cells incapable of swimming or fertilize the egg. The fertilizing ability will be acquired in a temporally controlled manner during different stages towards the encounter with the female gamete: (1) along the transit through the epididymal duct, (2) at the encounter with the seminal plasma during the ejaculation, (3) during the transit through the female reproductive tract, (4) during the interaction with oviductal epithelial cells and (5) during the interactions with the different female gamete structures. Sperm changes occurring in the male reproductive tract have been defined as maturational changes where as those occurring in the female counter part are known as capacitation-associated changes (Caballero
et al. 2010).
1.1.1 Hormonal Regulation of Spermatogenesis
The mammalian spermatogenesis regulation includes communication between the hypothalamus–hypophysis axis and the gonad itself (Aragon et al. 2005). The master control hormone is the gonadotropin releasing hormone (GnRH), a decapeptide produced by specialized neurons in the hypothalamus (Walker and Cheng 2005). Pulsatile GnRH reaches the anterior pituitary via the hypothalamo-pituitary portal vessels triggering the release of both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) that then act on the testes to regulate the spermatogenic potential (Figure 3) (Vander et al. 2001). The LH binds to receptors on the surface of leydig cells and stimulates testosterone production, a steroid hormone that diffuses into the seminiferous tubules. Within the seminiferous tubules only SCs possess receptors for testosterone and FSH (Walker and Cheng 2005). Thus, testosterone and FSH acts synergistically on SCs leading to the secretion of paracrine agents that are essential for spermatogenesis (Aragon et al. 2005).
Some negative feedbacks exerted by testicular hormones are also part of this regulation. The protein hormone inhibin secreted by the SCs can act on the anterior pituitary, decreasing the FSH levels, or on the hypothalamus decreasing GnRH levels. On the other
6
hand, testosterone inhibits mainly LH secretion. It can do so in two ways: acting on the hypothalamus to decrease the frequency of GnRH bursts, resulting in a decreased amount of GnRH reaching the pituitary and less secretion of the gonadotropins; or acting directly on the anterior pituitary leading to a decreased LH secretion in response to any given level of GnRH (Vander et al. 2001). Thus, testosterone is essential to maintain spermatogenesis at numerous levels. In the absence of testosterone or the androgen receptor, formation of the BTB is compromised, germ cells are unable to progress beyond meiosis, immature germ cells are prematurely displaced from SCs and mature spermatozoa cannot be released from SCs. The disruption of any of these testosterone-dependent steps results in the failure of spermatogenesis and infertility (Walker 2010).
Figure 3 - Summary of hormonal control of male reproductive function. Hypothalamus synthesizes the
gonadotropin releasing hormone (GnRH), which in turn stimulates the anterior pituitary to produce the luteinizing hormone (LH) and follicle-stimulating hormone (FSH). FSH acts only on the Sertoli cells (SCs) leading to the stimulation of spermatogenesis, whereas LH acts only on the Leydig cells that produce testosterone. The secretion of FSH is inhibited mainly by Inhibin, a proteic hormone secreted by the SCs, and the secretion of LH is inhibited mainly by testosterone. Solid line – Stimulates; Dashed line – inhibits.
7
Apart from gonadotropins and androgens, spermatogenesis is a highly regulated developmental process occurring under a complex multifactorial control (Carreau and Hess 2010) and estrogens are now recognized as potential regulators in numerous species including humans (Carreau et al. 2010). The aromatase cytochrome P450, which is the last step in the steroidogenesis pathway leading to the formation of estrogens from androgens, is localized in the cellular endoplasmic reticulum of numerous tissues (Carreau and Hess 2010). In the male reproductive tract, aromatase and estrogen receptors (ERs) are mainly found in germ cells including spermatocytes, round spermatids, and elongated spermatids, as well as in leydig and SCs (Cheng et al. 2010). In order to exert a biological effect, estrogens should interact with ERs which in turn can modulate the transcription of target genes (genomic effect) and/or activate different signalization pathways located on the membrane (non genomic effect) (Carreau et al. 2012). Two main types of nuclear ERs have been described: estrogen receptor α (ERα) and estrogen receptor β (ERβ) (Carreau and Hess 2010). The infertility in mice lacking a functional ERα (ERαKO) was the first definitive demonstration that estrogen was required for male fertility (Lubahn et al. 1993; Korach 1994; Eddy et al. 1996; Hess et al. 1997). The ERαKO mice are infertile primarily due to a defect in EDs development and function (Hess et al. 1997; Lee et al. 2000). Mice lacking a functional aromatase gene (ArKO) are also infertile; however, this appears to be primarily due to a specific defect in germ cell development (Robertson et al. 1999).
Estrogen is clearly involved in the negative feedback effects of testosterone on the brain to control pituitary gonadotropin secretion, and hence an absence of, or inappropriate exposure to, estrogens leads to disturbances in the delicate balance of the hypothalamo-pituitary-testis axis in both mice and men. Once the development and spermatogenic potential of the testis is reliant upon this axis, such disturbances are likely to have a deleterious effect on spermatogenesis and fertility (O'Donnell et al. 2001). So, to ensure the well-functioning of spermatogenesis it is required a regulated balance between androgens and estrogens, with aromatase serving as a modulator (Shaha 2008; Carreau and Hess 2010).
1.2 Sertoli cells
Sertoli cells are highly polarized epithelial cells that extend upwards from the basement membrane of the seminiferous tubule to its open lumen (Mruk and Cheng 2011). They play a central role in the functional development of the testis and hence in the expression of the male phenotype (Mackay 2000). From Enrico Sertoli works, in 1865, came out the concept that SCs function as “nurse cells”, that is, they provide nutrients and regulatory factors for the sustenance of germ cells (Griswold 1995). SCs also facilitate germ
8
cell movement and mature germ cell release (Mruk and Cheng 2004), and secrete the seminiferous tubular fluid (STF) that helps the transport of spermatozoa into the epididymis (Waites and Gladwell 1982; Sikka and Wang 2008).
The SCs fate is established in the embryonic gonad at the time of sex determination (Hacker et al. 1995; Lovell-Badge and Hacker 1995) and is followed by a phase of rapid cell proliferation and differentiation. At the onset of puberty, SCs become terminally differentiated postmitotic cell population that support spermatogenesis (Chaudhary et al. 2005). Maturational process is marked by a reduced ability or the total loss of proliferation and irreversible changes in SCs morphology and physiology (Jegou 1992), heralding the switch from an immature, proliferative state to a mature, non-proliferative state. Morphologically, the nucleus enlarges and becomes tripartite and the nucleolus becomes more prominent (Sharpe et al. 2003). The changes associated with terminal differentiation of SCs include exit from the cell cycle and the formation of the BTB (Norton and Skinner 1992; Bremner et al. 1994; Law and Griswold 1994; Schlatt et al. 1996; Ketola et al. 2002; Holsberger et al. 2003). Each SC has a fixed capacity for the number of germ cells that it can support (Orth et
al. 1988), though this capacity varies between species. The Sertoli:germ cell ratio is
approximately 1:50 in the adult rat testis (Weber et al. 1983; Wong and Russell 1983). The number of SCs will determine the number of germ cells that can be supported through spermatogenesis and hence the daily sperm production, a factor with obvious bearing on fertility (Orth et al. 1988; Sharpe et al. 2003).
The BTB is created by adjacent SCs that forms tight junctions (TJ) with each other such that nothing larger than 1000 daltons can pass from the outside to the inside of the tubule (Mruk and Cheng 2004; Cheng et al. 2010). At the beginning of meiosis, germ cells located outside of the barrier pass through the TJ. Once beyond the BTB, germ cells are dependent on SCs to supply nutrients and growth factors (Mruk and Cheng 2004). The BTB divides the seminiferous epithelium into the basal compartment (containing spermatogonia and spermatocytes) and adluminal (apical) compartment (containing different stages of meiotic spermatocytes, round spermatids, elongated spermatids, and spermatozoa) (Mruk and Cheng 2004; Su et al. 2011). In rats, the BTB forms at the age of 15–18 day’s post-partum (Mruk and Cheng, 2008). This barrier consists in three components: an anatomical/physical barrier to restrict entry of molecules into the adluminal compartment, an immunological barrier, that limits the movement of immune cells and regulates the level of cytokines in the seminiferous epithelium and a physiological barrier, that are highly dynamic to encounter the needs of germ cells (Sikka and Wang 2008; Mital et al. 2011). Together, these components are essential to the function of BTB in testes and the special microenvironment created is responsible for proper development of the spermatogenesis.
9
Sertoli cells provide factors required to fuel germ cell metabolism, including lactate, which is a key element for germ cells due to its anti-apoptotic effect and its role as energy source (Erkkila et al. 2002; Michael K. Skinner 2005). In SCs, glucose is efficiently metabolized via cytosolic glysolysis to pyruvate, which is likely channeled via gap junctions to germ cells to maintain their survival, and lactate, the preferred energy source of germ cells (Robinson and Fritz 1981; Grootegoed et al. 1986). It is well known that spermatogonia may utilise glucose as the major energy substrate (Nakamura et al. 1984), but spermatocytes and spermatids suffer a rapid decline in their ATP content in glucose-supplemented media, thus, they require lactate/pyruvate for the maintenance of their ATP concentrations (Jutte et al. 1981; Mita and Hall 1982). In contrast, spermatozoa use glucose/fructose as the major source of energy (Mann 1964). So, it appears that, at each stage of spermatogenesis, there is a change in the substrate required for energy (Bajpai et al. 1998).
The metabolism of SCs is under the complex control of hormones, growth factors and even paracrine, autocrine and juxtracrine reactions occurring between the various cell types in the testis (Guma et al. 1997). The FSH and insulin regulate glucose metabolism in SCs, thus stimulating lactate production (Jutte et al. 1982; Jutte et al. 1983; Oonk et al. 1985; Oliveira
et al. 2012). These stimulatory effects of FSH and insulin do not require protein synthesis,
suggesting a modulation of enzyme activity and/or regulation of glucose transport (Oonk et
al. 1985; Oonk and Grootegoed 1987). Insulin dysfunction (deficiency or resistance) has
attracted much attention since it spreads the outcome results to diabetes mellitus (DM), hypertension, dyslipidaemia, among others (Reaven 1988). The SCs can adapt to conditions of glucose deprivation to ensure the adequate lactate concentration in the microenvironment where germ cell develop and even when glucose levels are low or in the complete absence of glucose, the SCs still produce lactate (Riera et al. 2009; Oliveira et al. 2012). The regulation of glucose metabolism in SCs is crucial for normal spermatogenesis and fertility (Boussouar and Benahmed 2004), but it is not completely understood (Rato et al. 2012).
1.3 Mammalian Spermatozoon
A mature sperm cell, or spermatozoon, comprises a head and a tail region (flagellum) (Figure 4) (Vander et al. 2001). The head contains the nucleus, acrosome, cytoskeletal structures and a small amount of cytoplasm. The nucleus fills most of the head and contains a haploid set of condensed, genetically inactive chromosomes (Saladin 2003). It is covered by the acrosomal cap, a membrane-enclosed cytoplasmic vesicle containing hydrolytic enzymes (Vander et al. 2001). The flagellum is divided successively into the midpiece (body), principal piece and end piece regions (Saladin 2003). It contains a central complex of microtubules forming the axoneme, which is responsible for spermatozoon motility. It is essentially a long,
10
specialized cilium surrounded in turn by outer dense fibers extending from the neck into the principal piece. The midpiece contains the mitochondrial sheath, a tightly wrapped helix of mitochondria surrounding the outer dense fibers and axoneme (Rabbani et al. 2010). The end piece consists of the axoneme only and is the narrowest part of the sperm (Saladin 2003).
Figure 4 - General features of the mammalian spermatozoon. The mammalian spermatozoon has two parts: a pear-shaped head and a long tail (or flagellum). In the head, the acrosome is a lysosome in the form of a thin cap covering the apical half of the nucleus. The basal body of the tail flagellum is nestled in an indentation at the basal end of the nucleus. The regions of the flagellum are the middle piece which contains the mitochondrial sheath, the principal piece (major part of the tail) that consists of the axoneme surrounded by a sheath of fibers, and the end piece, which consists of the axoneme only and is the narrowest part of the spermatozoon. Adapted from Saladin (2003).
There are substantial species-specific differences in the size and shape of the head, in the length and relative amount of the different components of the flagellum (Rabbani et al. 2010). The rat sperm head is approximately 2.5 µm long and resembles a hook (Figure 5). It contains a dense nucleus and has a less dense tip referred to as the acrosome. The midpiece contains the centrioles and a spirally coiled sheath of mitochondrial material, while the tail contains a long axial filament that becomes vibratile for a brief period when the spermatozoon is mature (IRDG 2000).
11
The specialized structural features of mammalian spermatozoa reflect the unique functions of this cell type. The acrosome contains enzymes essential for penetrating the investments of the egg to achieve fertilization, while the flagellum contains the energy sources and machinery to generate the motility necessary for the sperm to reach the egg (Rabbani et al. 2010).
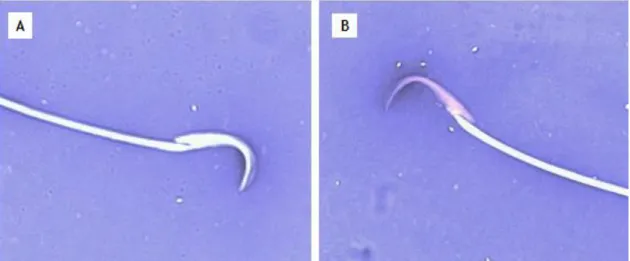
Figure 5- Lateral view of the falciform-shaped head of rat spermatozoa. A - Light microscopy image of a typical rat spermatozoon with Eosin/Negrosin staining (100× magnification). B – Schematic representation of the rat spermatozoa tail and head structures: acrosome and nucleous. Figure B adapted from Robaire et al. (2010).
12
2. The Epididymis
The epididymis is derived from the Wolffian duct and at birth consists mainly of mesenchymal tissue. The epididymis undergoes considerable remodeling including duct elongation and convolution, so that by puberty it has acquired its fully differentiated state (Carmen M. Rodriguez 2002). It consists of one convoluted and long tubule which, in humans and if uncoiled, measures about 5.5 m (Kent 2001). It links EDs to the vas deferens and is lined by a continuous layer of epithelial cells joined by TJ (Joseph et al. 2011). In humans, it can be morphologically divided into three principal regions (Figure 6): the caput and corpus regions, involved in the early and late sperm maturational events, respectively, and the distal cauda epididymis, which primarily serves as a storage site for functionally mature spermatozoa (Thimon et al. 2007; Dube et al. 2008; Cornwall 2009). The most proximal epididymal region, in some species such as the mouse, is also known as the initial segment (Figure 6) (Cornwall 2009). In the human, the initial segment is not as extensive, but a very tall epithelium is present (Yeung et al. 1991).
Figure 6 - Schematic and histological representation of the male reproductive tract and excurrent ducts. Schematic (left) showing relative orientation of the efferent ductules (EDs) and the proximal (IS and caput) and distal segments (corpus and cauda) of the epididymis. The cauda connects to the ejaculatory duct or vas deferens. Sagittal section (right) of the EDs and epididymis depicting its convoluted nature as well as its complex and changing epithelium. eff duct - efferent ductules; IS and init segment - initial segment; Bar = 1.5 m. Adapted from Joseph et al. (2011).
13
Histologically, the epididymis is a tube of smooth muscle lined by a pseudostratified epithelium. From proximal to distal, the muscular wall increases from a single circular layer to three layers. Proximally, the smooth muscle exhibits slow rhythmic contractility which gently moves spermatozoa towards the vas deferens, while distally, it is richly innervated by the sympathetic nervous system which produces intense contractions of the lower part of the epididymis during ejaculation (Kent 2001). The interstitial component that surrounds the duct contains blood vessels, connective tissue septa, lymphatic vessels, macrophages, and lymphocytes (Mital et al. 2011).
Each of the epididymal regions has a complex and different epithelium and can be further divided into intraregional segments or lobules of coiled tubule that are separated by septae (Turner et al. 2007; Joseph et al. 2011). Recently, microdissection of the mouse, rat and human epididymides has demonstrated that they are divided into 10, 19 and 7 intraregional segments (Figure 7), respectively and it has been shown that the connective tissue septae that separate the segments can establish borders for epididymal gene expression, protein presence and epithelial responses to lumicrine factors (Turner et al. 2007; Shum et al. 2011).
Figure 7 - Segmental structure of the epididymis. Typical schematic patterns of mouse (A) rat (B) and human epididymal segmentation (C). The mouse and rat epididymis are not drawn to scale. The average rat epididymis is approximately 2.5-times the length and has 40-times the total mass of the mouse epididymis (data not shown). Figure A and B adapted from Jelinsky et al. (2007), Figure C adapted from Dacheux et al. (2006).
14
Several different cell types can be identified in the epididymal epithelium (Figure 8): principal, narrow, apical, clear, basal cells and halo cells, surrounded by multiple layers of peritubular myoid cells (Joseph et al. 2011; Mital et al. 2011; Shum et al. 2011).Although the exact regional localization of the cell types differs between species (in part due to the inconsistency of identification of the different regions) in general, all the cell types appear to be present in all mammalian species (Rabbani et al. 2010). Each cell type contributes to the establishment and regulation of a unique luminal environment for the concentration, maturation, storage and viability of spermatozoa (Cornwall 2009; Shum et al. 2011).
The primary cell type throughout the tubule is the principal cell which constitutes approximately 80% of the epithelium and is responsible for the bulk of the proteins that are secreted to, and also endocyted from, the epididymal lumen (Cornwall 2009). The morphology of the principal cell reveals a prominent, branched, microvillus, absorptive border, but the cell changes dramatically from a tall columnar structure in the initial segment to low cuboidal cells in the cauda epididymis (Abe et al. 1983; Abou-Haila and Fain-Maurel 1984).
Figure 8 - Schematic view of the main epithelial cell types in the epididymis: narrow, clear, principal and basal cells. While principal and basal are present in all epididymal regions, narrow cells are located exclusively in the initial segment, and clear cells are present in the caput, corpus and cauda epididymis. Adapted from Shum et al. (2011).
15
Narrow cells are exclusively present in the initial segment (Shum et al. 2011). Their cytoplasm tapers between principal cells as it touches the basement membrane, but its apical cytoplasm may bulge slightly into the lumen with numerous vacuoles, endocytic vesicles, lysosomes and mitochondria, whereas apical cells have no such contacts (Hermo et al. 2000). Narrow cells are involved in endocytosis and secretion of protons (H+) into the lumen (Hermo
et al. 2000), while apical cells are also active in endocytosis and additionally contain many
proteolytic enzymes (Adamali and Hermo 1996).
Clear cells are large endocytic cells interspersed between principal cells and are found in the caput, corpus and cauda epididymis. They are characterized by their lack of microvilli, as well as an apical region with numerous coated pits, vesicles, endosomes, multivesicular bodies, lysosomes, as well as lipid droplets (Abou-Haila and Fain-Maurel 1984; Hermo et al. 1988; Rabbani et al. 2010). These are thought to be the epididymal cells with the most endocytic activity and they are particularly active in the cauda epidydimis (Sun and Flickinger 1979; Hermo et al. 1988). These cells are also responsible for the uptake of a number of different proteins excreted by the epididymal epithelium in a region-specific manner and also participate in the regulation of luminal fluid acidification (Sun and Flickinger 1979; Flickinger
et al. 1988; Hermo et al. 1988; Vierula et al. 1995).
Basal cells, as their name suggests, are situated on the basal side of the epithelium (Veri et al. 1993). They are present in all epididymal segments (L. Hermo 2002), and constitute 15%–20% of the total epithelium (B. Robaire 1988; Marengo and Amann 1990; Adamali and Hermo 1996). Basal cells do not access the luminal compartment and are in close association with the overlying principal cells, as indicated by the presence of basal cell cytoplasmic extensions between principal cells, and thus may regulate its functions (Veri et
al. 1993; Seiler et al. 1999). Their morphology is typical to the structure of endocytotic cells
and it is believed that they can endocytose factors derived from blood or principal cells and may help those in the regulation of electrolyte and water transportation (Veri et al. 1993; Hermo and Papp 1996). Some authors consider another type of epididymal cells, known as apical cells (Hermo et al. 2000; Cornwall 2009). They are "apical-reaching" basal cells (Shum
et al. 2011) that endocytose luminal components (Cornwall 2009). While a few of them are
present in the initial segment and caput, their numbers increase progressively in the corpus and reach a maximum in the distal corpus and proximal cauda regions (Shum et al. 2011). Halo cells appear to be the primary immune cell in the epididymis (Cheung et al. 2005). They are easily recognized in several histological stainings by their narrow rim of clear cytoplasm, and are present in all epididymal segments (Rabbani et al. 2010). These cells are usually located at the base of the epithelium and contain variable numbers of dense core granules. In young adult animals, halo cells consist of helper T lymphocytes, cytotoxic T lymphocytes, and monocytes (Flickinger et al. 1997; Serre and Robaire 1998; Serre and Robaire 1999).
16
2.1 Functions of the Mammalian Epididymis
The mammalian epididymis has two main functions. First, it creates a unique microenvironment within the lumen of the duct that helps transform immotile, immature testicular spermatozoa into fully fertile competent cells, and second, it stores fertile spermatozoa in a viable state within the cauda epididymis/vas deferens regions until they are ejaculated (Amann et al. 1993; Kirchhoff et al. 1998; Jones 2004).
From the rete testis to the end of the epididymis, spermatozoa are bathed in a continuously and progressively changing medium of fluid proteins and other chemical components (Guyonnet et al. 2011). Many luminal proteins may not play direct roles in sperm maturation but rather help to create the appropriate environment that is conducive for this process to occur (Cornwall et al. 2011). This can include proteins involved in the regulation of luminal pH, osmolality, regulation of oxidative stress (OS) and regulation of protein folding/misfolding. There are also mechanisms to remove secreted proteins from the lumen, presumably once their functions have been carried out or if misfolding occurs (JL Dacheux 2002). The formation of this luminal environment is the result of net secretory and absorptive processes of the epithelium, which continually changes along the duct (Hermo and Jacks 2002; Sullivan et al. 2007; Rabbani et al. 2010). It is a complex mixture of water, inorganic ions, small organic molecules and proteins, which accompanies the spermatozoa as they travel from the testis to the epididymis through the EDs, and is mostly composed of secretions by the SCs (Rato et al. 2010).
2.1.1 Epididymal Sperm Maturation
Epididymal sperm maturation, which is essential for the acquisition of progressive motility and the ability to fertilize (Rabbani et al. 2010), depends on the unique luminal environment created by the secretion and absorption of proteins and ions by the epithelium and the selective transport of molecules across the blood-epididymis barrier (BEB) (Hinton and Palladino 1995; Dacheux et al. 2003; Dacheux et al. 2006). This barrier comprises apical TJ between principal cells that create a seal between the cells and forces the selective transport of molecules across the epithelium (Dube et al. 2010).
The maturation process is androgen-dependent and conducts several biochemical and functional changes in spermatozoa. Epididymal originated proteins may change the membrane properties of the spermatozoa in several ways: they may bind to the sperm surface and/or
17
modify the structure or the arrangement of the existing membrane molecules (Vreeburg et al. 1992). Some of these proteins contribute to the stabilization of the sperm plasma membrane which prevents the occurrence of premature capacitation and are, therefore, known as “decapacitation factors”, whereas others have been implicated in the acquisition of the sperm ability to bind and recognize the egg (Lefebvre et al. 2009; Cohen et al. 2011; Joseph
et al. 2011).
2.1.2 Epididymal Sperm storage
Species presenting internal fertilization concentrate and store their maturing spermatozoa in the epididymis prior to ejaculation. This makes the spermatozoa amount in the ejaculate more controllable and less dependent on testicular production, once it can be maintained normally while spermatozoa are transported to the epididymis (Jones 1999). The normal transit time in mammals through the cauda epididymis is in the range of 3 to 10 days, but spermatozoa can be stored in this tissue for periods extending beyond 30 days (Rabbani et
al. 2010). The survival of spermatozoa in the cauda epididymis depends on the species and
incubation temperature (Jones 2004). In scrotal mammals, the combination of a unique luminal milieu and lower temperatures (30–32ºC) are thought to be major contributors to sperm survival. However, if spermatozoa are removed from the cauda and incubated at 32ºC
in vitro, their fertility and viability is measured in hours rather than days (Jones 2004).
Spermatozoa need to be protected from damage caused by OS and microorganisms, and their motility needs to be suppressed until it is required to conserve energy and maintain structural integrity (Jones 1999). The BEB isolates the contents of the epididymal lumen from the rest of the body, which is important as certain proteins in spermatozoa are recognized as foreign objects by the immune system (Itoh et al. 2005). However, since immune cells are isolated from the epididymal lumen, it is required an alternative method to protect the spermatozoa against harmful microorganisms. With the high incidence of sexually transmitted infections and effect on fertility in humans, increased interest towards such factors has resulted (Hall et al. 2002). It has been described that genes expressed in the epididymis encoding cysteine-rich secreted proteins have antimicrobial properties and are involved in sperm maturation and defense against microorganisms (Hall et al. 2007; Diao et al. 2011; Zhao et al. 2011).
One of the most important factors contributing to poor quality semen has been reported to be OS (Tuncer et al. 2010). It is a condition associated with an increased rate of cellular damage induced by oxygen and oxygen derived oxidants commonly known as reactive oxygen species (ROS) (Sikka et al. 1995). Uncontrolled production of ROS that exceeds the
18
antioxidant capacity of the seminal plasma leads to OS which is harmful to spermatozoa (Desai et al. 2010). All cellular components including lipids, proteins, nucleic acids, and sugars are potential targets of OS (Agarwal et al. 2008).
The production of ROS by spermatozoa correlates with lipid peroxidation, DNA oxidation, poor sperm function and reduced fertility (Moustafa et al. 2004; Aitken and Koppers 2011). However, recent evidence suggests that redox activity is physiologically important in promoting normal sperm function (de Lamirande and Gagnon 1992; Aitken 1999; Aitken 2000). Small amounts of ROS are required for sperm capacitation, the final maturation steps associated with hyperactive motility and a physiological acrosome reaction (Griveau and Le Lannou 1997; Agarwal et al. 2004). Thus, it is essential to the proper functionality of the spermatozoa to maintain a delicate balance in ROS production and recycling. It has been estimated that OS is a contributor in 30–80% of cases of male infertility thereby making it an important area of research (Tremellen 2008).
2.2 Epididymal sperm abnormalities
Sperm abnormalities have long been associated with male infertility and sterility in most species studied. These abnormalities vary from morphological defects that are evident upon clinical examination, to those, which are more subtly defective (Chenoweth 2005). Morphology can be defined as the structure and form of organisms to include the anatomy, histology and cytology at any stage of its life history. It is important to compare this to morphometry, or the measurement of the forms of organisms. The latter, while relating to some aspects of viability is less relevant to the function of an organism as an entity (IRDG 2000). Abnormal sperm morphology is a reflection of negative stress factors working on the body without affecting the overall health of a specific male (Menkveld et al. 2011). Most of the published data are on sperm concentration, being the semen characteristic most commonly assessed and the one least subject to methodological bias. In contrast, there are fewer studies reporting data on sperm motility, viability or morphology. The assessment of these characteristics is more subjective by nature with an overall noticeable inter-technician and inter-laboratory variability (Neuwinger et al. 1990; Cooper et al. 1992; Matson 1995; Ombelet et al. 1998; Auger et al. 2000). Otherwise, the preparation, fixation and staining methods of the semen smears, have a severe influence on viability and sperm morphology evaluation results. In 1971, Eliasson made an important contribution to the classification system, which has been commonly used. He stated that for the complete morphological rating of human spermatozoa, sperm abnormalities should be classified in three main groups: those of the sperm head, the mid-piece and the tail(Eliasson 1971).
19
3. Diabetes mellitus and male infertility
Diabetes mellitus represents one of the greatest threats to modern global health and its incidence is rising rapidly. In the year 2000, the World Health Organization (WHO) reported that 177 million people were affected by diabetes worldwide and that by 2025 this figure is estimated to rise to an incidence of over 300 million individuals (Agbaje et al. 2007; Bener et
al. 2009).
Diabetes mellitus describes a metabolic disorder characterized by hyperglycaemia (poor glucose in the blood) resulting from defective insulin secretion, resistance to insulin action, or both (Alberti 1998). Type 1 or insulin-dependent diabetes mellitus (T1DM), which generally develops at young age, is a multi-factorial immune-mediated disease characterized by the autoimmune destruction of insulin-producing pancreatic islet beta cells in genetically susceptible individuals, resulting in a dependency of exogenous insulin treatment (Burul-Bozkurt et al. 2010; Grieco et al. 2012). Type 2 or non-insulin-dependent diabetes mellitus (T2DM) is responsible for 90–95% of diabetes cases. In T2DM, liver, muscle and fat cells are resistant to insulin action. The compensatory attempt by the beta-cell to release more insulin is not sufficient to maintain blood glucose levels within a normal physiological range, finally leading to the functional exhaustion of the surviving beta-cells. Despite genetic predisposition, the risk of developing T2DM in humans increases with age, obesity, cardiovascular diseases and a lack of physical activity (Golay and Ybarra 2005; Carneiro et al. 2010).
Prospective studies of the natural history of T2DM have shown that the prediabetic state is characterized by impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), or both. The prediabetic state, which is associated with insulin resistance (IR) and that usually precedes T2DM, is also characterized by resistance to an insulin-mediated glucose disposal and a compensatory slight hyperinsulinemia. The transition from prediabetes to T2DM occurs when the secretory capacity of the pancreatic B cell is no longer able to compensate for the IR. However, frank hyperglycemia in patients with T2DM is not associated with absolute hypoinsulinemia and day-long circulating insulin concentrations in patients with T2DM are comparable in absolute terms to the values in non-diabetic patients (Reed et al. 2000). Pre-diabetes commonly associates with the metabolic syndrome, which in turn is closely associated with obesity (Ai et al. 2005). The progression from prediabetes to T2DM occurs over many years, strong evidence to support intervention to delay the progression from prediabetes to diabetes (Aroda and Ratner 2008).
20
Infertility according to the WHO is defined as the inability to conceive after 1 year of unprotected intercourse (Zegers-Hochschild et al. 2009) and affects about 13%–18% of couples (Dube et al. 2008). In about half of them, male factor is the sole cause or contributes to the infertility problem (Shukla et al. 2012). Male infertility can occur either as an isolated disease or as part of a complex syndrome. The main causes of infertility in men are diverse such as genetic, physiopathologic and anatomopathologic abnormalities, intense and prolonged physical exercise, aging, drugs and even excessive time of sexual abstinence (World Health Organization 2010). But in more than half of infertile men, the cause of their infertility is unknown (de Kretser 1997; Seshagiri 2001; Dohle et al. 2005). A large number of the male infertility cases are also associated to suboptimal sperm quality due to abnormal parameters - motility, morphology, concentration and DNA fragmentation (Iammarrone et al. 2003; du Plessis et al. 2011) and, thus, semen analysis represents the initial test for evaluating male-factor infertility.
Male fertility is compromised by the hormonal and metabolic changes associated with T1DM and T2DM (Bener et al. 2009; Mallidis et al. 2009), obesity (Pauli et al. 2008) and the metabolic syndrome, the latter sharing essential pathological features with the DM pathologies (Kasturi et al. 2008). The deleterious influence of DM and obesity on fertility is receiving increasing attention since their prevalence and incidence is escalating worldwide, while the age of first diagnosis for both diseases is in continuous decline (Lavizzo-Mourey 2007; Harjutsalo et al. 2008). Due to this situation, the fertility of a growing number of individuals is affected prior to and during their reproductive years (Delfino et al. 2007; Nguyen et al. 2007).
The prevalence of sexual dysfunction in diabetic male individuals has been reported to approach 50% of the cases (Kandeel et al. 2001). In particular, decreased sperm concentration and motility, abnormal sperm morphology and increased seminal plasma abnormalities were detected (Amaral et al. 2008). Furthermore, DM is associated with increased OS which damages sperm nuclear and mitochondrial DNA (Kort et al. 2006; Kasturi
et al. 2008), retrograde ejaculation, premature ejaculation, decreased libido, delayed sexual
maturation and impotence (Kandeel et al. 2001; La Vignera et al. 2011). Finally, spermatogenesis disruption and germ cell apoptosis in T1DM may relate to a local autoimmune damage, whereas IR, obesity and other related co-morbidities may impair sperm parameters and decrease testosterone serum levels in patients with T2DM (La Vignera et al. 2011). Low testosterone levels have also been found to predict IR and the future development of T2DM from a prediabetic state (Kapoor et al. 2007).