Carvacryl acetate, a novel semisynthetic monoterpene
ester, binds to the TRPA1 receptor and is effective in
attenuating irinotecan-induced intestinal mucositis in mice
Elenice M. Alvarengaa, Nayara A. Sousaa, Simone de Araujo a, Jose L. P. J unior a, Alyne R. Araujo b, Bruno Ilesa, Dvison M. Pacıficoc, Gerly Anne C. Britoc, Emmanuel P. Souzac, Damiao P. Sousa~ d and Jand Venes R. Medeirosa
aLaboratory of Pharmacology of Inflammation and Gastrointestinal Disorders (Lafidg), Federal University of Piau
ı, Parnaıba, PI, Brazil,
bBiotechnology and Biodiversity Center Research, BIOTEC, Federal University of Piauı, Parnaıba, PI, Brazil,cDepartment of Morphology, Faculty of
Medicine, Postgraduate Program in Morphofunctional Sciences, Federal University Ceara, Fortaleza, CE, Brazil anddDepartment of Pharmaceutical
Sciences, Federal University of Paraıba, Jo~ao Pessoa, Paraıba, Brazil
Keywords
carvacryl acetate; CPT-11; cytokines; molecular docking; oxidative stress
Correspondence
Jand Venes R. Medeiros, BIOTEC/LAFFEX/
UFPI, Av. S~ao Sebasti~ao, no 2819, CEP
64202-020, Parnaıba, PI, Brazil.
E-mail: jandvenes@ufpi.edu.br
Received July 5, 2017 Accepted August 26, 2017
doi: 10.1111/jphp.12818
Abstract
Objectives We aimed to determine whether carvacryl acetate acts as a TRPA1 receptor agonist and its effects against irinotecan (CPT-11) induced intestinal mucositis in mice.
Methods TRPA1 structure was obtained from a protein databank, and the 3D structure of carvacryl acetate was determined. Appropriate binding conforma-tions were discovered via automatic docking simulaconforma-tions. To determine the effect of carvacryl acetatein vivo, mice were treated with either DMSO 2%, CPT-11, carvacryl acetate followed by CPT-11, or HC-030031, a TRPA1 antagonist, fol-lowed by carvacryl acetate. Jejunum samples were taken and structural, inflam-matory and antioxidant parameters were studied.
Key findings Eight amino acids residues in TRPA1 established stable interactions with carvacryl acetate, which led to pharmacological efficacy against CPT-11-induced intestinal mucositis via reduction of both neutropenia and bacteremia, increase in villi height and crypt depth, decrease in pro-inflammatory cytokines (interleukin-1b, keratinocyte chemoattractant and tumour necrosis factor-a) and decrease in malondialdehyde and nitric oxide metabolite levels in the jejunum.
Conclusions Carvacryl acetate is a promising anti-inflammatory and antioxidant agent, a fact confirmed through observations of its interactions with TRPA1 in CPT-11-induced intestinal mucositis in mice.
Introduction
Carvacryl acetate (5-isopropyl-2-methylphenyl acetate, C12H16O2), a semisynthetic monoterpene ester, has shown
anti-inflammatory and antinociceptive effects in experi-mental models of peritonitis and paw oedema.[1]The main properties of carvacryl acetate include: molecular weight–
192.26; refractive index–1.497; boiling point–94.56°C at 760 mmHg; enthalpy of vapourization – 48.414 kJ/mol; density–0.994 g/cm3.[2]Due to the relatively high toxicity of its precursor carvacrol compared to that of other phenols and esters, carvacryl acetate was thought to be a less toxic semisynthetic derivative of carvacrol with the same or improved pharmacological properties. The difference
between carvacrol and carvacryl acetate is the replacement of the hydroxyl group by an ester group in the latter, which is attributable to carvacryl’s lower toxicity and higher sta-bility. Carvacryl acetate inhibits inflammatory mediators and neutrophil migration.[1] However, information about the effect of carvacryl acetate on specific inflammatory dis-eases is scarce, particularly on disorders of the gastrointesti-nal tract.
Intestinal mucositis, an inflammatory process that results in morphological and physiological changes in the small intestine mucosa, is generally caused by the use of antineo-plastic drugs, such as irinotecan hydrochloride (CPT-11). CPT-11 is a semisynthetic analogue of camptothecin and a selective inhibitor of the enzyme topoisomerase I.[5,6]It is one of the main neoplastic drugs in use today. As intestinal mucositis is severe, studies on how to minimize its negative effects, or that clarify how intestinal mucositis develops are essential, because they can help discover potential thera-peutic targets. Due to its wide distribution in the intestinal mucosa, TRPA1 is a molecule of interest in studies that attempt to uncover the mechanisms triggering mucositis.
The aim of this study was to investigate whether car-vacryl acetate is a TRPA1 receptor agonist. We also aimed to verify whether this interaction between carvacryl acetate and TRPA1 could reduce inflammation and increase muco-sal protection in the gut of mice with CPT-11-induced intestinal mucositis.
Materials and Methods
Molecular docking
Structures of the agonist carvacryl acetate and TRPA1 (PDB ID: 3J9P), determined using cryo-electronic micro-scopy at a resolution of 4.24A, were obtained from the Research Collaboratory for Structural Bioinformatics pro-tein databank. A docking simulation was used to study the binding of TRPA1 and carvacryl acetate. As previ-ously mentioned,[7]the area of the binding site was con-sidered the most desirable region for fitting the agonist. The binding ability of carvacryl acetate and its corre-sponding binding affinity scores were used to determine better molecular interactions. The results were visualized and analysed using ADT, Maestro, PyMOL and Discovery Studio.
Preparation of carvacryl acetate
Carvacryl acetate (98% purity; Figure 1a) was obtained via acetylation of carvacrol, using acetic anhydride for the acetylation and pyridine as a catalyst. Carvacryl acetate was obtained (4.779 g; 0.025 mol) at a 76% yield.[8,9] The structural identification of carvacryl acetate was performed by spectroscopic techniques (1H and 13C NMR, IR) and by comparison with the literature data.[1]
Animals
Female Swiss mice (25–30 g) were randomly maintained in appropriate cages at 252°C under a 12-h light/dark cycle with food and water provided ad libitum. All
experimental procedures were performed in accordance with the Guide for Care and Use of Laboratory Animals (National Institute of Health, Bethesda, MD, USA) and were approved by the local ethics committee (protocol no. 080/15). The animals were divided into experimental groups of 5–7 animals each.
Effect of carvacryl acetate on intestinal mucositis: experimental protocol
The experimental protocol to induce intestinal mucositis was based on a previously described protocol with modifi-cations.[10,11]In this study, which lasted 8 days, the mice in the experimental groups received only carvacryl acetate (25, 75, or 150 mg/kg, i.p.) on the first day.[1,12] Between the second and fifth days the mice received CPT-11 (75 mg/kg, i.p.), followed by carvacryl acetate 30 min later. From days 6–8, the mice received either carvacryl acetate or DMSO (2%, i.p.). To verify the putative involvement of TRPA1 receptors in the carvacryl acetate effect on mucositis, a dif-ferent group of mice was administered HC-030031 (0.025 mg/kg, i.p.) (4), a TRPA1 antagonist, 30 min before carvacryl acetate from day 2 onwards. Negative and positive controls received only DMSO (2%, i.p.) or only CPT-11 (75 mg/kg, i.p.), respectively.
In the experimental period, the mass and survival of the mice were measured daily. The mice were then killed with an overdose of ketamine/xylazine (>100/10 mg/kg, s.c.). Blood samples were obtained by cardiac puncture to per-form leucogram and bacterial counts, and segments of the jejunum (80–100 mg) were removed for further analysis described below.
Blood leucocyte and bacteria counts
Blood samples obtained by cardiac puncture were trans-ferred to test tubes, which were then heparinized and diluted with Turk’s solution (380ll of blood and 20 ll of diluent solution). The leucocytes were counted in a Neu-bauer Chamber with a light microscope.
To count the bacteria, blood samples obtained by cardiac puncture were quantified according to a previously devel-oped methodology[13]with modifications.
Histological and morphometric evaluation
Determination of Na+/K+-ATPase activity
Na+/K+-ATPase activity of enterocytes was measured in segments of mouse jejunum according to a previously described methodology with modifications.[16]Results are
expressed as a relation between the inorganic phosphate and protein concentration (lmolPi/mg protein/h).[17]
Effect of carvacryl acetate on inflammatory and oxidative stress markers in intestinal mucositis
Myeloperoxidase activity was assessed according to.[18] Cyto-kine levels (TNF-a, IL-1band KC) were measured using an enzyme-linked immunosorbent assay (ELISA) according to[15] and the manufacturer’s instructions (DuoSet ELISA Development kit R&D Systems, Minneapolis, MN, USA).
Glutathione (GSH) and superoxide dismutase (SOD) levels were determined according to the method described by[19] and,[20] respectively. The malondialdehyde (MDA) was used as an indicator of lipid peroxidation. The MDA concentration for jejunum tissue was measured using the method described previously by.[21]
The Griess reaction[22]was used to quantification of the nitric oxide (NO) metabolites, nitrate (NO3 ) and nitrite
(NO2 ) (collectively NOx), in mouse small intestine
according to.[22]
Immunohistochemical assay for NF-jB and
COX-2
Segments of mouse jejunum were incubated with goat anti-COX-2 (1:200, SC-1747; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit anti-NF-kB p50 NLS antibodies
Figure 1 Chemical structure of carvacryl acetate (5-isopropyl-2-methylphenyl acetate, C12H16O2). Models of molecular docking of carvacryl acetate
(1:200, SC-114; Santa Cruz Biotechnology). The quantita-tive estimation of DAB products from immunostaining was performed using digital images of at least ten different areas of each section.[23]
Statistical analysis
Results are expressed as meanSEM of 5–8 animals per group, and statistical analysis was performed using one-way analysis of variance (ANOVA) followed by the New-man–Keuls post hoc test, when appropriate. Statistical threshold was set atP<0.05.
Results
Molecular docking
The chemical structure of carvacryl acetate is shown in Fig-ure 1a. The protein–ligand complexes of carvacryl acetate were inserted at the TRPA1 cavity through Autodock 4.2 software, as shown in Figure 1c. Interactions between car-vacryl acetate and the TRPA1 receptor occurred at eight amino acids residues at the active site of TRPA1, (ILE803, LEU807, LEU848, LEU863, GLU864, LEU867, LYS868 and PHE947) (Figure 1b). A hydrogen bond formed from the interaction between the acetylate ring and LYS868. GLU864 interacted with carvacryl acetate through a pi-anionic inter-action. The other amino acid residues in TRPA1 interacted with carvacryl acetate via alkyl interactions. The binding energy that resulted in the carvacryl acetate-TRPA1 interac-tion was negative ( 6.1 kcal/mol), in a single conforma-tion with an inhibiconforma-tion constant of 36.8lM. These results
show that carvacryl acetate binds tightly to the active site of TRPA1. All molecular docking models of interactions between carvacryl acetate and the TRPA1 receptor are illus-trated in Figures 1b–d.
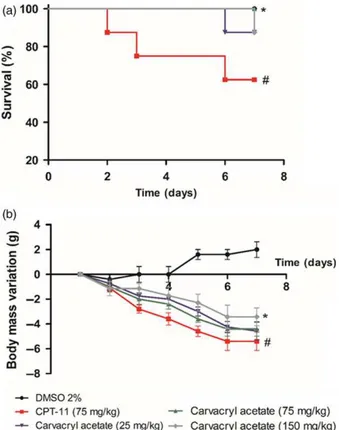
Effect of carvacryl acetate on survival and body mass variation
The mouse survival rate and changes in body mass were observed throughout the intestinal mucositis experiment (Figure 2). Mice treated with CPT-11 (75 mg/kg, i.p.) had the lowest survival rate (62.5%) compared to that of mice treated with DMSO 2% (negative control, 100% survival) or those treated with carvacryl acetate at any concentration (25, 75, or 150 mg/kg, i.p., 87.5%, 100% and 87.5% sur-vival, respectively). The only dose at which there were no deaths was 75 mg/kg of carvacryl acetate (100% survival).
Body mass increased only in mice treated with DMSO 2% (20.9 g at 7 days), while those treated with CPT-11 lost body mass (5.42.1 g at 7 days). Mice treated with carvacryl acetate (25, 75 and 150 mg/kg, i.p.) lost less mass
compared to that observed for the CPT-11 group (4.6 1.7 g, 4.41.7 g and 3.41.3 g, respectively). Therefore, as carvacryl acetate (75 mg/kg) caused no deaths and caused a small change in body mass, this dose was cho-sen for all further experimental procedures. Moreover, this dose and its route of administration have been previously reported as effective in other mice models of inflammation by our research group (1).
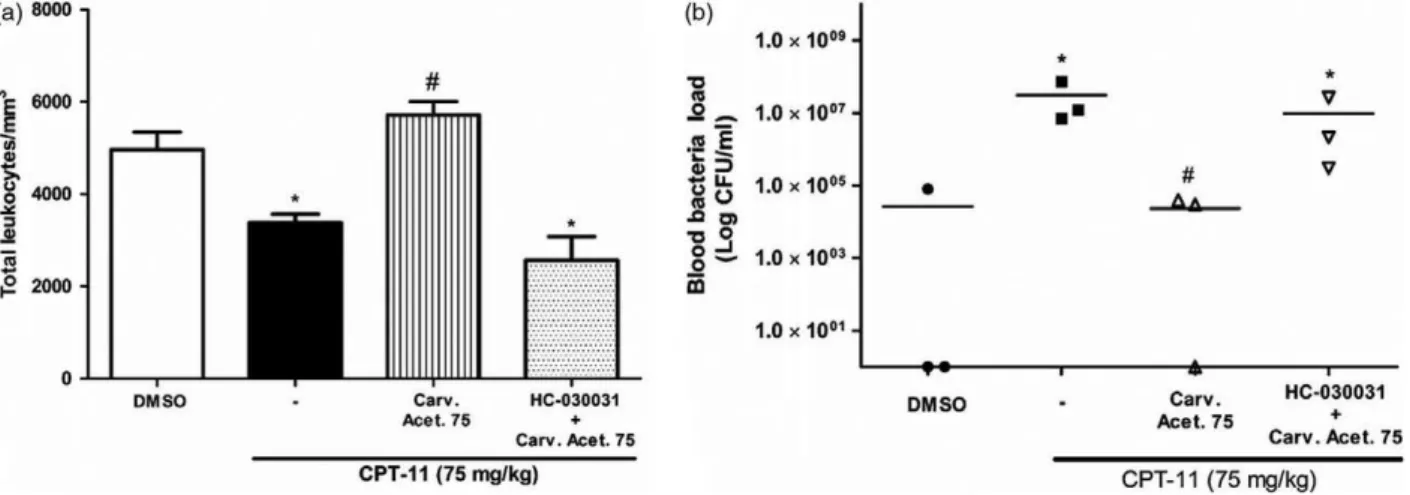
Effect of carvacryl acetate on leucogram and bacterial counts
After intestinal mucositis induction, leucograms were determined. CPT-11 treatment caused a decrease in total leucocyte number (3382 613.7 leucocytes/mm3) relative to that observed for DMSO 2% treatment (4967 1145 leucocytes/mm3). Conversely, the density of leucocytes in mice treated with carvacryl acetate was higher (5710660.9 leucocytes/mm3) than that in the CPT-11
Figure 2 Carvacryl acetate effect on survival and change in body mass in mice. Carvacryl acetate treatment (75 mg/kg) in mice increased the survival (a) and decreased body mass (b) of mice with induced mucositis compared to CPT-11 (75 mg/kg) treatment. Rows represent the
meanstandard error of the mean expressed as change in body mass
and were analysed using one-way analysis of variance (ANOVA) followed
by a Newman–Keuls test.*P<0.05 vs control group;#P
<0.05 vs CPT-11
and DMSO groups. Mice treated with HC-030031 (0.025 mg/kg) had the lowest density of leucocytes (2570 1142), as shown in Figure 3a.
Carvacryl acetate effectively reduced bacterial growth (Figure 3b) relative to that reported with CPT-11. The levels of bacterial growth in mice treated with carvacryl acetate were similar to those in mice treated with DMSO. Pretreatment with HC-030031 increased the bacterial count to the level observed after CPT-11 treatment.
Effect of carvacryl acetate on morphological and morphometric parameters
As determined by degrees of mucositis, mice treated with CPT-11 showed extensive loss of crypt architecture, includ-ing necrotic, shortened and flattened villi (Figure 4b). Mucositis of degree 3–4, necrotic cells and infiltration of inflammatory cells such as neutrophils into more than 20% of the lamina propria were observed. The injury scores in mice treated with carvacryl acetate (Figure 4c) were signifi-cantly lower (0–2) than those for the CPT-11 group, sug-gesting that carvacryl acetate significantly restored the histopathological alterations caused by intestinal mucositis. This result is corroborated by the observation of a reduc-tion in infiltrareduc-tion of inflammatory cells and the general alteration of architecture, largely restoring normal tissue structure of villi and crypts. Figure 4a shows that there was no injury to mouse jejunum in samples treated with DMSO 2% (0–1). Moreover, Figure 4d shows that pretreatment with HC-030031 nullified the beneficial action of carvacryl
acetate on intestinal mucosa, resulting in mucositis of degrees 2–4; this shows that carvacryl acetate likely acts through TRPA1 receptors.
Morphometric analysis also showed that treatment with carvacryl acetate restored villi length (185 37.2lm) and enlarged crypt depths (86.5 17.34lm), as shown in Fig-ure 5a,b. CPT-11 caused a significant shortening of the villi height (104.5 12.63lm) and a reduction in crypt depth (33 12lm), while DMSO left the intestinal architecture intact (villi height, 19913.4lm; crypt depth, 89.6 14.9lm). Pretreatment with HC-030031 reduced villi height (77.268lm) and crypt depth (40.79 5.9lm) to levels similar to those observed after CPT-11 treatment.
Na+/K+-ATPase activity
Treatment with CPT-11 reduced the activity of Na+/K+ -ATPase enzyme (1027 299 lmol/mg/h) compared to that reported with DMSO treatment (1894 514.6lmol/ mg/h). However, after treatment with carvacryl acetate, Na+/K+-ATPase activity returned to baseline values (2156884.3lmol/mg/h), as shown in Figure 6.
Effect of carvacryl acetate on inflammatory and oxidative stress parameters
Results related to the effects of carvacryl acetate (75 mg/kg) on MPO, GSH, MDA, NOx and cytokines (TNF-a, IL-1b
and KC) are shown in Table 1. Higher levels of MPO were
Figure 3 Carvacryl acetate effect on leucocyte levels and bacterial load in blood of mice. CPT-11 (75 mg/kg) treatment decreased the total num-ber of leucocytes in mice with induced intestinal mucositis, and treatment with carvacryl acetate (75 mg/kg) restored leucocyte levels to normal (a). Bacterial load, expressed as a logarithm of the number of bacterial colonies, was also measured in blood samples. In mice treated with CPT-11 (75 mg/kg), an increase in bacteremia was observed, which was reduced by treatment with carvacryl acetate (75 mg/kg) (b). In both cases,
pre-treatment with HC-030031 (0.025 mg/kg) prevented the action of carvacryl acetate. Bars and dots represent the meanstandard error of the
mean and were analysed using one-way analysis of variance (ANOVA) followed by a Newman–Keuls test.*P<0.05 vs control group;#P
(a) (c)
(b)
(e)
(d)
Figure 4 Photomicrographs from mouse jejunum mucosa stained with haematoxylin and eosin. (Panel a) DMSO 2% group (negative control) exhibits normal histological features, with intact tissue architecture (preservation of villi and crypts) and no evidence of mucositis. (Panel b) CPT-11 (75 mg/kg) group shows pronounced mucositis with shortened villi caused by vacuolated cells, loss of crypt architecture, and infiltration of inflam-matory cells into the lamina propria. (Panel c) Carvacryl acetate (75 mg/kg) partly restored the tissue architecture after induction of mucositis with some preservation of villi height and crypt depth. (Panel d) HC-030031 (0.025 mg/kg) pretreatment group shows prevention of the carvacryl acet-ate action, with villi shortening and loss of crypt architecture. Scale bars: 50 mm. (Panel e) Histological grading of mucositis for mouse jejunum treated with carvacryl acetate (75 mg/kg), which significantly reduced the grade of CPT-11-induced intestinal mucositis. Data represent median
values of scores and were analysed using a Kruskal–Wallis test.*P<0.05 vs control group;#P
<0.05 vs CPT-11 (75 mg/kg) group. [Color figure
observed in mice that received CPT-11 (4.082.1 U MPO/mg of tissue) than in mice treated with DMSO (0.951.4 U MPO/mg of tissue). Treatment with car-vacryl acetate decreased the levels of MPO in the jejunum (1.161.12 U MPO/mg of tissue) while pretreatment with HC-030031 induced a significant increase in MPO levels (4.011.6 U MPO/mg of tissue).
Treatment with CPT-11 significantly increased the levels of the cytokines IL-1b, KC and TNF-a(447.9 53.8 pg/ ml, 1024 147.9 pg/ml and 144.551 pg/ml, respec-tively) compared to that reported for the DMSO group (12.57 6.2 pg/ml, 8 9.98 pg/ml and 9.838.42 pg/ ml, for IL-1b, KC and TNF-a, respectively). Carvacryl acet-ate reduced the CPT-11-induced increase in cytokine levels (148.4 51.12 pg/ml, 497.9151.4 pg/ml and 46.2442.38 pg/ml for IL-1b, KC and TNF-a, respec-tively). Pretreatment with HC-030031 increased the cytoki-nes levels (42654.9 pg/ml, 810.9133.3 pg/ml, 139.140.91 pg/ml, for IL-1b, KC and TNF-a, respec-tively), close to those observed after CPT-11 treatment, clearly preventing carvacryl acetate action.
The presence of GSH, a cellular defence agent, is inferred by hepatic non-protein sulfhydryl (NPSH) levels. Thus, after intestinal mucositis induction with CPT-11, a reduc-tion of NPSH levels (165 90.91lg/ml) was seen com-pared to that with DMSO treatment (385.8 77.59lg/ ml). Treatment with carvacryl acetate increased NPSH levels (610.5 95.09lg/ml), and pretreatment with HC-030031 prevented this increase (216 92.96lg/ml).
The levels of SOD, another cellular defence agent, after intestinal mucositis induction with CPT-11 was reduced (2.72 0.49 U SOD/lg) if compared to that observed after DMSO treatment (3.92 0.18 U SOD/lg). Treat-ment with carvacryl acetate increased SOD levels (5.04 0.31 U SOD/lg), and pretreatment with HC-030031 prevented this increase (3.83 0.20 U SOD/lg).
Another commonly used marker of oxidative stress is MDA, a product of lipidic peroxidation. Mice treated with CPT-11 had higher MDA levels (280.1119.7 nmol/ml
Figure 5 Villi height (a) and crypt depth (b) of mouse jejunum mucosa showing villi shortening and decrease of crypt depth in mice subjected to induced intestinal mucositis. Carvacryl acetate (75 mg/kg) reverted the effects of CPT-11 (75 mg/kg) and pretreatment with HC-030031
(0.025 mg/kg) showed similar effects as CPT-11. Bars represent the meanstandard error of the mean and were analysed using one-way
analy-sis of variance (ANOVA) followed by a Newman–Keuls test.*P<0.05 vs control group;#P
<0.05 vs CPT-11 (75 mg/kg) group.
Figure 6 The Na+/K+-ATPase activity in the mouse jejunum was
measured by determining the release of inorganic phosphate from the hydrolysis of ATP. Pretreatment with carvacryl acetate (75 mg/kg)
increased the activity of Na+/K+-ATPase significantly as compared to
the CPT-11 group. Bars represent the meanstandard error of the
mean and were analysed using one-way analysis of variance (ANOVA)
followed by a Newman–Keuls test. *P<0.05 vs control group;
#P
than mice that received DMSO did (161.4 83.02 nmol/ ml). Treatment with carvacryl acetate reduced MDA levels (111.881.85 nmol/ml) to those observed in the DMSO group, which supports the protective action of carvacryl acetate. Mice pretreated with HC-030031 showed slightly elevated MDA levels (194.1 38.72).
NOx levels represent the amount of oxidative damage in inflamed tissues. Thus, mice that received CPT-11 had higher levels of NOx (0.47 0.16lM) than mice that
received DMSO treatment did (0.240.03lM). Carvacryl
acetate lowered NOx levels (0.240.15lM), to those
observed in the DMSO group, confirming its protective action on intestinal mucosa. Pretreatment with HC-030031 increased NOx levels (0.48 0.21lM), to those observed
in the CPT-11 group. This result confirms that carvacryl acetate exerts its protective action through interactions with the TRPA1 receptor.
Immunochemical detection of NF-jB and
COX-2
Jejunum sections submitted to immunochemical detection of NF-jB and COX-2 showed intensely stained areas in CPT-11-treated samples (33.8912.66 cells immunos-tained for NF-jB detection and 47.6 11.14 cells immunostained for COX-2 detection) compared to the results of the DMSO group (NF-jB, 7.263.63 cells immunostained; COX-2, 19.56.9 cells immunostained). Treatment with carvacryl acetate significantly reduced these immunostained areas (NF-jB, 23.5812.62 cells immunostained; COX-2, 12.143.6 cells immunos-tained); in the case of COX-2, the staining intensity decreased to levels close to those of the DMSO group. In the DMSO and carvacryl acetate groups, there were few immunostained cells in the epithelium. However, in the CPT-11 group, the epithelium and muscle layers showed intense immunostaining, as shown in Figures 7 and 8, respectively.
Discussion
Results showed that carvacryl acetate stably binds to TRPA1, both because of the number and the type of inter-actions between them. Carvacryl acetate interacted with TRPA1 and activates, acting as an agonist, through eight amino acids residues, which contributed to the high stabil-ity in this interaction. It bound to the TRPA1 receptor via hydrogen bonds and pi-anionic interactions, both of which are among the strongest kind of chemical interactions, which also contributed to the strength of the interaction. Hydrogen bonds are very strong due to the presence of high hydrogen electropositivity and high oxygen, nitrogen and fluorine electronegativity.[24]
Comparison of the molecular docking models shows that the interactions between carvacryl acetate and TRPA1 are more stable and lasting than those between carvacrol and TRPA1.[12]This is because carvacrol and TRPA1 interacted through only six amino acids residues using bonds weaker than hydrogen bonds.[12]Some molecules can show a large conformational variation, with even a few rotatable bonds creating many different conformations.[25]In this way, car-vacrol can bind to TRPA1 through seven different confor-mations.[12] Carvacryl acetate, however, only showed a single conformation in the TRPA1 receptor-binding site.
This stable and lasting interaction between carvacryl acetate and TRPA1 is also corroborated by observation of the inhibition constant, which is a parameter associated with the dissociation constant. Interactions between car-vacryl acetate and TRPA1 showed a smaller inhibition
constant than that reported for the carvacrol-TRPA1 inter-action.[12]
To demonstrate that the interaction between carvacryl acetate and TRPA1 could culminate in a relevant biological effect against intestinal mucositis, we observed some clini-cal parameters. Carvacryl acetate did not affect the mice’s survival and induced a loss in body mass.
Some of the main side effects of CPT-11 are neutropenia due to myelosuppression,[26]and the increase in bacteria in the blood due to tissue damage.[13,27]Results demonstrated that carvacryl acetate restored leucocyte levels in the blood and minimized bacteremia, which were again increased through HC-030031 pretreatment. Bacteremia may have been triggered by the disruption of the mucus barrier in the small intestine. Normally, bacteria are located in the loose mucus and cannot penetrate the inner mucus layer.[27,28]
(a)
(c)
(b) (d)
Figure 7 Photomicrographs from immunohistochemical staining for NF-jB in jejunum of mouse with induced intestinal mucositis. Moderate
NF-jB staining was seen in normal jejunum (a). A jejunum from a mouse treated with CPT-11 showed intense immunostaining for NF-jB in the
epithelium and muscular layer (b). Treatment with carvacryl acetate considerably reduced the immunostaining in the surface epithelium (c), which was demonstrated by a reduction in the number of immunostained cells following carvacryl acetate (75 mg/kg) treatment (d). Bars represent the
meanstandard error of the mean and were analysed using one-way analysis of variance (ANOVA) followed by a Newman–Keuls test.*P<0.05
vs control group;#P
However, in intestinal mucositis, because of the tissue destruction caused by CPT-11 treatment, there was damage to the mucus layer, which may have allowed bacteria to reach the bloodstream through contact with injured epithe-lial cells.
Furthermore, in intestinal mucositis, we observed high levels of MPO, indicating a large amount of neutrophil migration to the injured tissue. Treatment with carvacryl acetate reduced MPO levels in the jejunum tissue, although there was an increase in the systemic levels of leucocytes. This is because CPT-11 causes systemic neutropenia, along with an increase in neutrophil migration to the injured tis-sue to defend against inflammation. Oxidative stress, which is common in inflamed tissues, can induce p53 activation resulting in senescence, arrest cycle and cell death in the hematopoietic compartment.[29,30] This could explain the low leucocyte systemic levels after CPT-11 treatment and
its reversion after carvacryl acetate treatment. Moreover, as proposed in other studies,[30]the neutrophil migration to the injured site itself may help explain the decrease in leu-cocyte number in the blood.
To confirm that neutrophils trigger the release of inflam-matory mediators,[31]we observed the levels of some pro-inflammatory cytokines (IL-1b, KC – an ortholog of human IL-8 in mouse and TNF-a), which decreased after treatment with carvacryl acetate. It is well established that these cytokines have important functions in the develop-ment of intestinal mucositis by regulating and amplifying the immune response, contributing to tissue damage.[11,32] These actions are related to the stimulation of cytokine release by IL-1b; induction of metabolite generation from arachidonic acid by IL-1b, which can act as a trigger for inflammation; and activation by TNF-aof some proteases released by epithelial cells, fibroblasts and neutrophils,
(a)
(c)
(d)
(b)
Figure 8 Photomicrographs from immunohistochemical staining for COX-2 in jejunum mucosa in a mouse with induced intestinal mucositis trea-ted with DMSO 2% (a). The jejunum of a mouse that received CPT-11 showed intense immunostaining for COX-2 in the epithelium and muscular layer (b). Treatment with carvacryl acetate considerably reduced the immunostaining present in the surface epithelium (arrow, c). This was
demon-strated by a reduction in the number of immunostained cells following carvacryl acetate (75 mg/kg) treatment (d). Bars represent the mean
s-tandard error of the mean and were analysed using one-way analysis of variance (ANOVA) followed by a Newman–Keuls test. *P<0.05 vs
control group;#P
related to tissue injury.[11,31]Moreover, KC acts as a rele-vant chemokine recruiting neutrophils to the inflammation site[11,33]and, thus, acting as a feedback agent in this pro-cess, as more neutrophils causes more cytokine activation.
TNF-a, through interactions with TNF-R1, is also associ-ated with activation of the transcription factor NF-jB.[34] Additionally, the presence of bacteria in injured mucosa, as observed in intestinal mucositis, can lead to the activation of NF-jB.[35]The transcription factor NF-jB is associated with an increase in pro-inflammatory cytokine levels, such as IL-1b and TNF-a, and can induces apoptosis, contributing to the pathology of intestinal mucositis. Furthermore, NF-jB activates the expression of cell surface receptors to molecules related to the inflammation, cell adhesion molecules and molecules related to the response to oxidative stress.[34]
Another pro-inflammatory mediator shown to be highly expressed in CPT-11-induced intestinal mucositis was COX-2, which was also decreased by carvacryl acetate treat-ment. COX-2 is involved in the synthesis of prostaglandins, which are responsible for the development of inflamma-tion.[34,36] As in other studies, we noted that COX-2 and NF-jB levels seemed to follow a similar pattern of expres-sion; both increased after CPT-11 treatment and decreased after carvacryl acetate treatment. This agrees with the understanding that NF-jB upregulates COX-2 expression, which can also be increased by IL-1b.[37]
Results also showed a concomitant reduction in oxida-tive stress parameters (MDA and NOx) and increase in cel-lular defence agents (GSH and SOD) after treatment with carvacryl acetate. This is consistent with the fact that increasing levels of reactive oxygen and nitrogen species can activate many pro-inflammatory pathways and upregu-late pro-inflammatory cytokines.[29,36]The mechanisms by which carvacryl acetate reduced MDA levels and increased GSH and SOD levels may have been related to the decrease in neutrophil migration to the small intestine mucosa, or by the reduction in the direct toxic action of CPT-11 on the epithelial cells. The increase in GSH levels may also be secondary to the consumption of other reactive oxygen spe-cies.[36]
The enzyme Na+/K+-ATPase is responsible for the active transport of many electrolytes in the small intestine. Its inhibition prevents the absorption of Na+ and K+in the small intestine and, thus, induces intestinal fluid accumula-tion, which contributes to diarrhoea,[17,38] one of the side effects of CPT-11 therapy.[11] Information about
Na+/K+-ATPase activity in intestinal mucositis is scarce. This study showed that Na+/K+-ATPase activity was dimin-ished with CPT-11 treatment and returned after carvacryl acetate treatment. In this manner, carvacryl acetate may also help prevent diarrhoea found in intestinal mucositis, encouraging survival.
Based on these results, it is possible to infer that TRPA1 act as a sensitive channel for cell damage. Other authors showed this and the fact of its sensitivity are increased under inflammatory conditions in mouse sensory neurons, as demonstrated by other authors.[3,39] Through interac-tions with carvacryl acetate, which act as an agonist for this receptor, TRPA1 could act in the modulation of the cells responses to the damage made by ROS and stimulated for other inflammation related factors. When it is used an antagonist of TRPA1, it is possible to notice that this results are reverted and there is no response to the inflammation and cell damage, which is in concordance with the action of carvacryl acetate on TRPA1.
Conclusion
Results show that carvacryl acetate presented a strong and stable interaction with the TRPA1 receptor. This may be responsible for the anti-inflammatory and antioxidant action of carvacryl acetate in intestinal mucositis. There-fore, our results suggest that carvacryl acetate may be an effective drug to treat intestinal mucositis, especially because of its route of action through TRPA1.
Declarations
Conflict of interest
The Authors confirm that there is no conflict of interests.
Acknowledgements
This work was supported by the National Counsel of Tech-nological and Scientific Development (CNPq, grant no. 303032/2013-8). Dr. Brito, Dr. Sousa and Dr. Medeiros are recipients of CNPq fellowships. We thank Rivelilson Men-des de Freitas (in memoriam) for his assistance with the obtention of chemical compounds used in this work. The authors declare that no competing interests exist.
References
1. Damasceno SRBet al.Carvacryl acet-ate, a derivative of carvacrol, reduces nociceptive and inflammatory
response in mice. Life Sci 2014; 94: 58–66.
2. Fortes ACet al.Aplicacß~oes do acetato de carvacrol em formulacß~oes far-mac^euticas para o tratamento da
esquistossomose. BR 1 O 2012 007004-9 A2, 19 March 2012, 19 November 2013.
to pathophysiology. Pflugers Arch
2012; 464: 425–458.
4. Kim MJ et al. The TRPA1 agonist, methyl syringate suppresses food intake and gastric emptying. PLoS One2013; 8: e71603.
5. Yeoh A et al. Radiation therapy-induced mucositis: relationships between fractionated radiation, NF-jB, COX-1, and COX-2.Cancer Treat Rev2006; 32: 645–651.
6. Stringer AMet al.Irinotecan-induced mucositis manifesting as diarrhea cor-responds with an amended intestinal flora and mucin profile. Int J Exp
Pathol2009; 90: 489–499.
7. Takaishi M et al. 1,8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol Pain2012; 8: 1–12.
8. Vogel AI et al. Vogel’s Text Book of
Practical Organic Chemistry, 5th edn.
Englewood Cliffs: Prentice-Hall, 1996. 9. Moraes J et al. Anthelmintic activity
of carvacryl acetate against
Schisto-soma mansoni. Parasitol Res 2013;
112: 603–610.
10. Ikuno N et al. Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum (reports).
J Natl Cancer Inst 1995; 87: 1876–
1883.
11. Melo ML et al. Role of cytokines (TNF-a, IL-1aand KC) in the patho-genesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxi-fylline and thalidomide. Cancer
Che-mother Pharmacol2008; 61: 775–784.
12. Alvarenga EMet al.Carvacrol reduces irinotecan-induced intestinal mucositis through inhibition of inflammation and oxidative damage via TRPA1 receptor activation.Chem Biol Interact
2016; 260: 129–140.
13. Wong DVTet al.The adaptor protein Myd88 is a key signaling molecule in the pathogenesis of irinotecan-induced intestinal mucositis. PLoS One 2015; 10: e0139985.
14. Woo PCYet al.Clarithromycin atten-uates cyclophosphamide-induced mucositis in mice. Pharmacol Res
2000; 41: 526–532.
15. Lima-Junior RCet al.Involvement of nitric oxide on the pathogenesis of
irinotecan-induced intestinal mucosi-tis: role of cytokines on inducible nitric oxide synthase activation.
Can-cer Chemother Pharmacol 2012; 69:
931–942.
16. Bewaji CO et al. Comparison of the membrane-bound (Ca2+ +Mg2+
)-ATPase in erythrocyte ghosts from some mammalian species. Comp
Bio-chem Physiol1985; 82: 117–122.
17. Yakubu MT et al. Antidiarrhoeal activity of Musa paradisiaca Sap in wistar rats. Evid Based Complement
Alternat Med 2015; 1: 1–9; Article ID
683726.
18. Bradley PPet al.Cellular and extracel-lular myeloperoxidase in pyogenic inflammation. Blood 1982; 60: 618– 622.
19. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ell-man’s reagent.Anal Biochem1968; 25: 192–205.
20. Das Ket al.A modified spectrophoto-metric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys
2000; 37: 201–204.
21. Mihara M, Uchiyama M. Determina-tion of malonaldehyde precursor in tissues by thiobarbituric acid test.
Anal Biochem1978; 86: 271–278.
22. Green LC et al. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal Biochem 1982; 126: 131– 138.
23. Yeoh ASet al.Nuclear factor kappa B (NFjB) and cyclooxygenase-2 (Cox-2) expression in the irradiated colorec-tum is associated with subsequent histopathological changes.Int J Radiat
Oncol Biol Phys2005; 63: 1295–1303.
24. Levitt M, Perutz MF. Aromatic rings act as hydrogen bond acceptors.J Mol Biol1988; 201: 751–754.
25. Vieth M et al.Do active site confor-mations of small ligands correspond to low free-energy solution structures.
J Comput Aided Mol Des 1998; 12:
563–572.
26. Ribeiro RA et al. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives. Cancer
Chemother Pharmacol 2016; 78: 881–
893.
27. Lam W et al.The number of intesti-nal bacteria is not critical for the enhancement of antitumor activity and reduction of intestinal toxicity of irinotecan by the Chinese herbal med-icine PHY906 (KD018).BMC
Comple-ment Altern Med2014; 14: 490.
28. Johansson ME et al.The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria.
Proc Natl Acad Sci USA 2008; 105:
15064–15069.
29. Abbas HAet al.Mdm2 is required for survival of hematopoietic stemcells/ progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell
2010; 7: 606–617.
30. Arifa RD et al. The reduction of oxidative stress by nanocomposite Fullerol decreases mucositis severity and reverts leukopenia induced by Irinotecan. Pharmacol Res 2016; 107: 102–110.
31. Wang J, Arase H. Regulation of immune responses by neutrophils.
Ann N Y Acad Sci2014; 1319: 66–81.
32. Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterol-ogy1994; 106: 533–539.
33. Reaves TA et al. Neutrophil transep-ithelial migration: role of toll-like receptors in mucosal inflammation.
Mem Inst Oswaldo Cruz 2005; 100:
191–198.
34. Logan RM et al. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal mod-els and cytotoxic drugs. Cancer Treat Rev2007; 33: 448–460.
35. Kanarek N et al. Critical role for IL-1b in DNA damage induced
mucosi-tis.Proc Natl Acad Sci USA2014; 111:
E702–E711.
36. Yeoh ASet al. A novel animal model to investigate fractionated radiother-apy-induced alimentary mucositis: the role of apoptosis, p53, nuclear factor-jB, COX-1, and COX-2. Mol Cancer Ther2007; 6: 2319–2327.
against intestinal damage induced by gamma radiation in rats.Int J Radiat Biol2015;91:150–156.
38. Sousa NAet al.The efficacy of a sul-phated polysaccharide fraction from
Hypnea musciformis against diarrhea
in rodents. Int J Biol Macromol2016; 86: 865–875.
39. Cattaruzza F et al.Transient receptor potential ankyrin-1 has a major role
in mediating visceral pain in mice.
Am J Physiol Gastrointest Liver Physiol