Integrated Masters in Veterinary Medicine
Master’s degree thesis
Brachycephalic Airway Obstructive
Syndrome
Epidemiological study and its relation with anatomic abnormalities
Mariana Oliveira da Silva Almeida
Tutor: Professor Doctor Luis Miguel Maltez da Costa
University of Trás-os-Montes and Alto Douro Vila Real, 2015
iii
Integrated Masters in Veterinary Medicine
Master’s degree thesis
Brachycephalic Airway Obstructive
Syndrome
Epidemiological study and its relation with anatomic abnormalities
Mariana Oliveira da Silva Almeida
Tutor: Professor Doctor Luis Miguel Maltez da Costa
Jury members:
_____________________________________________________ _____________________________________________________ _____________________________________________________
University of Trás-os-Montes and Alto Douro Vila Real, 2015
iv All information present on this study is the entire responsibility of the author.
v
Acknowledgments
Foremost, I want to thank the whole staff from Gran Sasso clinic (Milan, Italy), where I performed great part of my final traineeship, for being such a great family to me. There, I was able to be involved in numerous wonderful surgical procedures what leaded me to be even keener in my pursuit to be a veterinary surgeon specialized in Small Animal Surgery.
In full gratitude I would like to acknowledge the following individuals who encouraged, inspired, supported, assisted, and sacrificed themselves to help me with this study:
Professor Doctor Luis Miguel Viana Maltez da Costa (UTAD, Portugal), my tutor and surgery professor, for being the best Master a beginner can possibly have.
Dr. Roberto Bussadori (Gran Sasso clinic, Milan, Italy), for being the main one who made possible this study to be performed, since he is the great surgeon behind the surgical procedures to which all these animals were submitted. Thank you one more time for allowing my access to the whole data.
Dr. Gabriele di Salvo (Gran Sasso clinic, Milan, Italy), for all the support given to me when planning my thesis and collecting the Data. You are such a great person, a great surgeon, a great professor and a great friend.
Dr. Laurent Findji (Fitzpatrick Referrals, UK), for the wisest advice.
Edgar Mesquita, for helping me with the most difficult part of this study, the statistical analysis.
vi
Abstract
Introduction Brachycephalic airway obstructive syndrome (BAOS) corresponds to a multifocal obstruction caused in first place by a shortened skull, which narrows the lumen of the upper respiratory tract. The diversity and the severity of the anatomic abnormalities present, whose anatomy is vastly described in the literature, depend on each individual case. Moreover, each individual case is characterized by epidemiological variables. Identify the role of the several anatomic anomalies in the prognosis of a certain dog, or patients that will have limited improvement or complications from standard surgical management are currently frustrating tasks for surgeons. Therefore, it was mandatory to study the association between epidemiological variables, as breed, gender and age, and the anatomical abnormalities present. Material and Methods Medical files from all cases that underwent surgical treatment of BAOS at the Gran Sasso clinic, Milan, Italy, between 2003 and 2014 were consulted for epidemiological data and anatomic abnormalities present. Statistical analysis was performed on SPSS version 22.0 (IBM Corporation, 2013). Results Sixty-six brachycephalic dogs from three different breeds (English bulldog, French bulldog and pug) presented at the veterinary practice mainly due to obstructive dyspnoea and respiratory stertor. English bulldogs were overrepresented (39 of 66 cases, 59.1%), as well as males (48 of 66 cases, 72.7%), which may justify the fact that most dogs aged less than 1 year old were male English bulldogs (13 dogs, 86.7% within age). Nevertheless, the earlier age of presentation of this breed and gender seemed to provide some protection effect from secondary anatomic abnormalities. On the other hand, half of the females group was presented at 4 or more years old (9 dogs, 50%. within gender). This gender presented a higher prevalence of animals affected by tonsillar hypertrophy (6 dogs, 33.3%. within gender) and 3rd LC (5 dogs, 27.8% within gender) with an 18 times higher risk of presenting this secondly referred anatomic abnormality than males. The majority of dogs presented stenotic nares (61 dogs, 92.4%) and everted saccules (48 dogs, 72.7%). There was a more than expected presence of everted saccules in young animals (12 dogs, 80% within age). Furthermore, elongated soft palate was observed in the entire population (66 dogs, 100%). French bulldog presented the highest prevalence of animals affected by RAC (11 dogs, 64.7%. within breed), tonsillar hypertrophy (6 dogs, 35.3%. within breed), 2nd LC (5 dogs, 29.4% within breed) and 3rd LC (4 dogs, 23.5%. within breed). Conclusion Some skull characteristics differ between each brachycephalic breed and the anatomic abnormalities presented seem to reflect it. French bulldogs were severely affected, especially by secondary changes, and age of presentation to surgery was considered a
vii worsening factor. Males and English bulldogs seemed to be more protected which can be attributable to an earlier age of presentation. However, this previously referred breed appears to be more affected than what it is indicated in the literature by some anatomic abnormalities. Conclusions about pug population were not reliable due to its small size.
viii
Resumo
Introdução A síndrome respiratória obstrutiva braquicefálica corresponde a uma obstrução multifocal causada em primeiro lugar pelo encurtamento marcado do aspecto rostral do crânio de animais braquicéfalos, o que leva à estenose e obstrução das vias aéreas superiores. O número e o grau de severidade das alterações anatómicas presentes, cuja anatomia se apresenta vastamente caracterizada na literatura, variam individualmente. No entanto, cada indivíduo é caracterizado pelas variáveis epidemiológicas. Identificar o papel da presença de determinadas alterações anatómicas no prognóstico de determinado animal ou, por outro lado, antever o resultado ou possíveis complicações após as técnicas cirúrgicas standard tornou-se uma tarefa frustrante para cirurgiões. Deste modo, é extremamente importante a elaboração de um estudo em que se associe a presença de alterações anatómicas com variáveis que caracterizem a população (sexo, raça e idade). Materiais e Métodos Os ficheiros médicos de animais que foram submetidos ao tratamento cirúrgico standard na clínica veterinária Gran Sasso, em Milão, Itália, entre 2003 e 2014, foram consultados para obter dados epidemiológicos e informações sobre as alterações anatómicas presentes. A análise estatística foi realizada usando o software SPSS versão 22.0 (IBM Corporation, 2013). Resultados Sessenta e seis animais das raças Bulldog inglês, Bulldog francês e pug apresentaram-se com dispneia obstrutiva e estertor respiratório. Os Bulldogs ingleses apresentaram-se em maioria (39 de 66 animais, 59.1%), assim como os machos (48 de 66 casos, 72.7%), o que pode explicar o facto de a maioria dos animais com idade inferior a 1 ano serem Bulldogs ingleses machos (13 cães, 86.7% dentro da idade). Metade das fêmeas apresentaram-se com 4 ou mais anos (9 cães, 50% dentro do género). Do mesmo modo, este género apresentou uma maior prevalência de animais afectados por hipertrofia das amígdalas (6 animais, 33.3%. dentro do género) e por colapso laríngeo de 3º grau (5 cães, 27.8% dentro do género). Grande parte da população apresentou estenose das narinas (61 cães, 92.4%) e eversão dos ventrículos laríngeos (48 cães, 72.7%). Verificou-se um número inesperado de animais com menos de 1 ano com eversão dos ventrículos laríngeos (12 animais, 80% dentro da idade). Além disso, toda a população apresentou palato mole longo. Os Bulldogs franceses apresentaram uma elevada prevalência de animais afectados por conchas aberrantes rostrais (11 cães, 64.7%. dentro da raça), hipertrofia das amígdalas (6 cães, 35.3%. dentro da raça), colapso laríngeo de 2º grau (5 cães, 29.4% dentro da raça) e colapso laríngeo de 3º grau (4 cães, 23.5%. dentro da raça). Conclusão As diferentes raças braquicéfalas apresentam variações morfológicas cranianas, levando a uma predisposição racial diferente para certas alterações anatómicas. Os
ix Bulldogs franceses apresentaram-se gravemente afectados por alterações secundárias, pelo que a idade média a que esta raça se apresentou para tratamento foi considerada um factor de agravamento. Machos e Bulldogs ingleses apresentaram menos alterações anatómicas secundárias, o que pode dever-se à idade mais precoce de apresentação. No entanto, esta raça apresentou-se afectada em maior grau em comparação com a literatura. Os resultados relativos ao pug não foram considerados fiáveis devido ao reduzido tamanho de sua população.
xi
Table of contents
1. The importance of this scientific study ... 1
2. Introduction ... 2 2.1. Epidemiology ... 2 2.1.1. Brachycephalic breeds ... 2 2.1.2 Age ... 2 2.1.3. Gender ... 3 2.1.4. Bodyweight ... 3
2.2. Skeletal anatomic abnormalities ... 3
2.2.1. General skull formation – embryology and growth pathology ... 3
2.2.2. Skeletal features ... 5
2.2.3. Morphometric measurements ... 6
2.3. The respiratory obstructive syndrome ... 8
2.3.1. Clinical Signs... 9
2.4. Multilevel obstruction ... 10
2.4.1. Nasal obstruction: Nostrils, vestibule ... 11
2.4.2. Conchae ... 14
2.4.2.1. Rostral aberrant conchae ... 16
2.4.2.2. Caudal berrant conchae ... 19
2.4.2.3. Mucosal conctact points ... 21
2.4.2.4. Thermoregulation ... 22
2.4.3. Elongated and thickened soft palate ... 23
2.4.4. Nasopharyngeal Obstruction ... 28
2.4.5. Laryngeal diseases ... 30
2.4.6. Tracheal Hypoplasia ... 37
2.4.7. Tracheal and bronchial collapse ... 41
2.4.8. Inspiratory resistance and negative pressure ... 42
2.4.9. Gastroesophageal disease ... 43
3. Objectives of the statistical study ... 45
4. Material and methods ... 46
4.1. Data collection... 46
4.2. Statistical analysis... 46
xii
5.1. Descriptive analysis ... 49
5.1.1. Epidemiological Data ... 49
5.1.2. Anatomic abnormalities ... 51
5.1.2.1. Laryngeal collapse ... 52
5.2. Statistical association between variables ... 53
5.2.1. Association between breed and age at time of surgery ... 53
5.2.1.1. 4 intervals of age at time of surgery ... 53
5.2.1.2. 2 intervals of age at time of surgery ... 53
5.2.1.3. Interpretative analysis ... 54
5.2.2. Association between gender and age at time of surgery ... 54
5.2.2.1. 4 intervals of age at time of surgery ... 54
5.2.2.2. 2 intervals of age at time of surgery ... 55
5.2.2.3. Interpretative analysis ... 55
5.2.3. Association between anatomic abnormalities and age at time of surgery ... 56
5.2.3.1. 4 intervals of age at time of surgery ... 56
5.2.3.2. 2 intervals of age at time of surgery ... 57
5.2.3.3. Interpretative analysis ... 59
5.2.4. Association between anatomic abnormalities and breed ... 61
5.2.4.1. Interpretative analysis ... 62
5.2.5. Association between anatomic abnormalities and gender... 68
5.2.5.1. Interpretative analysis ... 69
6. Discussion ... 72
7. Conclusion ... 79
xiii
Index of figures
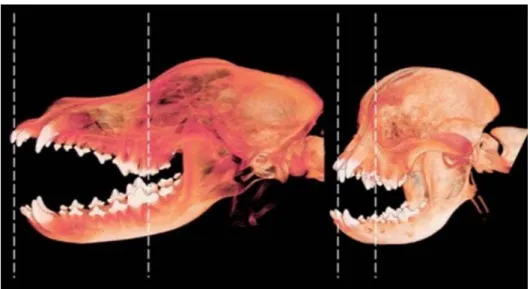
Figure 1. Three-dimensional CT reconstruction of a dolichocephalic (left)
and brachycephalic (right) skull………..…...….5
Figure 2. Craniofacial angle (left). Anatomic bases for skull ratios (right): skull width (SW) to skull length (SL) and cranial length (CL) to skull length (SL)………..……6
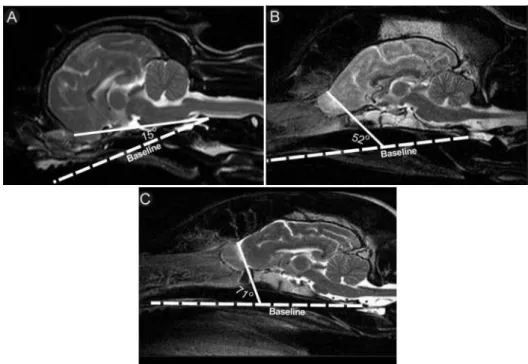
Figure 3. Sagittal T2-weighted MRI images illustrating typical olfactory bulb angles associated with brachycephalic, mesaticephalic, and dolichocephalic head conformations……….….……8
Figure 4. External nose - rostral aspect of planum nasale (left) and nasal cartilages (right)………..……….…………11
Figure 5. View of normal (left) and brachycephalic nares (right)…………...…….…11
Figure 6. Rostral aspect of nasal cavity………..……….12
Figure 7. Vertical wedge alaplasty technique……….……...…13
Figure 8. Vertical wedge alaplasty technique: final result………...……...….14
Figure 9. Endoscopic view of the vestibule in an English bulldog: the left image is normal and right image presents rostral aberrant concha (RAC)…..….…....…16
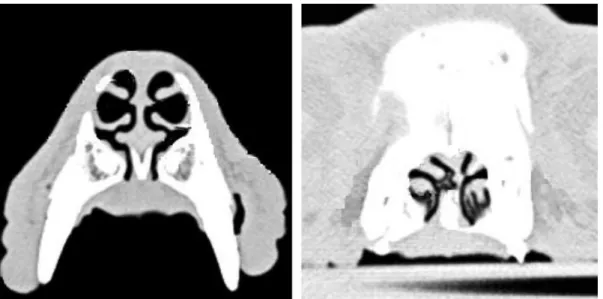
Figure 10.Transverse CT scans of the nose of a German shepherd dog (left) and of a pug (right), with severe deviation of the nasal septum and RACs present on its concave side………….…….………..…………...…..…….…...17
xiv
Figure 11. LATE surgery: endoscopic view of the diode laser fibre………..…….18
Figure 12. Sagittal computed tomography scans of a German shepherd (left) and a pug (right)………...………….……….……..19
Figure 13. Nasopharynx of a brachycephalic dog with nasopharyngeal turbinates protruding on each side of the vomer bone……….………..………...20
Figure 14. Nasal cavity with extensive mucosal contact………..………...21
Figure 15. Soft palate that extends beyond the tip of the epiglottis and tonsillar crypts……….….……….……....……….23
Figure 16. Excessively thickened portion of the soft palate removed after Folded flap palatoplasty………24
Figure 17. Folded flap palatoplasty technique – part 1………..………..…25
Figure 18. Folded flap palatoplasty technique – part 2……..………..………26
Figure 19. Folded flap palatoplasty technique – part 3………..………..………26
Figure 20. Folded flap palatoplasty technique – part 4……..………..………27
Figure 21. Folded flap palatoplasty technique – part 5…..………...…...27
Figure 22. Retrograde endoscopic view of the nasopharynx of a Poodle (left) and of a French bulldog (right)………..………..28
Figure 23. Larynx showing vocal and vestibular folds (the animal is in dorsal recumbency)………..….………30
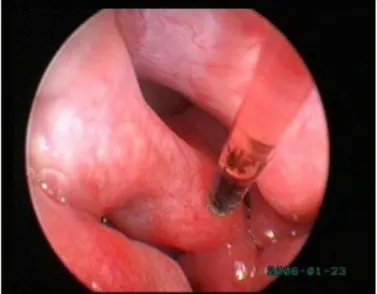
xv Figure 24. Everted saccules, swollen from inflammation and edema,
obstruct the ventral half of the rima glottidis (1 year old French bulldog)…...………….31
Figure 25. Normal larynx and both stages of laryngeal collapse..…………...32
Figure 26. Arytenoid lateralization technique………..34
Figure 27. Pre-operative view of laryngeal collapse (left), showing loss of rigidity and medial displacement of the corniculate processes of both arytenoid cartilages. Post-operative view of the same larynx following arytenoid laryngoplasty (right), showing good abduction of the left
arytenoid cartilage and enlargement of the rima glottides………....……...34
Figure 28. Everted laryngeal saccules in two dogs……….36
Figure 29. Cross section of a hypoplastic trachea, obtained by necropsy,
of an English bulldog puppy that died of bronchopneumonia……...………..37
Figure 30. Technique used for measuring thoracic inlet (TI) and tracheal
diameter (TD)………..………...39
Figure 31. Lateral thoracic radiograph from a Bulldog puppy with severe tracheal hypoplasia and bronchopneumonia (left) and the same bulldog at
xvi
Index of photos (Courtesy of Dr. Roberto Bussadori)
Photo 1. Overlong and thickened soft palate present in a female French
bulldog with 1 year old……….59 Photo 2. Tonsillar hypertrophy present in a male English bulldog with
6 years old………60 Photo 3. Overlong soft palate present in a male English bulldog with 2
years old………....63 Photo 4. Overlong and extremely thick soft palate present in a female
French bulldog with 3 years old……….………....………63 Photo 5. Eversion of laryngeal saccules present in a male English bulldog
with 6 years old………..………...63 Photo 6. 2rd grade laryngeal collapse present in a male French bulldog
with 2 years old………...64 Photo 7. 2rd grade laryngeal collapse present in a male French bulldog
with 3 years old………..…64 Photo 8. 3rd grade laryngeal collapse present in a female French bulldog
with 13 years old………...65 Photo 9. Rostral aberrant concha (RAC) present in a female French bulldog,
with 1 year old………...66 Photo 10. Rostral aberrant concha (RAC) present in a male English bulldog
with 2 years old……….66 Photo 11. Caudal aberrant concha (CAC) present in a male French bulldog
with 2 years old……….66 Photo 12. Tracheal hypoplasia present in a male English bulldog with
4 years old………...67 Photo 13. 2nd grade laryngeal collapse present in a female English bulldog
xvii
Index of tables
Table 1. Descriptive analysis of the variable age at surgery………..……50 Table 2. Descriptive analysis for the association between the variables
presence of everted saccules/laryngeal collapse and age at surgery. …………....……51 Table 3. Results of the statistical association between the variables breed
and 4 intervals division age at surgery………...….53 Table 4. Results of the statistical association between the variables breed
and 2 intervals division of age at surgery………53 Table 5. Results of the statistical association between the variables gender
and 4 intervals division of age at surgery………..…….54 Table 6. Results of the statistical association between the variables gender
and 2 intervals division of age at surgery………..…….55 Table 7. Results of the statistical association between the several anatomic
abnormalities and 4 intervals division of age at surgery……….………...56 Table 8. Results of the statistical association between the several anatomic
abnormalities and 2 intervals division of age at surgery……….……...57 Table 9. Risk of an animal present tonsillar hypertrophy by 2 age intervals
obtained by logistic regression……….…………...60 Table 10. Results of the statistical study for the association between the
several anatomic abnormalities and breed………...…61 Table 11. Risk of one breed present 2nd grade laryngeal collapse obtained by
logistic regression………...64 Table 12. Risk of one breed present 3rd grade laryngeal collapse obtained by
logistic regression………..………...65 Table 13. Risk of one breed present rostral aberrant conchae obtained by
logistic regression………...……65
Table 14. Risk of one breed present tonsillar hypertrophy obtained by logistic
xviii Table 15. Results of the statistical study for the association between the
several anatomic abnormalities and gender………..68 .
Table 16. Risk of one gender present 3rd grade laryngeal collapse obtained
by logistic regression……….…70 Table 17. Risk of one gender present tonsillar hypertrophy obtained by
logistic regression……….…71 Table 18. Frequency of several BAOS components………...73
Index of charts
Chart 1. Frequencies of the variable breed………...49 Chart 2. Frequencies of the variable gender……….49 Chart 3. Average age at surgery in all dogs of the study, by gender and by breed…….50 Chart 4. Frequency of dogs in each age at surgery interval……….51 Chart 5. Frequency of the presence of each anatomic abnormality……….52
xix
List of Acronyms and one Abbreviation
2nd LC – 2nd grade laryngeal colapse 3rd LC – 3rd grade laryngeal collapse
BAOS – Brachycephalic airway obstructive syndrome CAC – Caudal aberrant conchae
CNS – Central nervous system CT – Computed tomography
LAELS – Laser-assisted external laryngeal stiffening LATE – Laser-assisted turbinectomy
MRI – Magnetic resonance imaging OSAS - Obstructive sleep apnea syndrome RAC – Rostral aberrant conchae
SaO2 – Saturation level of oxygen in hemoglobin TD/TI ratio – Tracheal diameter to thoracic inlet ratio
TT/3R ratio - Ratio between the thoracic tracheal luminal diameter measured at the midpoint between the thoracic inlet and the carina (TT) and the width of the proximal third of the third rib (3R).
1
1. The importance of this scientific study
After an intensive research through scientific literature, it seems that the reason for limited improvement following BAOS (Brachycephalic airway obstructive syndrome) surgery is the presence of a number of anatomical abnormalities related to conformation that cannot be surgically corrected and the presence of irreversible secondary changes (Emmerson, 2014). If all locations are not addressed concurrently, one cannot expect a clinically healthy animal after surgery (certain stenoses remain untouched). Moreover, it is hard to differentiate what anatomic abnormalities contribute the most to clinical symptoms in a certain dog, and how those anatomic abnormalities vary between breeds, gender or age (Schuenemann & Oechtering, 2014 a).
Identify patients that will have limited improvement or complications from standard surgical management is currently a frustrating task for surgeons. Novel surgical options allied to standard surgical management offer a way to alleviate signs and can provide significant improvement. However, there is still a frustrating subset of cases with poor outcomes that surgeons struggle to identify pre-operatively (Emmerson, 2014).
Brachycephalic airway obstructive syndrome is a complex problem which currently is not fully understood. It is mandatory a study of the anatomical variations between individuals (Emmerson, 2014) and their association with breed, gender and age. Therefore, it is the main objective of the scientific study of this Master’s thesis.
2
2. Introduction
Brachycephalic breeds are widely popular and their popularity has risen in recent years, with the highest increase in terms of offspring in many parts of the world (Oechtering et al., 2007; Oechtering, 2010). There is no surprise since dogs like English, French bulldog and pug exhibit child-like characteristics and most people are instinctively attracted by this, not mentioning that these breeds show a very friendly and jolly character (Nöller et al., 2008). However, inappropriate breeding selection for extreme brachycephalic characteristics has led to structural deformity of the airways, causing severe malfunction and upper respiratory insufficiency. It is referred as Brachycephalic Airway Obstructive Syndrome (BAOS) (Oechtering et al., 2007; Dupré et al., 2013).
2.1. Epidemiology
2.1.1. Brachycephalic breeds
Dog breeds recognized as brachycephalic include English bulldog, French bulldog and pug as primary characters of this syndrome. Boston terrier, Pekingese, Maltese, Shih tzu, Lhasa apso and Cavalier king charles spaniel are secondary characters, due to specific and single anatomic alterations presented. Large-breed dogs such as Boxer, Sharpei, Dogue de bordeaux, Bullmastiff and some miniature breeds such as Yorkshire terriers, Chihuahua and miniature Pinschers may also show some degree of brachycephalic characteristics (Koch et al., 2003; Trappler & Moore, 2011; Dupré et al., 2013).
2.1.2 Age
According to the scientific literature, most brachycephalic dogs are evaluated with an average age between 2 and 4 years due to worsening of clinical signs (Harvey, 1982 b; Harvey, 1982 c; Helund, 2002 a; Huck et al., 2008; Dupré et al., 2013), while laryngeal collapse is associated with older animals (Harvey, 1982 b). Bulldogs tend to present with an average age between 1 and 2 years (Lorinson et al., 1997; Riecks et al., 2007). However, Pink et al. refer to 7 cases of dogs with less than 6 months with several grades of laryngeal collapse.
3 2.1.3. Gender
Some authors reported a higher prevalence of BAOS (2:1) in male dogs than females (Poncet et al., 2005; Poncet et al., 2006; Riecks et al., 2007; Huck et al., 2008). However, Torrez & Hunt (2006) reported a higher prevalence in female dogs (1.6:1).
2.1.4. Bodyweight
As stated by Riecks et al. (2007) and Grand & Bureau (2011), there were no significant differences among absent, minimal and severe BAOS groups with regard to bodyweight. However, an association between increased body weight and severity of general respiratory events is clinically recognized (Trappler & Moore, 2011).
2.2. Skeletal anatomic abnormalities
The dog’s skull presents many shapes and sizes, much more than any other mammal. The canine skull can be classified into three types: dolichocephalic (long and narrow), mesaticephalic (head of medium proportions) or brachycephalic (short, wide and with open orbitae) (Lauruschkus, 1942; Evans, H., 2013). Brachycephalic dogs’ phenotypic appearance is strongly related to breed specific skeletal features (Nöller et al., 2008).
2.2.1. General skull formation – embryology and growth pathology At birth, the visceral cranium, the middle face (nasal, incisive, maxilla, vomer, palatine, pterygoid, lacrimal, zygomatic, hyoid and mandibular bones), is smaller in comparison to the neurocranium (which includes both the cranial vault and the cranial base), constituted by the occipital, interparietal, sphenoid and ethmoid bones (Dupré et al., 2013). In non-brachycephalic animals, a pronounced growth of the visceral cranium starts postnatally and becomes more protuberant than the neurocranium (Nöller et al., 2008). The viscerocranium needs to adapt to the demand of space for the teeth. With the development of the permanent teeth, the face obtains the characteristic expression (Dupré et al., 2013). In brachycephalic dogs, there is a postnatal growth inhibition of the splanchnocranium, the visceral cranium, due
4 to premature closure that occurs in a synchondrosis of the base of the skull, leading to a shortened longitudinal axis of the skull (Koch et al., 2003; Oechtering, 2010; Schmidt et al., 2013). The early fusion of the spheno-occipital synchondrosis has been reported to be responsible for morphological variation in brachycephalic skull types because this synchondrosis primarily accounts for the longitudinal expansion of the skull base (Julien et al., 1957). The neurocranium, which includes both the cranial vault and the cranial base, expands rapidly during the postnatal period of dogs and other mammals (Evans, 1993). The main part of the longitudinal growth is dependent on the extension of the cranial base. Along the midline of the canine cranial base three synchondroses exist, and they are the spheno-ethmoidal synchondrosis, between the presphenoid and spheno-ethmoidal bones, the intersphenoid synchondrosis, between pre- and basisphenoid bone, and the spheno-occipital synchondrosis, between the sphenoid and basioccipital bones (Nickel et al., 1992; Evans, 1993). The cranial base synchondroses are analogous to the growth plates of long bones, as they facilitate rapid growth by endochondral ossification, in which the involved cartilage becomes progressively thinner during skeletal maturation and ultimately converted into bone (Evans, 1993). The fusion of these synchondroses results in their transformation into a synostosis with a continuity of the bony trabeculae and marrow spaces of the adjacent cranial base bones (Nickel et al., 1992).
In a study by Schmidt et al. (2013), brachycephalic dogs had an 80% probability for a closed spheno-occipital syncondrosis at 12 months of age, whereas mesaticephalic dogs had 80% probability of closure at 16 months. However, there was detected a significant difference in the probability of closure of the spheno-occipital synchodrosis between brachycephalic dogs and the Cavalier king charles spaniel. This synchondrosis therefore seemed to ossify at an earlier time point in the Cavalier king charles spaniel, with 80% probability of closure at 8 months. The intersphenoid and spheno-ethmoidal synchondrosis were open in all dogs examined in this study (dogs presented in this study were from neonates to 18 months old) (Schmidt et al., 2013).
In addition to the premature closure of cranial base synchondrosis, brachycephaly can also be caused by other growth defects. Achondroplasia may lead to disproportional dwarfism, as presented in the pug and in the French bulldog. More proportionate skeletal formation disturbances may be caused by a reduced level of growth hormones, such as those seen in the Chihuahua or the Yorkshire terrier. This form of dwarfism has been referred to as ateliotic
5 dwarfism (Stockard, 1941). It is well described in human medicine that growth hormone deficiency results in an immature facial appearance and a skull maintaining a child-like convexity, showing an overall general reduction in cranial base length (Konfino, Pertzeland, & Laron, 1975; Smith, Bannasch, & Young, 2008). There is a lack of histological and molecular studies describing the precise mechanisms responsible for the impaired cartilage growth leading to brachycephaly in different dog breeds (Konfino et al., 1975; Smith et al., 2008).
2.2.2. Skeletal features
Many of the complex alterations in brachycephalia arise from the marked shortening of the rostral aspect of the skull, particularly the facial bones, nasal cavity, and frontal sinuses (Figure 1). The orbits are widely spaced and round. Profound and severe brachycephalia is characterized by a dorsally rotated upper and lower jaw and an abnormal anatomic position of conchae with a steep course of intranasal airways and the nasolacrimal drainage system. Such dogs often have prognathism. The nose is distorted and reduced to almost nothing except for the rostral mobile part. These dogs have canine teeth positioned in a nearly horizontal orientation, an altered position of the nasal turbinates and small frontal sinuses (or absent frontal sinuses in dogs with extreme brachycephalia) (Hennet, 1992; Oechtering et al., 2007; Nöller et al., 2008; Dupré et al., 2013). In the French bulldog, the frontal sinuses are immensely small and in the pug they are completely absent (Nöller et al., 2008).
Figure 1: Three-dimensional CT reconstruction of a dolichocephalic (left) and
6 Dorsal rotation of the rostral maxilla in these dogs results in a smaller craniofacial angle, compared with dogs of other breeds (Regodo et al., 1993).
2.2.3. Morphometric measurements
Morphometric measurements of the skull report characteristic differences among brachycephalic dog breeds (Figure 2). An example, the pug has a shorter craniofacial skull angle than French bulldog and English bulldog (Oechtering et al., 2007).
Dogs are classified as brachycephalic when they present a skull width: length ratio of 0.81 or greater. Mesaticephalic and dolichocephalic dogs present a ratio of skull width to skull length of 0.52 and 0.39, respectively (Evans, 1993). Brachycephalic dogs have craniofacial angles (angle between the base of the skull (basilar axis) and facial skull (facial axis) of 9˚ to 14˚, when mesocephalic dogs have angles of 19˚ to 21˚ and dolichocephalic greyhounds have 25˚ to 26˚ (Regodo et al., 1993; Koch et al., 2003).
Figure 2: Craniofacial angle (left). Anatomic bases for skull ratios (right): skull width (SW) to
7 Similar head conformation index values correspond to similar head shape characteristics and predispositions to particular diseases. Dogs of brachycephalic breeds are predisposed to the development of a variety of diseases affecting the upper airways, eyes, facial morphology, and CNS (central nervous system). The predisposition to these diseases can be directly or indirectly attributed to the brachycephalic head phenotype. Some examples include cleft palate and lip, brachycephalic obstructive airway syndrome, quadrigeminal cysts and gliomas (Foley et al., 1979; Hendricks, 1992; Helund, 1998; Nöller, 2008).
Nevertheless, Hussein et al. (2011) defend that although head conformation indices provide objective measures of the degree of skeletal brachycephalia in dogs, they are not accurate measures of other physical characteristics related to brachycephalia. Development of such physical characteristics is presumably mediated through expression of modifying genes other than those responsible for brachycephalic head conformation. The head conformation characteristics associated with a small craniofacial angle (which affects the physical characteristics of eyes, upper airways and CNS) may not be predicted with traditional head conformation indices.
One physical CNS characteristic that seems to be related to head conformation and might be directly related to the position of ethmoid turbinates is the position of olfactory bulbs (Hussein et al, 2012). Olfactory bulbs are completely or partially housed within the ethmoidal fossa of the cribriform plate, depending on the depth of the ethmoidal fossa, and are separated from the rest of the forebrain by a visible groove (Williams & Warwick, 1973; Beitz & Fletcher, 1993). Olfactory bulb position may be best determined by evaluation of MRI images, T2-weighted images (Figure 3), in which fluid within olfactory bulb fissures can be identified, but can also be determined by evaluation of CT images of the head, identifying the position of the ethmoidal fossa, or lateral radiographic images of the head, identifying the cribriform plate (which outlines the ethmoid fossa). Olfactory bulb angle was determined by measuring the angle between the olfactory bulb fissure (groove separating an olfactory bulb from the rest of the brain) and the baseline of the cranial cavity (line from the oral aspect of the hard palate, rostrally, to the intercondylar notch of the foramen magnum, caudally). In this study by Hussein et al. (2011), olfactory bulb angle was typically smaller in brachycephalic dogs versus dogs with other head conformations. Results of linear regression indicated that olfactory bulb angle decreased significantly (p<0.001) with brachycephalic conformation. One of the physical abnormalities associated with brachycephalic obstructive airway
8 syndrome is the encroachment of nasal turbinates into the ventral nasal cavities, resulting in stenosis of nasal airways. The ethmoidal turbinates are attached caudally to the cribriform plate, and their orientation is directly related to the orientation of the ethmoidal fossa and olfactory bulbs. In dogs from the previous study, extreme brachycephalic conformation was significantly associated with a ventral orientation of olfactory bulbs and a small olfactory bulb angle. The consequence of a ventral orientation of olfactory bulbs is that the cribriform plate and ethmoid turbinates are ventrally oriented, potentially protruding into the nasal airways and possibly contributing to the pathogenesis of the obstructive airway syndrome (Hussein et al., 2012).
2.3.
The respiratory obstructive syndromeDogs with clinically significant BAOS typically present inspiratory dyspnea, stertor, snoring, exercise intolerance and in severe cases cyanosis and syncopal episodes, worsened with stress, exercise or heat (Wykes, 1991; Dupré et al., 2013). Labored breathing due to airway deformity and stenosis leads to a higher intrathoracic negative pressure, which tends to suck
Figure 3: Sagittal T2-weighted MRI images illustrating typical olfactory bulb angles associated with
brachycephalic (A), mesaticephalic (B) and dolichocephalic (C) head conformations. Figure adapted from Hussein et al. (2012).
9 the abdomen into the thoracic cavity and is accompanied by overdilation of the chest. Symptomatic animals suffer from lifelong respiratory distress, especially in elevated ambient temperatures. Additionally, studies have demonstrated a high incidence of gastroesophageal abnormalities, with signs of regurgitation, vomiting and dysphagia, in brachycephalic dogs presented for upper airway diseases (Poncet et al., 2005; Dupré et al., 2013).
Although upper respiratory syndrome is typically described in brachycephalic dogs, lower respiratory syndrome, with bronchial collapse, may also be seen (Oechtering, 2010; Dupré et al., 2013).
2.3.1. Clinical Signs
Clinical affected animals are characterized by snoring, stertorous breathing, inspiratory distress, gagging, productive coughing, difficulty in swallowing or sleeping, dyspnea, cyanosis and hyperthermia (Hendricks, 1992; Helund, 1998; Holt, 1998; Pink et al., 2006; Riecks et al., 2007; Huck et al., 2008; Papazoglou, 2011; Dupré et al., 2013). Owners report exercise intolerance and worsening of the clinical signs after excitement, stress or with some increase of the environmental temperature or humidity (Holt, 1998; Helund, 2002; Papazoglou, 2011). In most severe cases, clinical sings include syncopal episodes. Apnea can be observed during sleep (Farquharson & Smith, 1942). Suffocation is mainly observed during sleep due to the general muscular relaxation which narrows the respiratory passages (Koch et al., 2003). Several dogs may be admitted at veterinary practice with acute respiratory emergencies and require oxygen supplementation and cool environment in order to recover (Papazoglou, 2011).
If the upper respiratory tract is obstructed, inspiratory stertor is the mainly clinical sign. In each inspiration, the thoracic volume increases. Thereafter, pressure reduces in the lungs and respiratory airways. The resulting airflow leads to an additional pressure decrease in the respiratory airways (Bernoulli law). This, consecutively, brings the edges of the soft tissues close together in the stenotic area, causing the typical breathing sound. As a result of the positive pressure during expiration, the edges of the soft tissues come together only if stenosis is severe.
10 The respiratory tract is not the only structure exposed to increased negative pressure during inspiration. Due to their close vicinity to the airways the esophagus, auditory canals, central nervous system and lower respiratory tract should also be examined. An enlarged tongue, hiatal hernia, gastric bloating, otitis media, neurologic signs and bronchiectasia are common in brachycephalic breeds (Koch et al., 2003).
Dogs that undergo through an acute respiratory crisis should be specifically evaluated for evidence of aspiration pneumonia and non-cardiogenic pulmonary edema. It was reported an association between brachycephalic conformation and the presence of congenital cardiac defects. Therefore, a complete cardiac examination (electrocardiography and echocardiography) may be indicated in patients with physical or radiographic signals of cardiovascular system condition (Harvey, 1982 c; Coyne & Fingland, 1992).
Brachycephalic dogs with less than 50% reduction in the airway diameter show an obstructive breathing pattern with a slow inspiratory and rapid expiratory phase (Aron & Crowe, 1985; Hobson, 1995). In non-brachycephalic dogs, this pattern is often seen only with more than 50% reduction in airway diameter. This demonstrates the synergic role that stenotic nares and other BAOS components have on the respiratory function in affected dogs. Regardless of the respiratory patterns and general clinical signs observed, definitive diagnosis can only be established with an airway examination (Trappler & Moore, 2011).
2.4. Multilevel obstruction
Multifocal obstruction, which narrows the lumen of the upper respiratory tract, is composed by stenotic nares, aberrant turbinates, soft tissue thickening in the nasopharynx, enlargement of the root of the tongue, an elongated fleshy and thickened soft palate, narrowed rima glottides and tracheal hypoplasia (Koch et al., 2003; Oechtering et al., 2007). To obtain adequate amount of oxygen, brachycephalic dogs must produce higher negative pressure, by increasing labored breathing, distal to the obstruction. As a result of this high negative pressure, the soft tissues are drawn into the lumen and become hyperplasic. If negative pressure in the lumen is high enough, it might even exceed the resistance of these tissues, leading these structures to collapse: hypertrophied pharyngeal tonsils and everted laryngeal ventricles (stage I of laryngeal collapse) and, in advanced cases, laryngeal (stages II and III),
11 tracheal or bronchial collapse (Aron & Crowe, 1985; Hendricks, 1992; Koch et al., 2003; Pink et al., 2006; Poncet et al., 2006; Oechtering et al., 2007; Huck et al., 2008; Oechtering, 2010).
2.4.1. Nasal obstruction: Nostrils, vestibule
The openings of the nostrils are bounded medially by the nasal cartilaginous septum and laterally by the most mobile portion of the nose, the wing of the nostril or ala nasi (Figure 4), which contains most of the dorsal lateral and accessory nasal cartilages (Evans, 2013). The dorsolateral nasal cartilages support and shape the wings of the nostrils. The accessory nasal cartilages create the ventral aspect of the midlateral slit in the nose, beneath the wings of the nostrils (Tobias & Johnston, 2012).
In brachycephalic animals, the ala nasi is too large and presses against the septum from the lateral aspect, causing obliteration of the nasal vestibule (Figure 5). In dogs without brachycephalia, the ala nasi or nasal wing is very mobile (during inspiration, it is abducted to facilitate airflow into the nose). In brachycephalic animals, due to its size it appears to greatly restrict abduction, which further reinforces the anatomic stenosis (Dupré et al., 2013).
Figure 4: External nose - rostral aspect of planum nasale (left) and nasal cartilages (right). Figure
adapted from Evans (2013).
12 Stenotic nares have been considered to be the most common anatomic component of brachycephalic syndrome (Oechtering, 2010; Tobias & Johnston, 2012). By excessive selective breeding, the nose hole is being reduced in size to a vertical slit (Aron & Crowe, 1985; Wykes, 1991).
Early correction of stenotic nares has been advocated to minimize exacerbation of other components of brachycephalic airway syndrome, like rostral aberrant conchae (Aron & Crowe, 1985; Wykes, 1991). However, brachycephalic dogs with stenosis of the nostrils may exhibit unchanged nasal stridor even after generous wedge resection. A simple anatomical explanation is that, unlike humans, the canine nasal vestibule is not empty (Dupré et al., 2013). Inside the vestibule, the alar fold is a bulbous extension of the ventral nasal conchae that fuses with the ala nasi (Figure 6) (Evans, 1993; Tobias & Johnston, 2012).
Vertical wedge resection is the best known technique to correct stenotic nares. It was revised by Findji & Dupré in order to promote a deeper opening of the vestibule, being renamed of Vertical wedge alaplasty (Figures 7 and 8).
13
Figure 7: Vertical wedge alaplasty technique. A vertical wedge (A) is removed from the dorsal
lateral nasal cartilage, including the overlying mucosa and epithelium (Dupré, Findji, & Oechtering, 2013). In order to remove a pyramidal piece of tissue, two incisions are started at the apex of the wedge (B), which is positioned slightly dorsolateral to the dorsal limit of the slit-like opening of the stenotic nares, and made at an angle (40–70°) from the medial border. The tissue wedge must extend deep enough into the cartilage (C) and include a portion of the alar fold to fully relieve the obstruction and sufficiently enlarge the nares, not limiting the opening to the rostral part of the nostril (Wykes, 1991; Tobias & Johnston, 2012; Findji & Dupré, 2013; Dupré, Findji, & Oechtering, 2013). The two edges of the resulting wound are brought together and closed in direct apposition with a single layer of simple interrupted suture pattern using a 4/0 or 5/0 monofilament absorbable suture, which abrogates the need for suture removal. The initial suture is placed at the ventrolateral corner of the naris (D) in order to appose the tissue margin accurately. The resulting suture pattern promotes an abaxial movement of the wing of the nostril and opening of the nares (Tobias & Johnston, 2012; Findji & Dupré, 2013). In each case the size and exact direction of the wedge will vary depending on the desired amount of nasal opening. Another suture should be placed in-depth from medial to lateral (E), exiting through the skin just laterally to the caudal end of the alar notch (F). When gently tied, this suture should keep the deep portion of the naris wide during healing (G). Scalpel blades should be changed and the procedure is repeated on the other side (Findji & Dupré, 2013). According to Monet
et al. (2013), starting with the side of the surgeon’s dominant hand helps in controlling symmetrical
14 Other techniques to perform nasoplasty have been described and include vertical and lateral plane wedge resections with the use of a scalpel blade, electrosurgical device, and CO2 laser
(Hedlund, 2002 b; Monnet, 2003; Trappler & Moore, 2011; Lodato & Hedlund, 2012). In addition, the Trader’s technique which excises a section of the dorsolateral nasal cartilage is regaining popularity in clinical practice (Huck et al., 2008). A less commonly used technique that has been described is the alapexy, which secures the nostril laterally without alar wedge removal (Ellison, 2004).
2.4.2. Conchae
Inside the nose, there are first and foremost the nasal conchae, which have a termorregulation function in the cranial and middle part of the nasal cavity, as well as an olfactory function in the caudal part, and the nasal passageways. Unobstructed nasal passageways are crucial for nasal breathing (Oechtering, 2010).
The concha nasalis ventralis or maxiloturbinate emerges from the crista conchalis of the maxilla and fills almost entirely the main nasal cavity. It extends into the plica alaris within the nasal vestibule, finishing in the alar fold. Concha nasalis ventralis is covered with respiratory mucosa, and its main function is termorregulation. When breathing normally, air passes mainly through lamellae of the concha nasalis ventralis and the meatus nasi ventralis and communis. On the other hand, the concha nasalis media and the concha nasalis dorsalis, as well as endoturbinate III and IV, originate from the ethmoid. The concha nasalis dorsalis continues into the plica recta and lies dorsal to the concha nasalis ventralis. In the caudal
15 nasal cavity, the concha nasalis media lies between the conchae nasalis ventralis and the conchae nasalis dorsalis.
Endoturbinate III and IV, lying in the nasal fundus within the sphenoidal recess, and parts of the concha nasalis media, are covered with olfactory mucosa. Inspired air only reaches the olfactory mucosa when there is a forced inspiration (i.e., when sniffing) (Schuenemann & Oechtering, 2014 b).
The cause for the anatomically deformed conchae presented in brachycephalic dogs is the shortened nasal cavity. Some bones derived from the mesoderm, such as the nasal capsule forming the middle face (i.e., maxilla, incisive, palatine and nasal bones), are formed by membranous ossification and ossify earlier during development (day28). Furthermore, these bones tend to mold closely around adjacent structures (Evans, 1993). In the specific case of brachycephalic animals, there is a postnatal growth inhibition of the splanchnocranium, the middle face, leading to a shortened longitudinal axis of the skull (Koch et al., 2003; Oechtering, 2010). On the contrary, the cartilage substrate of endochondral bones continues to develop and ossify beyond the gestational period. Neural crest’s ectoderm derived bones formed by endochondral ossification are less plastic, tending to grow to their full extent. Nasal turbinates are formed by endochondral ossification, and they continue to grow beyond the adjacent structures (Trainor et al., 2003; Creuzet & Le Douarin, 2005). Thus, in brachycephalic breeds, nasal turbinates tend to protrude beyond their surroundings due to the limited space, worsened in their case, in an already ossified nasal capsule (Ginn et al., 2008). Due to their loss of space, conchae find different paths to grow and form so called rostral and caudal aberrant conchae (Schuenemann & Oechtering, 2014 a).
In contrast to normocephalic animals, whose turbinates grow until the lumen of the nasal cavity is filled out, but ceasing to grow before the mucosae of adjacent turbinate lamellae touch each other, a typical rhinoscopic finding in brachycephalic animals is the close contact between turbinate lamellae, leaving no space between mucosal surfaces for airflow. Schuenemann & Oechering (2014 a, b) observed a high prevalence of intranasal mucosal contact points with surrounding structures. Although brachycephalic dogs’ conchae are still smaller than normal, they are far too large for the given volume of the nasal cavity (
t
he comparison of the thickness of individual lamellae shows that they are twice as thick in a 10kg pug as in a 40kg German Shepherd, and it is called “relative conchal hypertrophy”)16 (Negus, 1958; Oechtering, 2010). Likewise, brachycephalic conchae were characterized as thickened and as having a lower degree of branching (Oechtering et al., 2007; Walter et al., 2008).
2.4.2.1. Rostral aberrant conchae
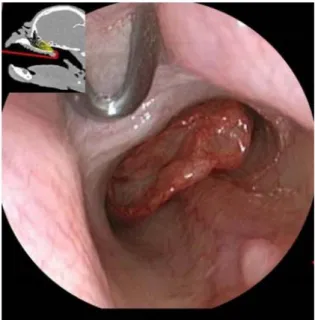
Rostral aberrant conchae (RACs) are defined as parts of the middle or ventral conchae that expand rostrally beyond the point where the plica alaris branches into the concha nasalis ventralis (Figure 9). Therefore, RACs obstruct the middle and ventral meatus nasi (Oechtering, 2010).
In humans, it is known that nasal septal deviation results in nasal obstruction caused by increased air resistance on the convex side of the deviated septum and hypertrophy of the nasal conchae on the concave side (Grützenmacher et al., 2006). Pugs frequently have pronounced septal deviations and show RACs from the concha nasalis media, a rostral growing turbinate, within the curves of the septal deviation (Figure 10). It may be due to the fact that pugs have the shortest nasal cavities of all brachycephalic breeds, leaving no space for a straight septum. (Oechtering et al., 2007; Schuenemann & Oechtering, 2014 a; Schuenemann & Oechtering, 2014 b)
Figure 9: Endoscopic view of the vestibule in an English bulldog: the left image is normal and
right imagepresents rostral aberrant concha (RAC).
1: Plica recta, 2: Plica alaris, 3: Plica basalis, 4: Ventral septal swell body, 5: Dorsal septal swell body, 6: RAC obstructing main nasal meatus (Oechtering, 2010).
17 In French bulldogs, the concha nasalis ventralis fills the main part of the nasal cavity (it is more capable of forming RACs in this breed), with the concha nasalis media usually being presented further back (right above the entrance to the meatus nasopharyngeus) (Schuenemann & Oechtering, 2014 b).
Although there may be intrinsic factors controlling conchal growth, they also adapt their growth to the airflow, resultant pressure and shear stresses. The dead space on the concave side of a septal deviation causes turbulence in the incoming airflow, leading to a relevant directional conchal growth (Schuenemann & Oechtering, 2014 b). Thus, it is possible that stenotic nares hinder the incoming air streams early in life, leading to loss of physical stresses inside the nasal cavity. It might lead to undirectional growth of conchal cells because of the missing information provided by those physical stresses (Schuenemann & Oechtering, 2014 a).
Early correction of obstructing nares might be able to influence conchal growth and prevent contact points by promoting better airflow and consequently the increase of shear stresses through the nasal cavity. Such surgery would be required prior to the end-point of conchal growth, what means as young as 5 months of age or even before (Schuenemann & Oechtering, 2014 a).
Figure 10: Transverse CT scans of a German shepherd dog nose (left) and of a pug nose
(right), with severe deviation of the nasal septum and RACs present on its concave side. Figure adapted from Oechtering et al. (2007).
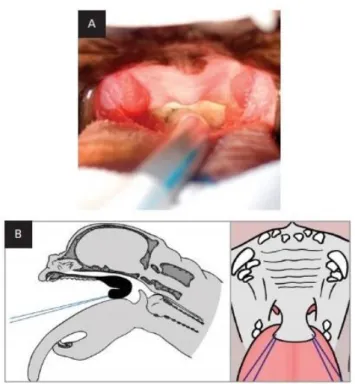
18 In order to correct intranasal
obstruction and reduce intranasal airway resistance, Oechtering et al. (2007) developed a novel-technique, the Laser assisted turbinectomy (LATE), using a diode laser (980 nm) introduced into the nasal cavity through a working channel of a 2,7 mm rigid endoscope (Figure 11). Most cases in which LATE was performed, the concha nasalis
ventralis was totally removed because it occupied the biggest part of the nasal cavity (French bulldogs as an example). In those cases in which the concha nasalis media contributed to the obstruction as a growing rostral aberrant turbinate, usually happening in pugs, it was removed as well (Schuenemann & Oechtering, 2014 b).
Although canine turbinates have the capacity to regrow after the LATE procedure, conchae develop significantly fewer contact points than before and not regularly lead to re-obstruction of the nasal cavity. Significant regrowth occurs in most cases from an intact concha nasalis media and it happens more in French bulldogs than pugs, probably because in most French bulldogs the conchae nasalis media is not removed. However, conchal remnants seen after LATE, and not causing obstruction of the nasal cavity, should not be removed. A certain amount of conchal tissue needs to remain intact for new conchae to grow, and a non-obstructive regrowth of conchae is desirable to fulfill their physiologic functions. Nasal crusting due to empty nose syndrome and atrophic rhinitis caused by the absence of normal nasal structures were reported in humans (Chhabra & Houser, 2009; Payne, 2009). Thereby, radical removal of turbinates should not be performed (Schuenemann & Oechtering, 2014 b). Conchal cells have a lifelong ability, by means of growth, to react to changes in airflow. After resection of the concha nasalis ventralis, airflow through the nasal cavity is turbulent, so that compensatory growth of the remaining turbinates may occur in order to reestablish the laminar airflow again. In the study by Schuenemann and Oechtering (2014b), French bulldogs, whose conchae nasalis ventralis obstructed more frequently the nasal cavity,
Figure 11: LATE surgery: endoscopic view of the diode
19 showed a much stronger conchal regrowth with a higher potential of re-obstruction after LATE than pugs (Schuenemann & Oechtering, 2014 b).
As in early life, correction of obstructed nares concurrently with LATE might be able to influence conchal growth and prevent mucosal contact points by promoting, postoperatively, better airflow and, consequently, the increase of shear stresses through the nasal cavity. It can explain the presence of mucosal contact points due to conchal regrowth in concurrence with re-collapse of nares. Therefore, aggressive correction of stenotic nares should be performed in conjunction with laser assisted turbinectomy (LATE) (Schuenemann & Oechtering, 2014 a; Schuenemann & Oechtering, 2014 b).
2.4.2.2. Caudal berrant conchae
Caudal aberrant conchae (CACs) are defined as parts of the middle or ventral nasal conchae that extend caudally through the choanas into the nasopharyngeal meatus (Figure 12). It causes a significant rise in intranasal airway resistance (Oechtering et al., 2007; Oechtering, 2010).
Figure 12: Sagittal computed tomography scan of a German shepherd skull (left) and a pug
skull (right). Plica alaris (a), plica recta (b), concha nasalis ventralis (c), concha nasalis media (d), concha nasalis dorsalis (e) and endoturbinate III (f) are present. Note the caudal aberrant turbinates of the concha nasalis media growing into the meatus nasopharyngeus in the pug. Figure adapted from Schuenemann & Oechtering (2014 b).
20 In normocephalic dogs, the wing of the vomer and the perpendicular lamellae of the hard palate form the floor of the nasal cavity caudally and separate at this level the nasal cavity from the nasopharynx. The basal lamina of the ethmoid bone forms a transition between the nasal cavity and nasopharynx further rostrally and dorsal to the wing of the vomer, and it is a point of attachment for the middle nasal concha. Otherwise, in brachycephalic dogs, the basal lamina of the ethmoid bone presents further rostrally. In the same way, the point of attachment of the middle nasal concha is also located further rostrally than in normocephalic dogs. Ventral to this point of attachment there is no closure of the floor of the nasal cavity, since it occurred further caudally. Instead, this area is occupied by conchal tissue, which extends freely into the choanae and nasopharynx (Figure 13) (Oechtering et al., 2007).
It has been reported that nasopharyngeal turbinates are highly represented in pugs (Creuzet & Le Douarin, 2005; Ginn et al., 2008). In the study performed by Ginn et al. (2008), 82% of canine cases presenting nasopharyngeal turbinates were pugs. However, the overall incidence of nasopharyngeal turbinates in the brachycephalic population is not truly determined. The reason is that brachycephalic animals included in studies about nasopharyngeal turbinates were those that had signs of BAOS severe enough to justify surgical intervention. There are no studies, with a population large enough, where both clinically normal brachycephalic
Figure 13: Nasopharynx of a brachycephalic dog with nasopharyngeal turbinates protruding on each
21 animals and those affected by BAOS have been submitted to rhinoscopy in order to screen for nasopharyngeal turbinates (Ginn et al., 2008).
2.4.2.3. Mucosal conctact points
Brachycephalic dogs show a high prevalence of intranasal mucosal contact points with the surrounding structures (Schuenemann & Oechtering, 2014 a; Schuenemann & Oechtering, 2014 b). Mucosal contact points in these dogs can be a result of either septal deviation or conchal hypertrophy (Mendonça & Bussoloti, 2005). A mixed conchal hypertrophy (mucosal and underlying bone hypertrophy) is likely to be present in brachycephalic dogs (Schuenemann & Oechtering, 2014 a). Chronic rhinitis of a thickened mucosa with enlarged vessels, often seen in these dogs, may develop secondarily and as a result of impaired nasal airflow thereby reducing mucociliary clearance (Mendonça & Bussoloti, 2005).
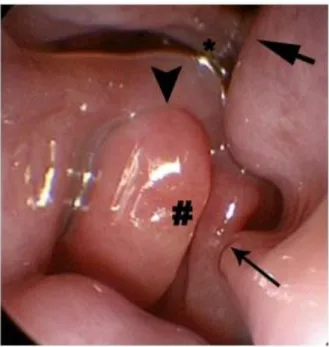
Figure 14: Nasal cavity with extensive mucosal contact. There is contact between the plica recta and
septum (thick arrow), between the lamella of the concha nasalis ventralis (CNV) and the septum (thin
arrow), between the lamellae of the CNV (#), between the CNV lamella and plica alaris (arrowhead), and between parts of the CNV and the plica recta (*) (Schuenemann & Oechtering, 2014 a).
22 French bulldogs show more frequently interlamellar mucosal contact of the concha nasalis ventralis, which is the main contributor to blockage of nasal airflow (Figure 14). There is a higher incidence of mucosal contact points in French bulldogs than in pugs. It is interesting that pugs, which have the shorter noses compared with French bulldogs, have less intranasal mucosal contact points (Schuenemann & Oechtering, 2014 a). However, French bulldogs tend to have thicker mucosal structures, as it happens with the soft palate in this breed. In the same way, it is possible the nasal mucosa to be hypertrophyed in French bulldogs (Grand & Bureau, 2011; Schuenemann & Oechtering, 2014 a). On the other hand, most pugs have a marked plica recta enlargement, often touching the septum (also frequently deviated in pugs) and the plica alaris. It causes obstruction in the nasal vestibule, worsened by RACs from the conchae nasalis media. In spite of that, pugs’ nasal cavities often seem to be less obstructed than those of French bulldogs (Schuenemann & Oechtering, 2014 a).
2.4.2.4. Thermoregulation
Deficiencies in thermoregulation explain why brachycephalic dogs show severe heat intolerance, even without effort, or need extremely long recovery periods after brief physical exercise (Dupré et al., 2013). While humans sweat to evacuate heat from the body, dogs pant. But contrary to common beliefs, dogs do not cool primarily using the surface of their tongue (Oechtering, 2010).
Conchae are extremely branched. The largest one, the ventral nasal concha (concha nasalis ventralis) is covered only by a respiratory epithelium, and has an extremely large, richly vascularized surface of mucous membrane rolled into very fine, space saving, spiral lamellae (Oechtering, 2010; Dupré et al., 2013).
For cooling by evaporation, it also requires arise of water by the excretory duct of the lateral nasal gland (glandula nasalis lateralis or Steno’s gland, located in the caudolateral part of the nose), which extends rostrally and opens laterally into the nasal vestibule. The secretion drips into the gutter-like channel formed by the plica alaris and runs caudally where the plica alaris branches into the concha nasalis ventralis. The liquid drips onto the broad ventral concha and is distributed across the whole surface by the inspired air, being then able to evaporate rapidly in the strong airflow, producing cooling by evaporation (Baker & Chapman,
23 1977). The excellent vascularization of the nasal mucous membranes enables heat to be exchanged rapidly and effectively.
In brachycephalic dogs, the tremendous changes in the conchae architecture mean that it loses its principal organ of thermoregulation. They are no longer capable of evacuate body heat efficiently in an event of physical effort, excitement or even in warm ambient temperatures (Oechtering, 2010).
2.4.3. Elongated and thickened soft palate
The literature on canine brachycephaly emphasizes the elongated soft palate (Wiestner et al., 2007; Dupré et al., 2013). Up to 100% of brachycephalic dog cases have been reported to suffer from elongated soft palate (Wykes, 1991; Poncet et al., 2006; Riecks et al., 2007). Ideally the caudal border of the soft palate and the tip of the epiglottis should just touch each other. Anatomically, the transition from hard to soft palate is caudal to the last molar in dolichocephalic and mesocephalic dogs, whereas it is more caudal in brachycephalic dogs. Therefore, the soft palate can be so elongated or pushed caudally against the maxillary bone that breathing is heavily hindered, leading to flutter audibly during inspiration. It may even be caught dorsal to the epiglottis, near the rima glottides (Figure 15), obstructing and causing edema, inciting suffocation (Koch et al., 2003; Dupré et al., 2013).
Figure 15: Soft palate that extends beyond the tip of the epiglottis and tonsillar crypts (Trappler &
24 In many brachycephalic dogs, it has long been
demonstrated that the soft palate is excessively thick (Figure 16), not only elongated, adding nasopharyngeal and oropharyngeal components to the airway obstruction (Grand & Bureau, 2011; Pichetto et al., 2011). Because of its thickness, it plays an even more significant role in the pathophysiology of the obstruction and leads to a major severity of the clinical signs
(Oechtering et al., 2007a; Oechtering et al., 2007b; Grand & Bureau, 2011; Dupré et al., 2013). The soft palate of a 10 kg brachycephalic dog can be 3 times thicker than the soft palate of a normal 40 kg dog, occupying a considerable space and leading to nasopharyneal obstruction dorsally (Dupré et al., 2013).
Brachycephalic dogs’ soft palates show peculiar features, present since the earliest grade of the respiratory syndrome, such as thickened superficial epithelium, extensive edema of the connective tissue and mucous gland hyperplasia. Moreover, several muscular alterations are evidenced (Pichetto et al., 2011). Similar microscopical findings characterize the soft palate in human patients with obstructive sleep apnea syndrome (OSAS) (Woodson et al., 1991; Hamans et al., 2000; Berger et al., 2002)
From a pathophysiological viewpoint, it is possible that the brachycephalic conformation may worsen the thickness and the stretching of the soft palate due to the higher intranasal airflow resistance (Grand & Bureau, 2011; Trappler & Moore, 2011). Mechanical stress and trauma caused by it can produce changes in epithelial surfaces, resulting in different modifications of the overlying epithelium or underlying connective tissue (Paulsen et al., 2002; Grand & Bureau, 2011). In the same way, a chronic and persistent traumatic insult to the palatine muscles leads to abnormal muscular activity and extensive degenerative lesions involving the majority of muscular fibers, contrasted with the extremely rare focal areas of muscular degeneration present in mesaticephalic dogs (Van der Touw et al., 1994; Arrighi et al., 2011) In humans with obstructive sleep apnea syndrome, obesity has its effect through fat deposition in the soft palate and neck (Horner et al., 1989). However, according to Grand & Bureau (2011), there were no significant differences among absent, minimal and severe BAOS groups
Figure 16: Excessively thickened portion of the
soft palate removed after Folded flap palatoplasty. (Findji & Dupré, 2008).