Acute and Sub-Chronic Toxicity of Aqueous Extracts
of
Chenopodium ambrosioides
Leaves in Rats
Marcel Gianni C. da Silva,
1Raimundo Neilson L. Amorim,
2Carlos C. Caˆmara,
2Jose´ Domingues Fontenele Neto,
2and Benito Soto-Blanco
31Secretariat of Public Health, Jardim do Serido´, Rio Grande do Norte, Brazil.
2Department of Animal Sciences, Federal Rural University of Semi-A´rido, Mossoro´, Rio Grande do Norte, Brazil. 3Department of Veterinary Clinic and Surgery, College of Veterinary Medicine, Federal University of
Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
ABSTRACT The present study aimed to evaluate the toxicity of aqueous extract ofChenopodium ambrosioidesleaves. To measure acute toxicity, rats were administered 0, 0.3, 1.0, or 3.0 g/kg of aqueous extract fromC. ambrosioidesleaves by gavage. To analyze sub-chronic toxicity, rats were treated by oral gavage for 15 consecutive days with 0, 0.3, or 1.0 g/kg of extract ofC. ambrosioidesleaves. No animals from either trial exhibited any signs of toxicity. In the acute study, the highest dose of the extract led to an increase in the serum activities of alanine transaminase (ALT) and aspartate transaminase (AST) and a decrease in the serum levels of urea. In the sub-chronic test, rats treated with 1.0 g/kg for 15 days exhibited increased serum ALT activity and creatinine levels and mild cytoplasmic vacuolation of hepatocytes. The results indicate that aqueous extract fromC. ambrosioidesleaves produce slight hepatotoxic lesions in rats.
KEY WORDS:ChenopodiaceaeChenopodium ambrosioidesmedicinal plantnematicidetoxicity
INTRODUCTION
C
henopodium ambrosioidesL., which is also known as Dysphania ambrosioides, is an herbal medicinal plant belonging to the Chenopodiaceae family that is native from Central and South America has been used as a traditional medicine in tropical and subtropical regions. The most com-mon traditional form of use as medicinal plant in Brazil is leaves blended with milk.1The therapeutic uses of C. am-brosioides include anthelmintic,2,3 abortifacient, emmena-gogue,4sedative, antipyretic, and antirheumatic applications. It has also been used for the treatment of digestive, respira-tory, urogenital, vascular, and nervous disorders, as well as diabetes, hypercholesterolemia,5and sickle cell anemia.6The pharmacological properties ofC. ambrosioidesinclude cyto-toxic,7,8,9antitumor,10anxiolytic, antipyretic,11analgesic, anti-inflammatory,12 immunostimulatory,13,14 molluscocidal,15 antiviral,16 antibacterial,9 antifungal,9,17,18 trypanocidal,7,8 antiplasmodial,19antileishmanial,20–22antischistosomal,23and anthelmintic2,3activities. The primary essential oil inC. am-brosioidesis the monoterpene ascaridole, which is most highly concentrated in the seeds.24,25 Other components include a -terpinene,p-cymene,22limonene, transpinocarveol,ascaridole-glycol, aritasone,b-pinene, myrcene, phelandrene, alcanphor, a-terpineol,25and other monoterpenes.26,27
The essential oil ofC. ambrosioidesis an irritant to the mucous membranes of the gastrointestinal tract and pro-motes lesions in the kidneys and liver. Presumably at high doses, the essential oil ofC. ambrosioideshas been reported to cause human fatalities, especially in children.16,20,28–30 Furthermore, the exposure of human lymphocytes to C. ambrosioidesextractsin vitrohas been shown to increase the frequency of chromosomal aberrations and to reduce the mitotic index.31 Furthermore, hepatocellular carcinomas were found in Egyptian toad (Bufo regularis) dosed with C. ambrosioidesoil for 3 months.32
AlthoughC. ambrosioidesexhibits anti-parasitic proper-ties in both humans2,3 and animals,33 its use as an anti-parasitic agent has been limited by its toxicity, which is promoted primarily by ascaridole. However, infusions of the plant have been shown to retain its parasiticidal activity.34 Thus, the present study aimed to determine the toxicity of aqueous extracts ofC. ambrosioides.
MATERIALS AND METHODS
Plant extract preparation
Fresh leaves of cultivatedC. ambrosioideswere obtained from the Medicinal and Toxic Plants Garden, Sector of Seedlings Production, Federal Rural University of Manuscript received 19 July 2013. Revision accepted 22 February 2014.
Address correspondence to: Benito Soto-Blanco, DVM, MSc, PhD, Departamento de Clı´nica e Cirurgia Veterina´ rias, Universidade Federal Rural do Semi-A´rido, Av. Antoˆnio Carlos 6627, Belo Horizonte, MG, 30123-970, Brazil, E-mail:benito.blanco@pq.cnpq.br J Med Food17 (9) 2014, 979–984
#Mary Ann Liebert, Inc., and Korean Society of Food Science and Nutrition DOI: 10.1089/jmf.2013.0134
Semi-A´ rido (UFERSA), in northeastern Brazil (51101500S
and 372003900W) at an altitude of 16 m above sea level. The
climate in the region is characterized as semi-arid. The mean annual temperature is 27.4C, and the average annual rain-fall and mean relative humidity are 674 mm and 68.9%, respectively. A reference specimen is deposited in the Da´rdano de Andrade Lima Herbarium at UFERSA under number MOSS 13814.
Fresh leaves (50 g) were blended with distilled water (200 mL) and then filtered, resulting in an aqueous solution with a final concentration of 0.25 g leaves/mL. Aqueous solutions were prepared daily for administration to rats.
Animals
The Wistar male rats used in the present study were ob-tained from the Department of Animal Sciences, UFERSA. The average age of the rats was 5 weeks old (weighing ~100–15 g). They were provided regular rodent chow (Labina, Purina, Sa˜o Lourenc¸o da Mata, PE, Brazil) ad libitumand given free access to tap water. During the entire study period, the animals were housed in cages under hy-gienic conditions in a controlled environment with a 12 h light/dark cycle that was maintained at 24–3C. Ethical procedures were based on the Standards for Didactic and Scientific Practice of Animal Vivisection and Ethical Prin-ciples for Use of Experimental Animals from the Brazilian College of Animal Experimentation (COBEA), Brazil, which are in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, March 18, 1986).
In vitrored blood cell lysis assay
The test was carried out as described earlier.35 Ery-throcytes were isolated from fresh horse blood, washed three times with a physiologic saline solution, and suspended in a physiologic solution at a hematocrit of 25%. C. am-brosioides leaves blended in physiologic solution were ad-ded to the suspenad-ded erythrocytes, and the suspension was diluted with physiologic solution to result in a final plant concentration of 0.5 g leaves/mL and a final hematocrit of 1%. The erythrocytes were then incubated for 20 min at room temperature. Non-lysed cells were removed by cen-trifugation at 3000 rpm for 5 min, and the hemoglobin in the supernatant was determined by its absorbance at 540 nm. Control samples exhibiting no lysis (with physiologic so-lution instead of plant soso-lution) and 100% lysis (with dis-tilled water instead of physiologic solution) were used in all experiments.
Acute toxicity trial
Twenty-four rats were randomly divided into four test groups of six animals each, which were treated by gavage with different concentrations of a C. ambrosioides extract corresponding to 0 (control), 0.3, 1.0, or 3.0 g of leaves/kg body weight. The rats were monitored closely for 24 h after dosing and then sacrificed under anesthesia (xylazine and
ketamine). Anticoagulant-free blood samples were collected from all animals and were centrifuged at 2000 rpm for ten minutes to separate sera. The serum levels of total protein, urea, creatinine, glucose, triglycerides, and cholesterol and the activities of alanine aminotransferase (ALT) and as-partate aminotransferase (AST) were determined using specific commercially available kits (Katal, Belo Horizonte, MG, Brazil) and an SBA-2000 automatic analyzer (Celm, Barueri, Sa˜o Paulo, Brazil).
After the rats were euthanized, tissue samples from liver and kidney were collected and fixed by immersion in 10% buffered formalin for 18 h at 4C. Next, samples were de-hydrated through a series of baths in increasing concentra-tions of ethanol, defatted in xylene, and, finally, embedded in paraffin (56C). Paraffin blocks were cut on a rotary microtome and 5 lm thick sections were obtained. The sections were stretched in water bath at 40C and placed on glass slides coated with Meyer’s albumin. The sections were stained with hematoxylin and eosin (H&E) and the sections were examined using a light microscope.
Sub-chronic toxicity trial
Thirty rats were randomly divided into three test groups of ten animals each and were treated for 15 consecutive days by gavage with different concentrations ofC. ambrosioides extract corresponding to 0 (control), 0.3, or 1.0 g of leaves/ kg body weight. The rats were monitored closely twice a day throughout the experimental period. Twenty-four hours after the final dosing, the animals were sacrificed under anes-thesia (xylazine and ketamine). Blood samples and tissue samples from liver and kidney were collected and handled as described at acute toxicity trial.
Statistical analyses
The results are expressed as the means–the standard er-ror of the mean (SEM). Comparisons between groups were performed by ANOVA followed by Dunnett’s multi-ple comparisons test. Data analysis employed Graphpad INSTAT software (version 2.0), and the level of statistical significance wasP<.05.
RESULTS
No hemolysis was observed in equine erythrocytes in-cubated withC. ambrosioides.
microscopic finding was mild congestion in the medullar region of kidneys from rats that were treated with the 3.0 g/ kg dose.
At the sub-chronic trial, none of the animals from all groups exhibited any signs of poisoning. The ALT activity and cre-atinine levels in the serum were significantly decreased in rats that were administered 1.0 g/kg ofC. ambrosioidesleaves for 15 days. The treatment withC. ambrosioidesleaves did not cause any significant changes in total protein, AST, urea, glucose, triglycerides, and cholesterol values (Table 2). Necroscopic examinations showed no gross lesions in any animal. Histopathological analyses revealed mild cytoplasmic vacuolation of hepatocytes (Fig. 1A), mild hepatic congestion and moderate congestion in the medullar region of the kidneys (Fig. 1C) of rats treated with 1.0 g/kg of C. ambrosioides leaves for 15 days. Mild congestion in the medullar region of the kidneys was observed in rats that were administered the 0.3 g/kg dose.
DISCUSSION
C. ambrosioidesoil has been reported to be toxic because it is an irritant to the mucous membranes of the gastro-intestinal tract and it promotes lesions in the kidneys and liver. Several human fatalities have been attributed to the use of high doses of C. ambrosioidesoil.16,20,28–30 Carva-crol, caryophyllene oxide, and ascaridole, which are the major compounds of this plant, have been shown to inhibit the mitochondrial electron transport chain. Carvacrol and caryophyllene oxide have been reported to affect mitochon-drial electron transport by directly inhibiting the electron-transferring complex I, whereas the effects of ascaridole have been shown to depend strongly on the availability of redox-active Fe2+.36
The red blood cell lysis assay used in the present study is based on the fact that the short-term exposure of erythrocytes to cytotoxic agents results in hemoglobin release, which is measured as the endpoint of the as-say.35,37 In the present study, C. ambrosioides did not promote hemolysis in horse erythrocytes. These data suggest that this plant does not promote membrane dis-ruption but are insufficient to conclude that it does not exhibit any cytotoxic effects.
It has been suggested that the use of C. ambrosioides infusions in traditional medicine, especially as vermifuge, is safer than the use of the essential oil.34Ascaridole has been shown to relax rat gastrointestinal tissue contracted by carbachol in vitro, but C. ambrosioidesinfusion at nema-tocidal concentrations was reported to have no effect at these contractions.34In our study, both the acute and chronic administration of an aqueous extract of C. ambrosioides leaves resulted in mild toxic effects in the liver and kidneys, which were associated with changes in serum biochemistry and histological abnormalities. These observed effects are far less toxic than those that have been reported with use of the essential oil.30,38 Similarly, the administration of a hy-droalcoholic extract from C. ambrosioides leaves to mice for 15 days led to reductions in the blood levels of albumin, VLDL cholesterol, and triglycerides,39 effects that might result from disturbances in hepatocyte function. In our study, the observed lowered serum ALT activities in rats treated with 1.0 g/kg ofC. ambrosioidesleaves for 15 days might be attributed to an initial liver damage, that was his-tologically observed as cytoplasmic vacuolation of hepato-cytes and mild hepatic congestion. In fact, previous studies showed reduced serum ALT activity associated to sub-chronic exposure to hepatotoxins.40,41 In the same experi-mental group, the observed congestion in the medullar
Table2. Serum Levels of Total Protein, Urea, Creatinine, Glucose, Triglycerides, and Cholesterol
and Serum Activities of Alanine Aminotransferase and Aspartate Aminotransferase in Rats After15
Consecutive Days of Administration of Extract ofChenopodium ambrosioidesLeaves
Parameter Control 0.3 g/kg 1.0 g/kg
Total protein (g/dL) 9.48–0.44 9.37–0.39 8.30–0.50
ALT (U/L) 72.0–4.98 64.1–3.76 58.6–1.78*
AST (U/L) 170.0–14.5 146.6–9.20 137.6–8.59
Urea (mg/dL) 52.2–1.77 50.5–1.28 51.9–1.48
Creatinine (mg/dL) 0.88–0.05 0.87–0.05 0.69–0.03* Glucose (mg/dL) 163.6–9.90 211.3–23.9 168.9–17.3 Triglycerides (mg/dL) 314.0–32.5 333.1–44.0 272.2–26.5 Cholesterol (mg/dL) 72.9–2.85 73.3–3.65 69.0–4.0
*Statistically significant as compared with control (P<.05).
Table1. Serum Levels of Total Protein, Urea, Creatinine, Glucose, Triglycerides, and Cholesterol and Serum Activities of Alanine Aminotransferase and Aspartate Aminotransferase
in Rats After a Single Dose of Extract ofChenopodium ambrosioidesLeaves
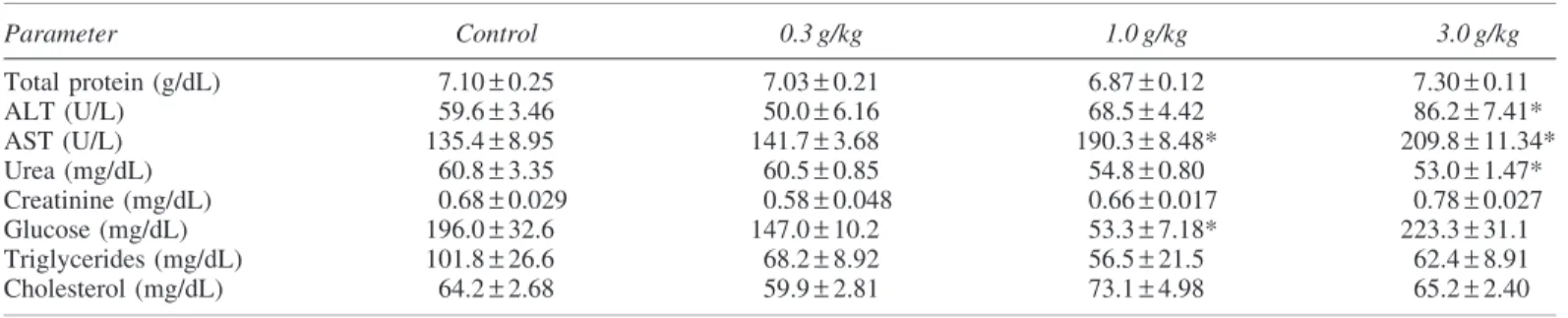
Parameter Control 0.3 g/kg 1.0 g/kg 3.0 g/kg
Total protein (g/dL) 7.10–0.25 7.03–0.21 6.87–0.12 7.30–0.11
ALT (U/L) 59.6–3.46 50.0–6.16 68.5–4.42 86.2–7.41*
AST (U/L) 135.4–8.95 141.7–3.68 190.3–8.48* 209.8–11.34*
Urea (mg/dL) 60.8–3.35 60.5–0.85 54.8–0.80 53.0–1.47*
Creatinine (mg/dL) 0.68–0.029 0.58–0.048 0.66–0.017 0.78–0.027
Glucose (mg/dL) 196.0–32.6 147.0–10.2 53.3–7.18* 223.3–31.1
Triglycerides (mg/dL) 101.8–26.6 68.2–8.92 56.5–21.5 62.4–8.91
Cholesterol (mg/dL) 64.2–2.68 59.9–2.81 73.1–4.98 65.2–2.40
region of the kidneys might be produced by renal vaso-constriction, which could predispose to renal failure.42,43 Increased cell numbers in the lymph node and bone marrow and reductions in the number of peritoneal cells were also reported in response to treatment with this hydroalcoholic extract.39
It is possible that compounds found inC. ambrosioides interact with DNA.In vitrostudies with human lymphocytes have shown thatC. ambrosioidesextracts increase the fre-quency of chromosomal aberrations and reduce the mitotic index.31 Furthermore, the administration of this oil to the Egyptian toad, B. regularis, induced hepatocellular carci-nomas after 3 months of treatment.32 Thus, the potential oncogenic effects ofC. ambrosioidesshould be evaluated in future studies.
CONCLUSIONS
The results of the present study indicate that aqueous so-lutions ofC. ambrosioidesleaves produce only slight hepa-totoxic and nephrotoxic effects in rats. These low levels of toxicity may not be significant in healthy individuals, but they may exacerbate pre-existing hepatic and renal disturbances.
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
Plantas Medicinais Brasileiras. 2nd ed.Editoria UFC, Fortaleza, Brazil, 2004. (In Portuguese.)
2. Kliks MM: Studies on the traditional herbal anthelmintic Che-nopodium ambrosioides L.: ethnopharmacological evaluation and clinical field trials.Soc Sci Med1985;21:879–886. 3. Giove Nakazawa RA: Traditional medicine in the treatment of
enteroparasitosis.Rev Gastroenterol Peru´ 1996;16:197–202. 4. Conway GA, Slocumb JC: Plants used as abortifacients and
emmenagogues by Spanish New Mexicans. J Ethnopharmacol 1979;1:241–261.
5. De Feo V, Senatore F: Medicinal plants and phytotherapy in the Amalfitan Coast, Salerno Province, Campania, Southern Italy.J Ethnopharmacol1993;9:39–51.
6. Ameh SJ, Tarfa FD, Ebeshi BU: Traditional herbal management of sickle cell anemia: lessons from Nigeria.Anemia2012;2012: 607436.
7. Nibret E, Wink M: Trypanocidal and cytotoxic effects of 30 Ethiopian medicinal plants.Z Naturforsch C2011;66:541–546. 8. Borges AR, Aires JR, Higino TM, et al.: Trypanocidal and
cy-totoxic activities of essential oils from medicinal plants of northeast of Brazil.Exp Parasitol2012;132:123–128.
9. Sousa ZL, de Oliveira FF, da Conceic¸a˜o AO, et al.: Biological activities of extracts fromChenopodium ambrosioidesLineu and Kielmeyera neglecta Saddi. Ann Clin Microbiol Antimicrob 2012;11:20.
10. Nascimento FR, Cruz GV, Pereira PV,et al.: Ascitic and solid Ehrlich tumor inhibition by Chenopodium ambrosioides L. treatment.Life Sci2006;78:2650–2653.
11. Bum EN, Soudi S, Ayissi ER,et al.: Anxiolytic activity evalu-ation of four medicinal plants from Cameroon. Afr J Tradit Complement Altern Med2011;8(5S):130–139.
12. Ibironke GF, Ajiboye KI: Studies on the anti-inflammatory and analgesic properties ofChenopodium ambrosioidesleaf extract in rats.Int J Pharmacol2007;3:111–115.
13. Rossi-Bergmann B, Costa SS, Moraes VLG: Brazilian medicinal plants: a rich source of immunomodulatory substances. Cieˆnc Cult (Sa˜ o Paulo)1997;49:395–401. (In Portuguese.)
14. Cruz GV, Pereira PV, Patrı´cio FJ, et al.: Increase of cellular recruitment, phagocytosis ability and nitric oxide production induced by hydroalcoholic extract from Chenopodium am-brosioidesleaves.J Ethnopharmacol2007;111:148–154. 15. Hmamouchi M, Lahlou M, Agoumi A: Molluscicidal activity
of some Moroccan medicinal plants. Fitoterapia 2000;71: 308–312.
16. Zanon SM, Ceriatti FS, Rovera M, Sabini LJ, Ramos BA: Search for antiviral activity of certain medicinal plants from Co´rdoba, Argentina.Rev Latinoam Microbiol1999;41:59–62.
17. Kishore N, Mishra AK, Chansouria JP: Fungitoxicity of essential oils against dermatophytes.Mycoses1993;36:211–215.
18. Prasad CS, Shukla R, Kumar A, Dubey NK:In vitroandin vivo antifungal activity of essential oils ofCymbopogon martiniand Chenopodium ambrosioidesand their synergism against derma-tophytes.Mycoses2010;53:123–129.
19. Pollack Y, Segal R, Golenser J: The effect of ascaridole on thein vitro development of Plasmodium falciparum. Parasitol Res 1990;76:570–572.
20. Franc¸a F, Lago EL, Marsden PD: Plants used in the treatment of leishmanial ulcers due toLeishmania(Viannia)braziliensisin an endemic area of Bahia, Brazil.Rev Bras Med Trop1996;29: 229–232.
21. Monzote L, Montalvo AM, Scull R, Miranda M, Abreu J: Com-bined effect of the essential oil fromChenopodium ambrosioides and antileishmanial drugs on promastigotes of Leishmania amazonensis.Rev Inst Med Trop Sao Paulo2007;49:257–260. 22. Monzote L, Nance MR, Garcı´a M, Scull R, Setzer WN:
Com-parative chemical, cytotoxicity and antileishmanial properties of essential oils fromChenopodium ambrosioides.Nat Prod Com-mun2011;6:281–286.
23. Kamel EG, El-Emam MA, Mahmoud SS, Fouda FM, Bayaumy FE: Parasitological and biochemical parameters inSchistosoma mansoni-infected mice treated with methanol extract from the plants Chenopodium ambrosioides, Conyza dioscorides and Sesbania sesban.Parasitol Int2011;60:388–392.
24. Okuyama E, Umeyama K, Saito Y, Yamazaki M, Satake M: Ascaridole as a pharmacologically active principle of ‘‘Paico,’’ a medicinal Peruvian plant. Chem Pharm Bull (Tokyo) 1993;41: 1309–1312.
25. Sagrero-Nieves L, Bartley JP: Volatile constituents from the leaves of Chenopodium ambrosioides L. J Essential Oil Res 1995;7:221–223.
26. Ahmed AA: Highly oxygenated monoterpenes from Chenopo-dium ambrosioides.J Nat Prod2000;63:989–991.
27. Kiuchi F, Itano Y, Uchiyama N, Honda G, Tsubouchi A, Nakajima-Shimada J, Auki T: Monoterpene hydroperoxides with trypanocidal activity from Chenopodium ambrosioides. J Nat Prod2002;65:509–512.
28. Levy RL: Oil of Chenopodium in the treatment of hookworm infections.J Am Med Assoc1914;63:1946–1949.
29. Paget H: The determination of ascaridole inChenopodium oil. Analyst1926;51:170–176.
30. Montoya-Cabrera MA, Escalante-Galindo P, Meckes-Fisher M, Sa´nchez-Vaca G, Flores-Alvarez E, Reynoso-Garcia M: Fatal poisoning caused by epazote oil:Chenopodium graveolus.Gac Me´ d Me´x1996;132:433–437.
31. Gadano A, Gurn˜ı´ A, Lo´pez P, Ferraro G, Carballo M: In vitro genotoxic evaluation of the medicinal plant Chenopodium am-brosioidesL.J Ethnopharmacol2002;81:11–16.
32. el-Mofty MM, Khudoley VV, Sakr SA, Ganem NF: Induction of neoplasms in Egyptian toadsBufo regularisby oil of Chenopo-dium.Oncology1992;49:253–255.
33. Lans C, Turner N: Organic parasite control for poultry and rab-bits in British Columbia, Canada.J Ethnobiol Ethnomed2011; 7:21.
34. MacDonald D, VanCrey K, Harrison P, et al.: Ascaridole-less infusions ofChenopodium ambrosioidescontain a nematocide(s) that is(are) not toxic to mammalian smooth muscle. J Ethno-pharmacol2004;92:215–221.
35. Pequeno NF, Soto-Blanco B: Toxicidade in vitro de plantas to´xicas: avaliac¸a˜o do teste de ac¸a˜o hemolı´tica. Acta Scientiae Veterinariae2006;34:45–48. (In Portuguese.)
36. Monzote L, Stamberg W, Staniek K, Gille L: Toxic effects of carvacrol, caryophyllene oxide, and ascaridole from essential oil of Chenopodium ambrosioides on mitochondria. Toxicol Appl Pharmacol2009;240:337–347.
39. Pereira WS, Ribeiro BP, Sousa AIP, et al.: Evaluation of the subchronic toxicity of oral treatment with Chenopodium am-brosioidesin mice.J Ethnopharmacol2010;127:602–605. 40. Guzman RE, Solter PF: Hepatic oxidative stress following
pro-longed sublethal microcystin LR exposure.Toxicol Pathol1999; 27:582–588.
41. Guzman RE, Liu Z, Solter PF: Decreased hepatic ALT synthesis is an outcome of subchronic microcystin-LR toxicity. Toxicol Appl Pharmacol2000;164:216–220.
42. Brenner BM, Meyer TH, Hotstetter TH: Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of glomerular sclerosis in agina, renal ablation and intrinsic renal disease. N Eng J Med 1982;307:652– 659.