CONTINUOUS ETHANOL PRODUCTION USING IMMOBILIZED YEAST CELLS ENTRAPPED IN LOOFA-REINFORCED ALGINATE CARRIERS
Phoowit Bangrak1, Savitree Limtong2, Muenduen Phisalaphong1*
1
Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Bangkok 10330, Thailand;
2
Department of Microbiology, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand.
Submitted: February 22, 2010; Approved: January 13, 2011.
ABSTRACT
A culture of Saccharomyces cerevisiae M30 entrapped in loofa-reinforced alginate was used for continuous
ethanol fermentation in a packed-bed reactor with initial sugar concentrations of 200-248 g/L. Maximum
ethanol productivity of 11.5 g/(L·h) was obtained at an ethanol concentration of 57.4 g/L, an initial sugar
concentration of 220 g/L and a dilution rate (D) of 0.2 h-1. However, a maximum ethanol concentration of
82.1 g/L (productivity of 9.0 g/(L·h)) was obtained at a D of 0.11 h-1. Ethanol productivity in the continuous
culture was 6-8-fold higher than that in the batch culture. Due to the developed carrier’s high
biocompatibility, high porosity, and good mechanical strength, advantages such as cell regeneration,
reusability, altered mechanical strength, and high capacity to trap active cells in the reactor were achieved in
this study. The immobilized cell reactor was successfully operated for 30 days without any loss in ethanol
productivity. The average conversion yield was 0.43-0.45 throughout the entire operation, with an
immobilization yield of 47.5%. The final total cell concentration in the reactor was 37.3 g/L (17.7 g/L
immobilized cells and 19.6 g/L suspended cells). The concentration of suspended cells in the effluent was
0.8 g/L.
Key words: ethanol, loofa, alginate, immobilization, continuous
INTRODUCTION
Ethanol demand has continued to grow, both as an
alternative fuel and as a petroleum fuel extender because of
gasoline shortages. Ethanol production from renewable
carbohydrate materials has attracted worldwide interest, and
much research has focused on ethanol production using
immobilized viable microbial cells in continuous systems.
Continuous fermentation using immobilized cell (IC) carriers
offers many advantages, such as higher productivity, relative
ease of product separation, biocatalyst reuse, and high
productivity. The immobilization of yeast cells inside the
porous support material enhances cell stability (5).
Fermentative ethanol production by S. cerevisiae
immobilized within alginate beads has been found to have a
higher productivity than in a batch system (8). However, some
limitations, such as gel degradation, low physical strength and
severe mass transfer restrictions, were often observed when
using alginate-based carriers. Dias et al. (3) reported a lower
specific growth rate of immobilized yeast cells due to oxygen
diffusion problems caused by entrapment in a Ba-alginate gel.
In contrast, the loofa sponge was shown to be an excellent cell
carrier for ethanol fermentation by flocculating the cells in a
bubble column with an external loop for the recirculation of the
fermentation broth (11). Its strength, abundance, low price,
biodegradability, and natural origin are of great interest.
However, a low-shear environment and a large aggregate of
cells were required during the application of the loofa sponge
to prevent excessive cell sloughing from the carrier (9, 10). In
our previous study, immobilized yeast cells entrapped in
loofa-reinforced alginate matrix (ALM) carriers were successfully
developed for repeated batch ethanol fermentations in a
500-mL shake flask system (13). The carriers were simply
fabricated by gelating a peripheral loofa sponge that had been
previously dipped in an alginate/cell mixture. An ALM with
dimensions of 9×9×3 mm3, which was comparable to a
2-mm-diameter alginate bead, was found to be effective for yeast
immobilization. Moreover, after storage for 4 months, the
ALM-immobilized cell culture was still active, and the stability
of IC cultures in the ALM was higher than that of the
suspended culture (13). The ALM structure proved to be more
porous and less dense than a typical alginate bead, allowing for
better internal mass-transfer diffusion. The aim of present
investigation is to use an ALM carrier with dimensions of
20×20×3 mm3 for continuous ethanol fermentation in a
packed-bed reactor (PBR) and evaluate its performance for long-term
operation.
MATERIALS AND METHODS
Microorganisms and media
Saccharomyces cerevisiae M30 was selected for this study because of its high efficiency in ethanol production from
molasses at high temperatures. Starter cultures were prepared
by transferring cells from stock PDA slants to 150 mL of
sterilized medium followed by incubation at 33°C at 150 rpm
for 20 h. The medium for the starter culture contained 0.05%
ammonium sulfate and 5% inverse sugar from palm sugar at
pH 5.0. Subsequently, the resulting cell suspension was
concentrated by decantation and then transferred to the main
culture.
Cell immobilization
Sodium alginate (3% w/v) solution was formulated by
dissolving Na-alginate powder in 0.9% (w/v) NaCl solution. It
was autoclaved for 5 min at 121°C and stored overnight at 4°C
to facilitate deaeration. To form an alginate-cell mixture, 5 mL
of cell suspension was added to 50 mL of 3% (w/v) alginate
solution. Loofa sponges were cut into small thin square pieces
with dimensions of 19 x 19 x 2 mm3 using scissors. To form
ALMs, 2 g of sterilized thin square loofa sponges were dipped
into the alginate/cell mixture. The gel carriers were transferred
to 1.5 % (w/v) CaCl2 solution and left to harden in this solution
with mild stirring for 15 min. The carriers were then rinsed 3
times with 0.9% (w/v) NaCl solution. The carriers were
prepared under aseptic conditions, and the average ALM
dimensions were 20 x 20 x 3 mm3.
Fermentations
A 1-L (Ø= 5.7 cm; height= 43.4 cm) reactor column
containing an immobilized cell bed of ALM carriers was used
for the study. The experimental setup for the PBR with a
packed volume of 32% (v/v) of the total bed volume is shown
in Fig. 1. The temperature of the system was controlled at 32 ±
1°C by passing 28°C cooling water inside the reactor jacket.
Initial sugar concentrations of 200, 220, and 240 g/L were
continuously fed into the bottom of the reactor at dilution rates
of 0.11, 0.16, 0.20, and 0.30 h-1. Samples (5 mL) were
collected every 8 hours. The samples were frozen before
determining the sugar, ethanol, and cell concentrations to
enable simultaneous analysis of all samples.
Analytical methods
Free cell dry weight was determined from the absorbance
measured at 660 nm by a UV-2450 UV-visible
using a corresponding standard curve. For immobilized cells, a
known mass of cell carriers was dissolved in 0.05 M sodium
citrate. After the sponge was removed, immobilized cell
concentrations were determined in a manner similar to that
used for the free cells. Yeast cell viability was determined
using methylene blue staining (2). The concentration of ethanol
was determined using a gas chromatograph (model GC-7AG;
Shimadzu, Kyoto, Japan) equipped with a flame ionization
detector. To measure reducing sugar concentration, the sample
solution was hydrolyzed in 33% HCl at 100ºC for 10 min and
neutralized with NaOH solution. Reducing sugar content was
then determined using the dinitrosalicylic acid method (6).
Figure 1. Schematic diagram of the immobilized-cell PBR.
RESULTS
Batch fermentation
The purpose of this work was to expand upon our previous
work (13) and develop an efficient, continuous process for
producing ethanol from sugarcane molasses using an
immobilized S. cerevisiae M30 culture. ALM was chosen
based on its strong potential as a cell carrier. The ALM carrier
has many advantages, including high regeneration ability,
reusability, altered mechanical strength, and high ethanol
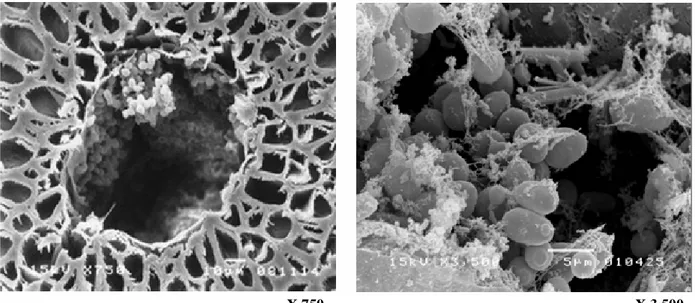
productivity. Inspection of the cross section of the ALM carrier
(Fig. 2) reveals the carrier’s structure, which is composed of
loofa fiber, a layer of alginate gel, and a hollow space between
the loofa fiber and alginate gel. The hollow space between the
alginate and loofa fiber was found to be an ideal space for cell
growth (13). Fig. 3 and 4 reveals that yeasts can also grow well
in the alginate layer and in the hollow space of the core fiber.
The cells had a normal oval shape, and filaments were
Feed pump
Feed tank
Product pump
Water cooler tank
Water cooler pump
Product tank
Sampling valve Carbon dioxide
vent
Immobilized cell reactor
Jacket
Sampling ports
Cotton filter
Vent
Cotton filter
Vent
Sampling ports
(5th)
5th
3rd
4th
2nd
observed connecting cells both to other cells and to cellulose
fibers (Fig. 4). This filamentous structure likely promoted firm
attachment of the cells both to the carrier and to cell
aggregations.
For more convenient preparation, the ALM carrier used in
the PBR was modified into a square with dimensions of 20 x
20 x 3 mm3. Pre-examination was performed in 500-mL
Erlenmeyer flasks containing 250 mL sterilized medium under
controlled conditions of 220 g/L of initial sugar, initial pH of
5.0, 150 rpm, and 33°C. Ethanol production, growth rate, and
immobilized yield (YI) using the squares with dimensions of 20
x 20 x 3 mm3 and 9 x 9 x 3 mm3 were comparable (Fig. 5). An
ethanol concentration of 89-90 g/L was produced within 60
hours of batch fermentation, whereas the IC and free-cell
concentrations were about 4.0 g/L and 1.0 g/L, respectively.
No significant differences were observed in the data obtained
from the ALM carriers of the two different sizes.
(A) (B)
Figure 2. (A) Cross-section of the ALM after 72 h of ethanol fermentation. The ALM consisted of loofa fiber (I), a hollow space between the loofa fiber and alginate gel (II), and alginate gel (III). (B) A higher-magnification view of the hollow space.
X 750 X 3,500
X 750 X 3,500 Figure 4. Yeast cells in the hollow core fibers of the ALM after 72 h of ethanol fermentation.
0 50 100 150 200 250
0 8 16 24 32 40 48 56 64 72
Time (hour) S u g a r c o n c e n tr a ti o n ( g /l ) 0 20 40 60 80 100 E th a n o l c o n c e n tr a ti o n (g /l )
Time (h)
S
u
g
a
r
c
o
n
c
en
tr
a
ti
o
n
(
g
/l
)
E
th
a
n
o
l
c
o
n
c
en
tr
a
ti
o
n
(
g
/l
)
Figure 5. Batch fermentations using ALM carriers with dimensions of 9 x 9 x 3 mm3 (open symbols) and 20 x 20 x 3 mm3 (filled
symbols); , = sugar: , = ethanol.
Continuous fermentation
Effect of dilution rate and initial sugar concentration:
Continuous ethanol fermentations using cane molasses in a 1-L
packed-bed reactor were performed at a temperature of 32 ±
1°C and an initial pH of 5.0. The initial reducing sugar
concentrations were 202, 222, and 248 g/L at dilution rates
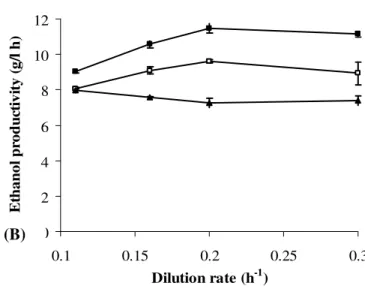
ranging from 0.11-0.30 h-1. The effects of dilution rate on
ethanol concentration and ethanol productivity are presented in
Fig. 6 and Fig. 7, respectively. The ethanol concentration
commonly observed. When the initial sugar concentration (S0)
was at 200–220 g/L, the ethanol productivity increased linearly
with the dilution rate, from 0.1 to 0.2 h-1, and then remained
nearly constant. However, at the very high initial sugar
concentration of 248 g/L, the ethanol productivity was limited
to 8.0 g/(L·h). The optimal S0 for ethanol productivity was 220
g/L. Under steady-state conditions and at varying dilution rates
of 0.11, 0.16, 0.20 and 0.30 h-1, the average ethanol
concentrations in the effluent were 82.1, 66.1, 57.4 and 37.2
g/L, respectively, corresponding to ethanol productivities (PE)
of 9.0, 10.6, 11.5 and 11.2 g/(L·h), respectively. The ethanol
conversion yield (YE/S) was almost constant at 0.45±0.02. An
optimal ethanol productivity of 11.5 g/(L·h) was obtained at an
ethanol concentration of 57.4 g/L, an initial sugar concentration
of 220 g/L, and D of 0.2 h-1, whereas the maximum ethanol
concentration of 82.1 g/L (PE = 9.0 g/(L·h)) was obtained at a
D of 0.11 h-1. At the end of the procedure (360 h), the
concentrations of immobilized cells, suspended cells in the
reactor, and suspended cells in the effluent were 16.0±0.4,
12.3±0.5 and 0.6±0.1 g/L, respectively, with an average
immobilized yield of 56.5%.
0 10 20 30 40 50 60 70 80 90 100
0.1 0.15 0.2 0.25 0.3
Dilution rate (h-1)
E th a n o l co n ce n tr a ti o n ( g /l )
Figure 6. Ethanol concentration at dilution rates of 0.1-0.3 h-1 and initial sugar concentrations of 200 g/L ( ), 220 g/L ( ), and 248
g/L ( ).
0 2 4 6 8 10 12
0.1 0.15 0.2 0.25 0.3
Dilution rate (h-1)
E th a n o l p ro d u ct iv it y ( g /l h )
Figure 7. Ethanol productivity at dilution rates of 0.1-0.3 h-1 and initial sugar concentrations of 200 g/L ( ), 220 g/L ( ),
and 248 g/L ( ).
Effect of superficial velocity: The design of packing materials that achieve ideal conditions, which correspond to
plug flow and a uniform distribution of fluid across the
cross-section of the column, is important for a PBR. The ethanol and
reducing sugar concentrations measured from the five sampling
ports on both sides of the PBR after the system reached steady
state revealed a satisfactorily uniform distribution of the fluid.
The ethanol concentration profiles in a plug-flow reactor
packed with immobilized cells entrapped in ALM are shown in
Fig. 6. Usually, for anaerobic ethanol fermentation with high
substrate concentrations, it is likely that external mass transfer
is not limiting, at least for a large portion of the packed bed
(15). The external mass transfer coefficient can be enhanced by
increasing the liquid superficial velocity. In this study, the
effect of liquid superficial velocity was examined. Liquid
superficial velocity (VS, cm/h)) was calculated from:
A
Q
V
Sϕ
=
,where Q is the volumetric flow rate of the fluid (cm3/h), A is
the cross-sectional area of the bed (cm2), and is the
packed-bed porosity (the volume of voids per volume of reactor).
Based on the results, the effect of superficial velocity on
ethanol concentration profile can be divided into two
sub-effects, one with S0 of 200-220 g/L and the other with S0 of
248 g/L. Fig. 8(A) and Fig. 8(B) depict the results with S0 at
200 g/L and 220 g/L, respectively. The results revealed an
increase in ethanol concentration as the liquid superficial
velocity was increased from 4.8 cm/h to 6.9 cm/h. However, no
significant enhancement in ethanol production was observed
when the liquid superficial velocity was further increased to
8.7-13.0 cm/h. Based on the result with S0 at 200-220 g/L, the
external mass transfer resistance exhibited some negative
effects on the overall fermentation rate of the packed bed
reactor when the liquid creeping over the static solid particles
had a velocity of less than about 7 cm/h. However, at a high
initial sugar concentration at S0 of 248 g/L, decreases in sugar
consumption and ethanol production were observed as the
liquid superficial velocity increased (Fig. 8(C)). The inhibitory
effects of high initial sugar and high ethanol concentrations
have been previously reported (12). Therefore, mass transfer
resistance had a positive effect on ethanol fermentation at very
high sugar concentrations due to a reduction of sugar-induced
inhibition.
0
20
40
60
80
100
0
2
4
6
8
10
Retention time (h)
E
th
a
n
o
l
co
n
ce
n
tr
a
ti
o
n
(
g
/L
).
0
20
40
60
80
100
0
2
4
6
8
10
Retention time (h)
E
th
a
n
o
l
co
n
ce
n
tr
a
ti
o
n
(
g
/L
).
0 20 40 60 80 1000 4 8 12 16 20
Retention time (h)
E th a n o l co n ce n tr a ti o n ( g /L ).
Figure 8. The steady-state ethanol concentration profiles at initial sugar concentrations of 200 g/L (A), 220 g/L (B), and
248 g/L (C) and at superficial velocities of 4.8 cm/h ( ), 6.9
cm/h ( ), 8.7 cm/h ( ), and 13.0 cm/h( ).
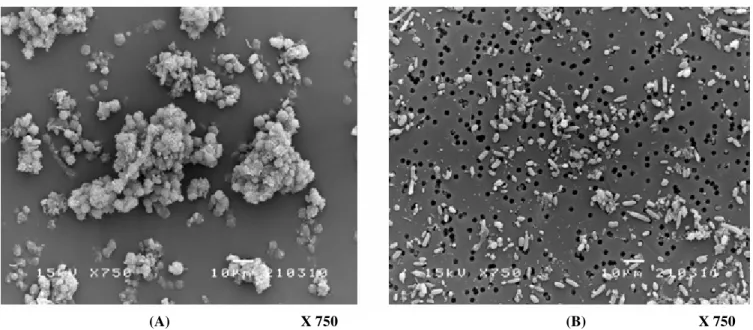
Stability test: The long-term stability of the immobilized yeast cells entrapped in ALM carriers during continuous
ethanol fermentation was examined using an initial sugar
concentration of 220 g/L at a constant D of 0.11 h-1. After 30
days of operation, the degradation of alginate films due to cell
growth and CO2 production was observed and resulted in cell
leakage. Such leaks could also be observed from the cell
suspensions in the reactor and in the effluent. Fig. 9(A) and
Fig. 9(B) depict the suspended yeasts in the reactor and in the
(A)
(B)
effluent, respectively. The suspended cells in the reactor
appeared healthy and retained their normal oval shape. In
addition, aggregations of yeast cells with filamentous
connections were observed. These cell filaments were barely
observed in the alginate gel layer. Therefore, it is possible that
the formation of these filaments may be activated by the
cellulose fibers of the loofa sponge. It was found that almost all
of the aggregated cells were trapped in the packed bed.
However, the suspended cells in the effluent were thinner,
much smaller, and non-aggregated. The use of a
hemacytometer and methylene blue staining to discriminate
between dead and live cells revealed that almost all of the cells
(>95%) in the reactor were alive, but more than 70% of cells in
the effluent were dead.
The robust performance of the immobilized cells in the
alginate-loofa cube during continuous ethanol fermentation in
the PBR was confirmed by satisfactory operational stability
during 30-day fermentation at a D of 0.11 h-1. There was no
significant decline in productivity during continuous operation,
and an average ethanol productivity of 8.7 g/ (L·h) was
achieved at an average ethanol concentration of 79.3 g/L. The
average conversion yield was 0.43-0.45 throughout the entire
operation, with an immobilization yield of 47.5%. The final
total cell concentration in the reactor was 37.3 g/L (17.7 g/L
immobilized cells and 19.6 g/L suspended cells). The
concentration of suspended cells in the effluent was 0.8 g/L.
(A) X 750 (B) X 750
Figure 9. Suspended cells in the PBR (A) and in the effluent (B) after 30 days of continuous fermentation.
DISCUSSION
The data presented in this work demonstrate that
continuous ethanol production from molasses using
immobilized S. cerevisiae M30 cells entrapped in ALM (20 x 20 x 3 mm3) is promising. Compared to the batch fermentation,
higher ethanol productivity (6-8-fold) was obtained by
continuous fermentation in the PBR. A maximum productivity
of 11.5 g/L h at an ethanol concentration of 57.4 g/L was
obtained using an initial sugar concentration of 220 g/L at a D
of 0.20 h-1, whereas a maximum ethanol concentration of 82.1
concentration in the effluent of the packed-bed column
obtained in this work was reasonably high compared to those
described in previous reports (1, 4, 7, 8, 14, 16). The
experimental results revealed that the ALM was suitable for
yeast immobilization in a continuous PBR. With its favorable
mechanical properties, high biocompatibility, and superior
porous structure, the ALM is a system with high cell density,
high ethanol production, and high stability. Furthermore, the
ALM should be applied as a cell carrier for efficient production
in other fermentation systems.
ACKNOWLEDGEMENTS
This work was supported by the Thailand Research Fund
(TRF) and Chulalongkorn University (contract grant number
RSA5080011).
REFERENCES
1. Amutha, R.; Gunasekaran, P. (2001). Production of ethanol from liquefied cassava starch using co-immobilized cells of Zymomonas mobilis and Saccharomyces diastatcus. J. Biosci. Bioeng. 92, 560-564. 2. Bai, F. W.; Chena, L. J.; Zhangc, Z.; Andersona, W. A.; Moo-Young, M.
(2004). Continuous ethanol production and evaluation of yeast cell lysis and viability loss under very high gravity medium conditions. J. Biotechnol. 110, 287-293.
3. Dias, J.C.T.; Rezende, R.P.; Linardi, V.R. (2001). Effects of immobilization in Ba-alginate on nitrile-dependent oxygen uptake rates of Candida guilliermondii. Braz. J. Microbiol. 32, 221-224.
4. Goksungur, Y.; Zorlu, N. (2001). Production of ethanol from beet molasses by Ca-alginate immobilized yeast cell in a packed-bed reactor. Turk. J. Biol. 25, 265-275.
5. Kiyohara, P.K.; Lima, U.A.; Santos, H.S.; Santos, P.S. (2003). Comparative study between yeasts immobilized on alumina beads and on membranes prepared by two routes. Braz. J. Microbiol. 34, 129-137. 6. Miller, G.L. (1959). Use of dinitrosalicylic acid reagent for
determination reducing sugar. Anal. Chem., 31, 426–428.
7. Monte Alegre, R.; Rigo, M.; Joekes, I. (2003). Ethanol fermentation of a diluted molasses medium by Saccharomyces cerevisiae immobilized on chrysotile. Braz. Arch. Biol. Technol. 46, 751-757.
8. Najafpour, G.; Younesi, H.; Syahidah, K.; Ismail, K. (2004). Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresour. Technol. 92, 251-260.
9. Ogbonna, J.C.; Tomiyama, S.; Tanaka, H. (1996). Development of a method for immobilization of non-flocculating cells in loofa (luffa cylindrical) sponge. Process. Biochem. 31, 737-744.
10. Ogbonna, J.C.; Tomiyama, S.; Liu, Y.C.; Tanaka, H. (1997). Efficient Production of ethanol by cells immobilized in loofa (Luffa cylindrical) sponge. J. Ferment. Bioeng. 84, 271-274.
11. Ogbonna, J.C.; Mashima, H.; Tanaka, H. (2001). Scale up of fuel ethanol production from sugar beet juice using loofa sponge immobilized bioreactor. Bioresour. Technol. 76, 1-8.
12. Phisalaphong, M.; Srirattana, N.; Tanthapanichakoon, W. (2006). Mathematical modeling to investigate temperature effect on kinetic parameters of ethanol fermentation. Biochem. Eng. J. 28, 36-43. 13. Phisalaphong, M.; Bundiraharijo, R.; Bangrak, P.; Mongkolkajit, J.;
Limtong, S. (2007). Alginate-Loofa as carrier matrix for ethanol production. J. Biosci. Bioeng. 104, 214-217.
14. Valach, M.; Navratil, M.; Horvathova, V.; Zigova, J.; Sturdik, E.; Hrabarova, E.; Gemeiner, P. (2006). Efficiency of a fixed-bed and gas-lift three-column reactor for continuous production of ethanol by pectate and alginate immobilized Saccharomyces cerevisiae cells. Chem. Pap. 60, 154-159.
15. Vega, J.L.; Clausen, E.C.; Gaddy, J.L. (1988). Biofilm reactors for ethanol production. Enzyme Microb. Technol. 10, 390-402.
16. Wang, B.; Ge, X.M.; Li, N.; Bai, F.W. (2006). Continuous ethanol fermentation coupled with recycling of yeast flocs. Chin. J. Biotechnol. 22, 816-821.