Clinical trial of lamivudine in children with chronic hepatitis B.
Texto
Imagem

Documentos relacionados
Results: The behavior of blood pressure, heart rate and respiratory rate was similar in both groups, but, at moment 2, the patients in the sufentanil group (Group S) had a
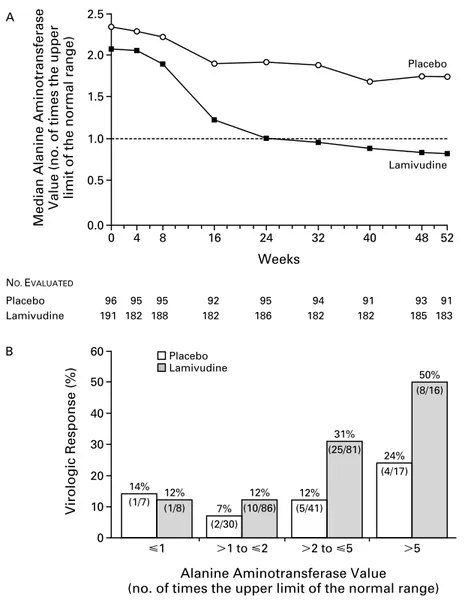
Summing up, two important conclusions arose from our observations: first, patients positive for HBeAg and with a high viral load are less prone to respond to lamivudine as
Given the loss of therapeutic efficacy associated with the development of resistance to lamivudine (LMV) and the availability of new alterna- tive treatments for chronic hepatitis
We also determined the critical strain rate (CSR), understood as the tangent of the inclination angle between the tangent to the crack development curve and the crack development
The goal of this study was to survey the presence of resistant mutations and other substitutions in the NS3 serine protease in patients with chronic hepatitis C who had
We report the case of a male patient with RA and chronic hepatitis B, on lamivudine and tenofovir, who used IFX for 30 months and had no HBV reactivation during the whole
included 38 patients with a median of four previous treat- ments, 87% of whom had received an auto-SCT, the objective overall response rate and complete response rate were 19% and
The m ortality rate of recurring patients was 85.7 percent (12 patients), and the 5-year survival rate was 15.3 percent (2 patients).. The purpose of the present study was the