Effects of sodium chloride supplementation for Pacific white shrimp Penaeus
vannamei diets, cultured in low salinity waters
Efeitos da suplementação do cloreto de sódio em rações para o camarão
branco do Pacífico, Penaeus vannamei, cultivado em águas oligohalinas
DOI:10.34117/bjdv5n12-391
Recebimento dos originais: 30/11/2019 Aceitação para publicação: 27/12/2019
Manuella Gazzineo de Moraes
Mestre pela Universidade Federal do Ceará
Programa de Pós-Graduação em Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: manugazzineom@hotmail.com
Rommel Rocha de Sousa
Doutor pela Universidade Federal do Ceará
Programa de Pós-Graduação em Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: rommelpesca@gmail.com
Carlos Henrique Profírio Marques
Doutor pela Universidade Federal do Ceará
Instituto Federal de Educação, Ciência e Tecnologia do Acre, 69.980-000, Cruzeiro do Sul, Acre, Brasil
E-mail: chnanet@yahoo.com.br
José William Alves da Silva
Doutor pela Universidade Federal do Ceará
Instituto Federal de Educação, Ciência e Tecnologia do Ceará, 62.930-000, Aracati, Ceará, Brasil E-mail: jose.william@ifce.edu.br
Rafael Lustosa Maciel
Doutor pela Universidade Federal do Ceará
Instituto Federal de Educação, Ciência e Tecnologia do Amazonas, 69.800-000, Humaitá, Amazonas, Brasil
E-mail: maciel.rlm@hotmail.com
Ítalo Régis Castelo Branco Rocha
Doutor pela Universidade Federal do Ceará
Instituto Federal de Educação, Ciência e Tecnologia do Ceará, 60.115-282, Morada Nova, Ceará, Brasil
João Felipe Nogueira Matias
Doutor pela Universidade Federal do Ceará
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: jfn.matias@gmail.com
José Renato de Oliveira César
Doutor pela University of Hawaii
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: renatocesarufc@gmail.com
Elenise Gonçalves de Oliveira
Doutora pela Universidade Estadual de São Paulo
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: elenisego@yahoo.com.br
Francisco Hiran Farias Costa
Doutor pela Universidade Federal do Ceará
Departamento de Engenharia de Pesca, Campus do Pici, Universidade Federal do Ceará, 60.455-760, Fortaleza, Ceará, Brasil
E-mail: hiranfcosta@gmail.com
ABSTRACT
The culture of Penaeus vannamei in low-salinity waters is a recent development in Brazilian Northeast. The purpose of this study was to investigate the effects of NaCl dietary supplementation on shrimp growth, survival, haemolymph osmolality and total hemocyte counts. In trial 1, earthen pond-reared shrimp ranging from 5 to 20 g were sampled at intervals for determination of haemolymph osmolality. Haemolymph osmolality was not influenced by the increased weight of shrimp. Juveniles shrimp (5-20 g) haemolymph osmolality were then evaluated 24 h after they had been transferred from pond water (0.5 ppt) to the seawater (35 ppt). Shrimp haemolymph osmolality increased with external salinity and ranged from 661.0 ± 25.2 mOsm/kg (D1) and 916.9 ± 40.1 mOsm/kg (D4). In trial 2, shrimp reared in low-salinity water (0.3-0.5 ppt) were distributed into five groups (control, D1, and treatments, D2, D3, D4 e D5) with four replicates. Diets consisted of the basal diet supplemented with 0 g/kg, 5 g/kg, 10 g/kg, 20 g/kg, and 40 g/kg of NaCl, respectively. After 22 days, differences in survival, final weight, specific growth rates (SGR) and absolute growth rate (AGR) among treatments were significant. However, there were no significant trends in haemolymph osmolality and shrimp total hemocyte counts within the five tested groups.
Keywords: Shrimp, low salinity water, dietary modification, haemolymph osmolality. RESUMO
O cultivo de Penaeus vannamei em águas oligohalinas tem sido um desenvolvimento recente na região Nordeste. O objetivo deste estudo foi investigar os efeitos da suplementação de NaCl em dietas no crescimento, sobrevivência, osmolalidade da hemolinfa e contagem total de hemócitos dos camarões. No experimento 1, camarões cultivados em águas oligohalinas, com peso variando entre 5 a 20 g, foram utilizados para a determinação da osmolalidade da hemolinfa, não tendo sido influenciada pelo aumento do peso do camarão. Após aclimatação em água do mar (35 ppt), a
osmolalidade da hemolinfa foi avaliada em camarões com peso entre 5-20 g, tendo sido incrementada para a salinidade da água do mar (35 ppt), variando de 661,0 ± 25,2 mOsm/kg (D1) a 916,9 ± 40,1 mOsm/kg (D4). No experimento 2, camarões cultivados em águas oligohalinas (0,3-0,5 ppt) foram distribuídos em cinco tratamentos (D1, D2, D3, D4 e D5), com quatro repetições. As dietas consistiram de uma dieta basal suplementada com 0 g/kg, 5 g/kg, 10 g/kg, 20 g/kg e 40 g/kg de NaCl, respectivamente. Após 22 dias, os parâmetros de sobrevivência, peso final, as taxas específicas de crescimento (SGR) e taxa de crescimento absoluto (TCA) apresentaram diferenças significativas entre os tratamentos. No entanto, não houve diferenças significativas na osmolalidade da hemolinfa e contagem total de hemócitos dos camarões distribuídos nos cinco grupos.
Palavras-chave: Camarão, água de baixa salinidade, modificação da dieta, osmolalidade da
hemolinfa.
1 INTRODUCTION
Crustaceans inhabit several aquatic environments including freshwater, brackish water and saltwater. This characteristic requires them continuous haemolymph osmolyte modulation, in order to control osmotic pressure from haemolymph, allowing them to successfully inhabit a particular environment (FREIRE et al., 2008; CHARMANTIER et al., 2009).
The consequences of marine crustaceans culture at low salinities can include low survival, reduced growth and/or high feed conversion rates, which can be attributed to the energy requirements increase in order to maintain a relative haemolymph osmotic homeostasis, due to the increase in active ion transportation (YE et al., 2009).
Most of the existing studies have focused on physiology, and therefore an assessment of the implications for crustacean aquaculture, as well as a discussion of possible approaches that can improve crustacean osmoregulatory capacity should be conducted towards to benefit the aquaculture industry (ROMANO; ZENG, 2012).
Although the best growth rates for P. vannamei juveniles have been observed at salinities above 20 ppt (PONCE-PALAFOX et al., 1997), this species has been cultured in low salinity waters (ATWOOD et al., 2003; SAOUD et al., 2003). P. vannamei is an eurihaline species that can tolerate a broad salinity range (0,5-45 ppt) (BRAY et al., 1994), being capable of growing in salinities below 0.5 ppt (CUVIN-ARALAR et al., 2009).
Under these culture conditions, due to mineral deficiencies in oligohaline waters, it has been postulated that ions shortage at the water-gill interface can be compensated by mineral supplementation (Na, Mg e K) in P. vannamei diets, increasing its availability and absorption in the digestive tract (XIE et al., 2014; ZHOU et al., 2014). Therefore, the general objective of this research was to investigate the effects of diets supplemented with NaCl on juvenile P. vannamei growth performance, in oligohaline waters
2 MATERIALS AND METHODS
This study was carried out at Marine Fish Farming Research Unit (UPPMAR), from the Center for Environmental Coastal Studies (CEAC) of the Institute of Marine Sciences (LABOMAR) at the Federal University of Ceará (UFC) (Eusébio, Ceará, Brazil).
Two batches of P. vannamei juveniles were obtained from a commercial company (Joli Aquicultura Ltda, Russas, Ceará, Brazil), from April to June 2015. At this farm, crops are grown in oligohaline waters with salinity ranging between 0,1 and 0,5 ppt. The specimens were transported in
plastic bags containing approximately 25% water and 75% pure oxygen to
UPPMAR/CEAC/LABOMAR/UFC
The April/2015 batch, called Lot 1 (salinity 0.4 ppt and pH 8.2), with an average weight between 5 and 20 g, was immediately used in experiments to evaluate osmolality. For the June/2015 batch, called Lot 2 (salinity 0.5 ppt and pH 8.1), specimens were stored in 3.000 L tanks equipped with an air diffuser, with a constant freshwater flow (previously adjusted to 28.0 ± 2.5 °C), under natural photoperiod and were fed twice a day (10:00 am and 4:00 pm) with pelleted feed containing 35% crude protein (Malta Cleyton®, Pernambuco, Brazil) at a 2.5% body weight feeding rate. Animals were cultured under these conditions for a 14 days period before experimental use, under a normal feeding pattern during this stage.
The experimental procedure was carried out using 5 diets (D1, D2, D3, D4 and D5), supplemented with different levels of NaCl (in proportions of 0 g, 5 g, 10 g, 20 g and 40 g/kg), generating 5 treatments with 4 repetitions each. Five aquatic recirculation systems (S1, S2, S3, S4 and S5) (SARs) were independently used (one per diet), with four 1.000 L circular tanks in each system. All recirculation systems were connected with a mechanical filter model Dancor® model DFR-11 with an electric motor of 0.25 CV and biological filter (3,000 L, including cascade of oysters and corals) interconnected. For the biological filter colonization of each system, 20 g of the probiotic Sanolife PRO-W (INVE Aquaculture, Inc., Salt Lake City, USA) in a solution containing 200 g of sugarcane and 15 L of freshwater were used, 14 days before the beginning of the experiment. The water flow was maintained at 6.5 L/minute per experimental unit, ensuring an 800% daily renewal.
A total of 500 juveniles shrimp (9.4 ± 0.1 g) were stocked at a density of 25 individuals per tank. The study was conducted during a 22 days period. Animals were fed with pelleted diets (D1, D2, D3, D4 and D5), containing 35% crude protein, at 10:00 and 16:00 h, using 2.5% body weight feeding rate. Tanks were siphoned daily to remove excess food, released carapaces and dead individuals. Animals were sampled (100% from each tank) every 7 days intervals to assess weight growth. After each sampling, feeding was adjusted accordingly to each tank biomass. At the end of the experiment, shrimps were captured and both final survival (%), final weight (g), weight gain (final
weight - initial weight, g), specific growth rate (SGR = (ln final weight - ln initial weight)/days x 100,%/day), and the absolute growth rate (AGR, g/shrimp/week), for each tank and treatment were calculated.
Formulation and chemical composition analysis of diets used in the experiment are shown in Table 1. For all diets (D1, D2, D3, D4 and D5), supplementation with both cholesterol (2 g/kg of feed), probiotic (Sanolife PRO-W, 2 g/kg of feed) and sugarcane molasses (20 g/kg of feed) were made. With the exception of the D1 diet, supplementation of the diets with MgO (8 g/kg of feed) and KCl (5 g/kg of feed) was also performed as described in Gong et al. (2004). Supplementation with NaCl was done in diets D1, D2, D3, D4 and D5 at concentfeeds of 0 g, 5 g, 10 g, 20 g, 40 g/kg of feed, respectively. All diets were mixed, pelleted, dried at room temperature (26-28 °C), and maintained at 20°C for immediate use. Subsequently, samples of the diets were subjected to chemical composition analyses by using a rapid count analyzer NIR (Model XDS monochromator type XM-1000, Foss NIR SDS2500, NIRSystems Inc., Sweden).
Osmolality analysis in Lot 1 was carried out immediately after shrimp specimens arrival in the laboratory, with the removal of the hemolymph from hemocele ventral region, at the beginning of the first abdominal segment, using 1.0 mL syringes and transferred to 1.0 mL micro-centrifuge tube. Each hemolymph sample was centrifuged at 6,000 x g for 15 minutes. Supernatant was transferred to another 1.0 mL micro-centrifuge tube, using an automatic pipette with a disposable plastic tip, and the resulting sample was kept frozen until osmolality analysis. After weight sampling and verification of the molting stage, according to Chan et al. (1998), specimens in the intermolt stage were used to hemolymph sampling.
Water samples from the earthen pond where shrimp were collected from Joli farm (Joli Aquicultura Ltda), were obtained, cooled, transported to UPPMAR and stored for osmolality measurement. Both hemolymph and water samples osmolalities were measured by freezing point depression in a Cryoscopic Osmometer model Osmomat 030 (Gonotec Gmbh Inc., Germany).
To determine shrimp osmotic regulation capacity, animals grown at Joli farm, between 5 and 20 g, were randomly distributed in four 50 L aquariums with 35 ppt water salinity. Dissolved oxygen was maintained above 5 mg/L throughout the experiment. Approximately 10 shrimp were stocked in each aquarium and no food was provided. After 24 h, shrimp hemolymph was collected using the same protocol described above for osmolality analysis.
Osmolality evaluation in Lot 2 followed the same protocol described for Lot 1, and samples were obtained in all experimental tanks at the beginning and at the end of the experiment. Similarly, water samples from all experimental tanks were evaluated for osmolality at the beginning and at the end of the experiment. For each of the tested diets, a 10 g sample was placed in a 50 mL beaker with
10 mL of distilled water, and kept under slight agitation. After 2 hours, a sample of the solution was obtained, by using an automatic pipette with a disposable plastic tip and used for osmolality measurement.
The total hemocyte count (THC) was individually determined using a Neubauer chamber and optical microscope, from direct hemolymph collection in an anticoagulant solution (1: 2) (0.45 M NaCl, 0.1 M glucose, 30 mM sodium citrate, 26 mM citric acid, 10 mM EDTA, pH 4.6) as described by Söderhäll and Smith (1983).
Temperature (oC) and dissolved oxygen (OD, mg/L) were monitored twice a day, at 8:00 am and 5:00 pm with an oximeter (YSI model 55). Physical-chemical parameters of water samples from the 5 experimental systems were analyzed weekly (APHA, 1995) for: alkalinity, hardness, pH, salinity, conductivity, total ammoniacal nitrogen (NHS), nitrite, nitrate, calcium, magnesium and potassium.
All data were expressed as mean ± SD. One-way analysis of variance (ANOVA) was used to determine the effects of stocking density on the water quality and growth performance and Tukey test was used for post hoc comparisons.
3 RESULTS
Results of zootechnical parameters are shown in Table 2. Survival was moderate and significantly (p<0.05) increased by the use of different diets supplemented with NaCl. Both final weight, weight gain, SGR and AGR of P. vannamei juveniles fed diets supplemented with NaCl (D2, D3, D4 and D5) were higher than the values observed for the control diet (D1).
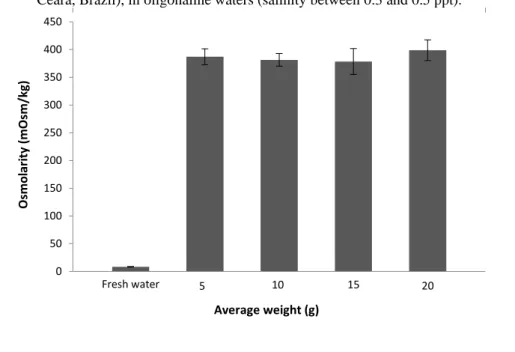
Hemolymph osmolality of P. vannamei specimens in Lot 1, grown at Joli farm, in oligohaline waters (salinity between 0.3 and 0.5 ppt), was not significantly (p>0.05) influenced by weight increase in sampled individuals (Figure 1). After being acclimated to sea water, P. vannamei specimens (Lot 1) showed an increase in hemolymph osmolality, in all sampled weights (between 5 and 20 g), with significant differences (p<0.05) between individuals weighing around 5 g and the other sampled weights (10, 15 and 20 g) (Figure 2).
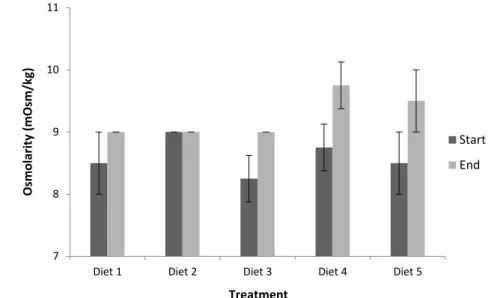
P. vannamei specimens from Lot 2, at the beginning of the experimental procedure, did not show significant differences (p>0.05) between treatments with respect to hemolymph osmolality (382.5 ± 16.0 and 400.8 ± 7.3 mOsm/kg) (Figure 3). However, at the end of the experiment, there was a significant increase (p<0.05) in the hemolymph osmolality of diet D5, which reached a value of 421.3 ± 10.6 mOsm/kg compared to diets D1 (control diet), D2 and D3, whose values were 402.5 ± 6.5, 408.3 ± 4.8, 410.3 ± 1.3 mOsm/kg, respectively. In addition, the hemolymph osmolality of D5
was similar to D4 (418.8 ± 7.8 mOsm/kg). The water culture osmolality, was not significantly different (p>0.05) between treatments (Figure 4), by comparing the beginning and the end of the experimental procedure, demonstrating that there was no ions loss from the diets to the medium. On the other hand, osmolality values were statistically different between diets (p<0.05) among all tested treatments, having increased from 797.3 ± 12.3 mOsm/kg, for the control diet (D1), up to 2,127.3 ± 31.3 mOsm/kg, for D5 (Figure 5).
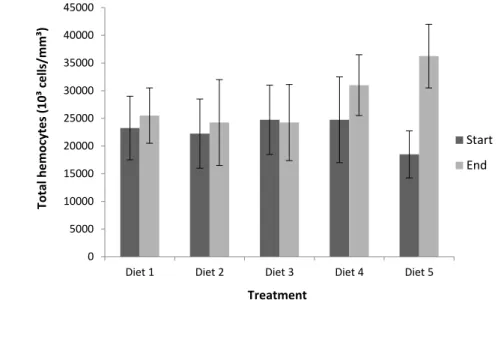
The total hemocyte count in juveniles of P. vannamei was not significantly different (p>0.05) between treatments, from the beginning to the end of the experimental procedure, which may indicate favorable culture conditions (Figure 6).
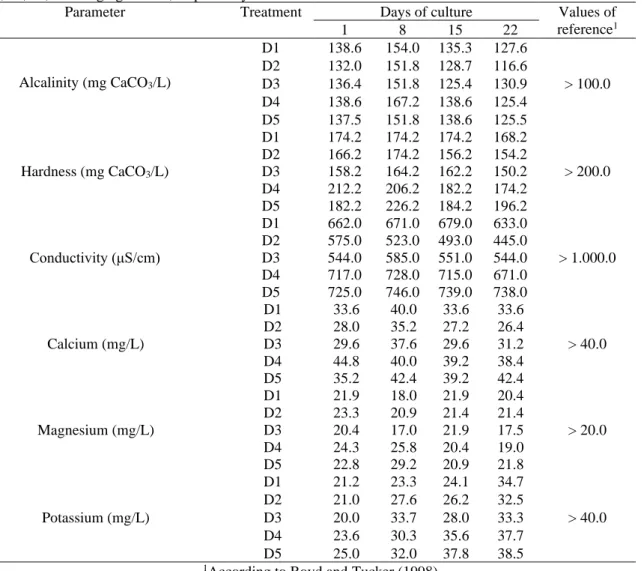
The water characteristics showed no significant changes between the different treatments, although they slightly varied during the experimental period. Daily monitoring in all SARs systems indicated that the water temperature ranged from 27.3 to 31.4 °C (average 29.6 °C), while dissolved oxygen ranged from 4.2 to 5.7 mg/L (average 5.1 mg/L). Salinity varied between 0.3 and 0.5 ppt (average 0.4 ppt) and pH remained between 7.9 and 8.6 (average 8.3). Ammonia (0.00 and 0.24 mg / L), nitrite (0.00 and 0.11 mg/L) and nitrate (0.00 and 0.05 mg/L) levels remained negligible throughout the period experimental for all treatments. The values of alkalinity, hardness, conductivity, calcium, magnesium and potassium are shown in Table 3.
4 DISCUSSION
Salinity is an important abiotic factor that affects metabolism, growth, survival, osmotic capacity, molting frequency and the immune system of penaeid shrimp (XIE et al., 2014). Culture of penaeid shrimp in oligohaline waters can present high mortality rates when individuals reach the juvenile phase, due to mineral deficiencies in these waters (ATWOOD et al., 2003; GONG et al., 2004).
Survival rates ranging from 27.0 ± 1.5% (D1) to 67.0 ± 3.5% (D5), can be explained by the use of oligohaline waters, between 0.3 and 0.5 ppt, which can harm osmotic regulation in P. vannamei, although this species is able to tolerate a wide range of salinities (0.5 to 60 ppt) (ATWOOD et al., 2003; SAOUD et al., 2003).
The D2, D3, D4 and D5 diets showed survival rates significantly different (p<0.05) from the control (D1), indicating the importance of combined NaCl, MgO and KCl supplementation. In this sense, researchers have used the same strategy when modifying diets to improve P. vannamei osmoregulatory capacity, cultured in oligohaline waters (GONG et al., 2004; ROY et al., 2007).
In the present experiment, progressive increases in NaCl supplementation in the diets was not able to increase survival. On the other hand, Zhou et al. (2014) did not observe survival increase when P. vannamei was fed, with diets supplemented with NaCl during 50 days. In that case, survivals ranged between 81.7% for the control diet and 85.8% for the diet with NaCl in the concentfeed of 40 g/kg of feed. Likewise, Roy et al. (2007) found no changes in survival rate when juveniles P. vannamei were fed different supplemented diets containing NaCl, MgO and KCl, compared to the basal diet, cultivated in oligohaline waters (4 ppt), during 49 days .
Final weights ranged between 11.8 ± 0.2 g for the control diet (D1) and 12.7 ± 0.2 g for D5 and were significantly different (p<0.05). However, there were no significant differences (p>0.05) for the final weights between treatments D2, D3, D4 and D5, regardless the NaCl concentration in the diets used. These results are partially similar to those obtained by Zhou et al. (2014). In that case, at the end of P. vannamei culture (initial weight between 4.4 and 4.5 g), final weights of 11.0, 12.6, 13.4 and 14.6 g were obtained, using of diets supplemented with NaCl at concent feeds of both 0, 10, 20, and 40 g/kg of feed, respectively.
Roy et al. (2007) found no significant differences in final weight of P. vannamei juveniles grown in oligohaline waters (4 ppt) when fed with diets supplemented with NaCl at 0, 10 and 20g/kg of feed. Likewise, both cholesterol and soy lecithin supplementation in diets was not able to increase final weights in P. vannamei cultured in oligohaline waters (4 ppt), during 42 days (ROY et al., 2006). The values of weight gain, SGR and AGR obtained in the present study were similar to those observed in other studies with P. vannamei grown in the same weight range (ROY et al., 2006; ROY et al., 2007; XIE et al. , 2014; ZHOU et al., 2014).
Euryhalines aquatic animals are able to perform osmoregulation in their body fluids within a narrow range of osmotic concent feed, while living in environments with wide salinity variation. The life cycle of penaeid shrimps, from the post-larval stage to the juvenile stage, occurs in estuaries and coastal bays, where salinity can vary from 0 to more than 50 ppt. When these shrimp reach the sub-adult stage, the migration to the sea begins, where salinities vary between 30-35 ppt. In this environment, osmotic concent feed decreases with the increase in the size of the animals (PEQUEUX, 1995; GONG et al., 2004; ROY et al., 2006).
The hemolymph osmolality of P. vannamei specimens (Lot 1), in oligohaline waters (salinity between 0.3 and 0.5 ppt), was not significantly (p>0.05) influenced by increase in body weight of the individuals sampled, and the reduction in osmoregulatory capacity has not been demonstrated with the increase in the weight of individuals (GONG et al., 2004). Previous studies have demonstrated a difference of 219 mOsm/kg in hemolymph osmolality, between juveniles and adults of Litopenaeus
stylirostris, maintained at 10.8 ppt, and a difference of 83 mOsm/kg, between juveniles and adults of L. setiferus, maintained at 9.8 ppt sea water (CASTILLE; LAWRENCE, 1981).
In the present study, values found for osmality are below those observed by other authors for P. vannamei (GONG et al., 2004; ROY et al., 2007). Likewise, in a culture of P. latisulcatus, the hemolymph osmolality values ranged from 664 to 1,202 mOsm/kg, for the salinities of 10 and 46 ppt, respectively (SANG; FOTEDAR, 2004). Silva et al. (2010) found hemolymph osmolality values varying between 238 mOsm/kg, for 5 ppt salinity, and 752.5 mOsm/kg, for 45 ppt salinity for Farfantepenaeus subtilis. Similar results were found for breeders of L. stylirostris, with hemolymph osmolality values ranging between 680 and 805 mOsm/kg, when the animals were kept in culture tanks with the medium osmolality ranging between 400 and 1,050 mOsm/kg (WABETE et al., 2006). Gong et al. (2004) analyzing the osmolality of P. vannamei hemolymph in oligohaline waters (2.7 ppt), found increasing values between 500 and 600 mOsm/kg, for individuals weighing between 10.0 and 30.0 g. However, when using a diet supplemented with NaCl (5 g/kg of feed), MgO (8 g/kg of feed) and KCl (5 g/kg of feed) in the diet of shrimp grown in oligohaline waters (5.0 ppt ), the observed values remained in a stable range between 650 and 700 mOsm/kg, regardless of the individuals' weight (10.0 and 30.0 g). Similarly, Roy et al. (2007) found values of 586 and 674 mOsm/kg for shrimps cultured in oligohaline waters (4 ppt), fed with diets supplemented with NaCl, however, there were no significant differences between treatments (10 and 20 g/kg feed) and the control group.
Despite the high concentfeed of osmotically active particles in the diets used, osmolality levels of water used within the treatments did not change, remaining between 9.0 and 9.8 mOsm/kg, indicating that supplementation of NaCl in diets has the potential to provide benefits for aquatic species, without impacting the growing environment (ROY et al., 2007).
The stress caused by captivity can influence several hemato-immunological parameters, including a total and differential hemocyte count, one of the parameters most affected by stress conditions, whether environmental or by infection, or by ecdysis period, as an indicator of the animal's health status (SÁNCHEZ et al., 2001; PERAZZOLO et al., 2002; COSTA; MARTINS, 2009). In this sense, decrease in the total hemocyte count is often related to marine crusts exposed to stress conditions (PERAZZOLO et al., 2002).
In the present study, observed values for the total hemocyte count varied between 18,500 ± 4,250 x 103 cells/mm3 (D5) and 27,500 ± 7,750 x 103 cells/mm3 (D4), at the beginning of the experiment and 24,250 ± 7,750 x 103 cells/mm3 (D5) and 36,250 ± 5,750 x 103 cells/mm3 (D4), at the end, being similar to those found by other authors, investigating P. vannamei (COSTA; MARTINS, 2009), F. paulensis (PERAZZOLO et al., 2002) and L. stylirostris (MUGNIER et al., 2008).
In general, water physical-chemical parameters during the experiment were kept within the tolerance range for most penaeid shrimp species used in aquaculture, indicating that the aquaculture recirculation systems (SARs) employed worked effectively (BOYD; TUCKER, 1998).
During the experimental period, water temperature, dissolved oxygen, salinity, pH, ammonia, nitrite and nitrate are kept within the ideal ranges, as suggested by Boyd and Tucker (1998). For each analyzed period, alkalinity values were maintained above reference value (> 100 mg CaCO3/L),
according to the same authors. Increase in alkalinity levels after 7 days of the experimental period may be related to denitrification processes, as suggested by Ray et al. (2011). Like curve tendencies for hardness values, they are similar to those seen for alkalinity. However, hardness levels are kept below reference value (> 200 mg CaCO3/L) recommended by Boyd and Tucker (1998). The
conductivity values kept below the reference value (> 1,000 μS/cm) suggested by Boyd and Tucker (1998), with the highest values found refer to the D4 diets, between 671 and 728 μS/cm, and D5, between 725 and 746 μS/cm.
Calcium is one of the most important minerals in crustaceans osmoregulation, comprising a significant fraction of the exoskeleton, and low levels of calcium can compromise molting frequency, hemolymph osmolality, carapace mineralization and survival rates (GREENAWAY, 1993). Calcium levels in treatments D1, D2 and D3 remained slightly below the reference value (> 40 mg/L) recommended by Boyd and Tucker (1998), whereas for diets D4 and D5, values observed were around 40 mg/L.
Ions including magnesium (Mg2+) and potassium (K+), has been shown to limit growth and
survival of penaeid shrimp grown in oligohaline waters (SAOUD et al., 2003; DAVIS et al., 2005). Magnesium levels remained slightly above the reference value (> 20 mg/L) recommended by Boyd and Tucker (1998). On the other hand, the potassium concentration, despite having remained below the reference value (> 40 mg/L) recommended by Boyd and Tucker (1998), showed a tendency to increase during the experimental procedure.
5 CONCLUSIONS
The current study presents an analysis of NaCl supplementation in diets used to feed Penaeus vannamei cultured in oligohaline waters. Survival was moderate and increased by the use of different diets supplemented with NaCl, however the progressive increase in NaCl supplementation in the diets was not able to increase survival rates. In addition, there were no differences for final weights in different treatments, regardless the NaCl concentration. At the end of the experiment, there was a
significant increase in the hemolymph osmolality in tratment D5, compared to D1 (control diet), D2 and D3, indicating the importance of combined NaCl, MgO and KCl supplementation.
ACKNOWLEDGEMENTS
We acknowledge Ceará State Foundation for Scientific and Technological Research Support (FUNCAP) who provided M.G. de Moraes with the scholarship for her M.Sc. degree.
Table1 Formulation and proximate composition (g/kg) for the treatments used in the experiment.
Ingredient (g/kg) Treatment
D1 D2 D3 D4 D5
Fish meal 200,0 200,0 200,0 200,0 200,0
Soybean meal 380,0 380,0 380,0 380,0 380,0
Wheat flour 125,0 125,0 125,0 125,0 125,0
Degreased rice bran 108,0 108,0 108,0 108,0 108,0
Maize starch 53,0 35,0 30,0 20,0 0,0
Soy lechtin (70%) 15,0 15,0 15,0 15,0 15,0
Fish oil 50,0 50,0 50,0 50,0 50,0
Vitamin premix1 30,0 30,0 30,0 30,0 30,0
Premix mineral – traces2 10,0 10,0 10,0 10,0 10,0
Cholesterol 2,0 2,0 2,0 2,0 2,0
Binder (inert) 5,0 5,0 5,0 5,0 5,0
Probiotic Sanolife PRO-W3 2,0 2,0 2,0 2,0 2,0
Molasses 20,0 20,0 20,0 20,0 20,0 MgO 0,0 8,0 8,0 8,0 8,0 KCl 0,0 5,0 5,0 5,0 5,0 NaCl 0,0 5,0 10,0 20,0 40,0 Composition (g/kg) Protein 358,0 354,3 352,0 351,1 349,8 Lipids 90,6 93,3 95,1 93,7 94,8 Ashes 96,0 103,6 105,3 108,5 113,3 Moisture 107,5 113,8 113,6 115,4 117,1
1Vitamin A 9,000 UI/kg; vitamin D3 2,700 UI/kg; vitamin E 180 UI/kg; vitamin K3 17 mg; vitamin B1 26 mg; vitamin B2 18 mg/kg; niacin 89 mg/kg; pantotenic acid 53 mg/kg; vitamin B6 17 mg/kg; folic acid 6 mg/kg; biotin 0.30 mg/kg; vitamin B12 90 mcg/kg; vitamin C 1,000 mg/kg. All values are referred as the minimum, according to the
manufacturer. 2Calcium 20 g/kg; phosphorus 13 g/kg; sodium 4,000 mg/kg; cupper 75 mg/kg; manganese 40 mg/kg; zinc 127 mg/kg; iodine 0,30 mg/kg; cobalt 5 mg/kg; selenium 0,30 mg/kg; chrome 0,01 mg/kg. All values are referred as the minimum, according to the manufacturer. 3Bacillus licheniforms (minimum) 1,5x108CFU/kg; Bacillus subtilis (minimum) 1,5x108CFU/kg; Saccharomyces cerevisiae (minimum) 5,0x107CFU/kg; Lactobacillus acidophillus
(minimum) 3,0x107CFU/kg; Bacillus pumilus (minimum) 7x107CFU/kg. All values are referred as the minimum, according to the manufacturer.
Table 2 Survival, weight (initial and final), weight gain, specific growth rate (SGR) and absolute growth rate (AGR) of Penaeus vannamei juveniles fed for 22 days with different ration supplemented with NaCl. Supplementation with NaCl was done in treatments D1, D2, D3, D4 and D5 at 0, 5, 10, 20 and 40 g/kg of feed, respectively.
Parameter Treatment D1 D2 D3 D4 D5 Survival 27.0 ± 1.5a 52.0 ± 2.0b 66.0 ± 4.0c 63.0 ± 1.5c 67.0 ± 3.5c Initial weight (g) 9.4 ± 0.2 9.3 ± 0.1 9.3 ± 0.1 9.4 ± 0.1 9.4 ± 0.1 Final weight (g) 11.8 ± 0.2a 12.5 ± 0.1b 12.5 ± 0.1b 12.6 ± 0.1b 12.7 ± 0.2b Weight gain (g) 2.4 ± 0.3a 3.2 ± 0.1b 3.1 ± 0.2b 3.2 ± 0.2b 3.4 ± 0.1b SGR (%/day) 1.1 ± 0.1a 1.4 ± 0.1b 1.4 ± 0.1b 1.4 ± 0.1b 1.5 ± 0.1b AGR (g/shrimp/week) 0.8 ± 0.1a 1.1 ± 0.1b 1.0 ± 0.1b 1.1 ± 0.1b 1.1 ± 0.1b
Figure 1 Osmolality levels according to weight in Penaeus vannamei juveniles grown at Joli Aquiculture farm (Russas, Ceará, Brazil), in oligohaline waters (salinity between 0.3 and 0.5 ppt).
Figure 2 Osmolality levels according to weight in Penaeus vannamei juveniles grown at Joli Aquiculture farm (Russas, Ceará, Brazil), in oligohaline waters (salinity between 0.3 and 0.5 ppt) acclimated to saltwater (35 ppt).
Fresh water 5 10 15 20 0 50 100 150 200 250 300 350 400 450 O smo lar ity ( mO sm/kg ) Average weight (g) Sea water (35 ppt) 5 10 15 20 0 200 400 600 800 1000 1200 1400 O smo lar ity ( mO sm/kg ) Average weight (g)
Table 3 Water quality parameters in juvenile Penaeus vannamei culture, fed for 22 days with different rations
supplemented with NaCl. Supplementation with NaCl was done in treatments D1, D2, D3, D4 and D5 at concentrations of 0, 5, 10, 20, and 40 g/kg of feed, respectively.
Parameter Treatment Days of culture Values of
reference1 1 8 15 22 Alcalinity (mg CaCO3/L) D1 138.6 154.0 135.3 127.6 > 100.0 D2 132.0 151.8 128.7 116.6 D3 136.4 151.8 125.4 130.9 D4 138.6 167.2 138.6 125.4 D5 137.5 151.8 138.6 125.5 Hardness (mg CaCO3/L) D1 174.2 174.2 174.2 168.2 > 200.0 D2 166.2 174.2 156.2 154.2 D3 158.2 164.2 162.2 150.2 D4 212.2 206.2 182.2 174.2 D5 182.2 226.2 184.2 196.2 Conductivity (μS/cm) D1 662.0 671.0 679.0 633.0 > 1.000.0 D2 575.0 523.0 493.0 445.0 D3 544.0 585.0 551.0 544.0 D4 717.0 728.0 715.0 671.0 D5 725.0 746.0 739.0 738.0 Calcium (mg/L) D1 33.6 40.0 33.6 33.6 > 40.0 D2 28.0 35.2 27.2 26.4 D3 29.6 37.6 29.6 31.2 D4 44.8 40.0 39.2 38.4 D5 35.2 42.4 39.2 42.4 Magnesium (mg/L) D1 21.9 18.0 21.9 20.4 > 20.0 D2 23.3 20.9 21.4 21.4 D3 20.4 17.0 21.9 17.5 D4 24.3 25.8 20.4 19.0 D5 22.8 29.2 20.9 21.8 Potassium (mg/L) D1 21.2 23.3 24.1 34.7 > 40.0 D2 21.0 27.6 26.2 32.5 D3 20.0 33.7 28.0 33.3 D4 23.6 30.3 35.6 37.7 D5 25.0 32.0 37.8 38.5
1According to Boyd and Tucker (1998).
Figure 3 Osmolality levels in juveniles Penaeus vannamei culture, fed for 22 days with different rations supplemented with NaCl, at the beginning and at the end of the experimental procedure. Supplementation with NaCl was done in treatments D1, D2, D3, D4 and D5 at concentrations of 0, 5, 10, 20, and 40 g/kg of feed, respectively.
330 340 350 360 370 380 390 400 410 420 430 440
Diet 1 Diet 2 Diet 3 Diet 4 Diet 5
O smo lar ity ( mO sm/kg ) Treatment Start End
Figure 4 Water osmolality levels in Penaeus vannamei juveniles fed for 22 days with different rations supplemented with NaCl, at the beginning and at the end of the experimental procedure. Supplementation with NaCl was done in treatments D1, D2, D3, D4 and D5 at concentrations of 0, 5, 10, 20, and 40 g/kg of feed, respectively.
Figura 5 Osmolality levels in diets used to feed juveniles Penaeus vannamei, at the beginning and at the end of the experimental procedure. Supplementation with NaCl was done in treatments D1, D2, D3, D4 and D5 at concentrations of 0, 5, 10, 20, and 40 g/kg of feed, respectively.
7 8 9 10 11
Diet 1 Diet 2 Diet 3 Diet 4 Diet 5
O smo lar ity ( mO sm/kg ) Treatment Start End 0 500 1000 1500 2000 2500
Diet 1 Diet 2 Diet 3 Diet 4 Diet 5
O smo lar ity ( mO sm/kg ) Treatment
Figura 6 Total hemocyte count in Penaeus vannamei juveniles fed for 22 days with different rations supplemented with NaCl, at the beginning and at the end of the experimental procedure. Supplementation with NaCl was done in
treatments D1, D2, D3, D4 and D5 at concentrations of 0, 5, 10, 20, and 40 g/kg of feed, respectively.
REFERENCES
APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater. Washington, DC, 1995.
ATWOOD, H. L.; YONG, S. P.; TOMASSO, J. R.; BROWDY, C. L. Survival and growth of Pacific white shrimp Litopenaeus vannamei postlarvae in low-salinity and mixed-salt environments. J. World Aquacult. Soc., v. 34, p. 518-523, 2003.
BOYD, C. E.; TUCKER, C. S. Pond Aquaculture Water Quality Management. Kluwer Academic Publishers, Norwell, MA, 700 p., 1998.
BRAY, W. A.; LAWRENCE, A. L.; LEUNG-TRUJILLO, J. R. The effect of salinity on growth and survival of Penaeus vannamei, with observation on the interaction of IHHN virus and salinity. Aquaculture, v. 122, p. 133–146, 1994.
CASTILLE, F.L.; LAWRENCE, A.L. A comparison of the capabilities of juvenile and adult Penaeus setiferus and Penaeus stylirostris to regulate the osmotic, sodium, and chloride concentfeeds in the hemolymph. Comp. Biochem. Physiol. A, v. 68, p. 677–680, 1981.
CHAN, S. M.; RANKIN, S. M.; KEELEY, L. L. Characterization of the molt stages in Penaeus vannamei: setogenesis and hemolymph levels of total protein, ecdysteroides, and glucose. Biological Bulletin, v. 175, p. 185–192, 1988. 0 5000 10000 15000 20000 25000 30000 35000 40000 45000
Diet 1 Diet 2 Diet 3 Diet 4 Diet 5
To tal h e mo cy te s ( 10³ c e lls/mm³ ) Treatment Start End
CHARMANTIER, G.; CHARMANTIER-DAURES, M.; TOWLE, D. Osmotic and ionic regulation in aquatic arthropods. In: EVANS, D.H. (Ed.), Osmotic and Ionic Regulation. Cells and Animals. CRC Press, Boca Raton, FL, New York, NY, Oxford, UK, pp. 165–230, 2009.
COSTA, A. M.; MARTINS, P. C. C. Analysis of total hemocytes counting and capacity of hemolymph coagulation of shrimp Litopenaeus vannamei (Boone, 1931) in ponds with occurrence of myonecrosis. B. Inst. Pesca, v. 35, p. 545–551, 2009.
CUVIN-ARALAR, M. L. A.; LAZARTIGUE, A. G.; ARALAR, E. V. Cage culture of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) at different stocking densities in a shallow eutrophic lake. Aquaculture Research, v. 40, p. 181–187, 2009.
DAVIS, D. A.; SAOUD, I. P.; BOYD, C. E.; ROUSE, D. B. Effects of potassium, magnesium, and age on growth and survival of Litopenaeus vannamei post-larvae reared in inland low salinity well waters in west Alabama. J. World Aquac. Soc., v. 36, p. 403–406, 2005.
FREIRE, C. A.; AMADO, E. M.; SOUZA, L. R.; VEIGA, M. P. T.; VITULE, J. R. S.; SOUZA, M. M.; PRODOCIMO, V. Muscle water control in crustaceans and fishes as a function of habitat, osmoregulatory capacity, and degree of euryhalinity. Comparative Biochemistry and Physiology, v. 149A, 435–446. 2008.
GONG, H.; JIANG, D. H.; LIGHTNER, D. V.; COLLINS, C.; BROCK, D. A dietary modification approach to improve the osmoregulatory capacity of Litopenaeus vannamei cultured in the Arizona desert. Aquaculture Nutrition, v.10, p. 227–236, 2204.
GREENAWAY, P. Calcium and magnesium balance during molting in land crabs. Journal of Crustacean Biology, v. 13, p. 191–197, 1993.
MUGNIER, C.; ZIPPER, E.; GOARANT, C.; LEMONNIER, H. Combined effect of exposure to ammonia and hypoxia on the blue shrimp Litopenaeus stylirostris survival and physiological response in relation to molt stage. Aquaculture, v. 274, p. 398–407, 2008.
PEQUEUX, A. Osmotic regulation in crustaceans. J. Crustacean Biol., v. 15, p. 1–60, 1995.
PERAZZOLO, L. M.; GARGIONI, R.; OGLIARI, P.; BARRACCO, M. A. Evaluation of some hemato-immunological parameters in the shrimp Farfantepenaeus paulensis submitted to environmental and physiological stress. Aquaculture, v. 214, p. 19–33, 2002.
PONCE-PALAFOX, J.; MARTINEZ, P. C. A.; ROSS, L. G. The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp, Penaeus vannamei, Boone, 1931. Aquaculture, v.157, p. 107-115, 1997.
RAY, A. J.; DILLON, K. S.; LOTZ, J. M. Water quality dynamics and shrimp (Litopenaeus vannamei) production in intensive, mesohaline culture systems with two levels of biofloc management. Aquacultural Engineering, v. 45, p. 127–136, 2011.
ROMANO, N.; ZENG, C. Osmoregulation in decapod crustaceans: implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture, v. 334–337, p. 12–23, 2012.
ROY, L. A.; DAVIS, D. A.; SAOUD, I. P. Effects of lecithin and cholesterol supplementation to practical diets for Litopenaeus vannamei reared in low salinity waters. Aquaculture, v. 257, p. 446– 452, 2006.
ROY, L. A.; DAVIS, D. A.; SAOUD, I. P.; HENRY, R. P. Supplementation of potassium, magnesium and sodium chloride in practical diets for the Pacific white shrimp, Litopenaeus vannamei, reared in low salinity waters. Aquaculture Nutrition, v. 13, p. 104-113, 2007.
SANG, H. M.; FOTEDAR, R. Growth, survival, haemolymph osmolality and organosomatic indices of the western king prawn (Penaeus latisulcatus Kishinouye, 1896) reared at different salinities. Aquaculture, v. 234, p. 601-614, 2004.
SÁNCHEZ, A.; PASCUAL, C.; SÁNCHEZ, A.; VARGAS-ALBORES, F.; Le MOULLAC, G.; ROSAS, C. Hemolymph metabolic variables and immune response in Litopenaeus setiferus adult males: the effect of acclimation. Aquaculture, v. 198, p. 13– 28, 2001.
SAOUD, I. P.; DAVIS, D. A.; ROUSE, D. B. Suitability studies of inland well waters for Litopenaeus vannamei culture. Aquaculture, v. 217, p. 373–383, 2003.
SILVA, E.; CALAZANS, N.; SOARES, M.; SOARES, R.; PEIXOTO, S. Effect of salinity on survival, growth, food consumption and haemolymph osmolality of the pink shrimp Farfantepenaeus subtilis (Pérez-Farfante, 1967). Aquaculture, v. 306, p. 352–356, 2010.
SÖDERHÄLL, K.; SMITH, V. J. Sepafeed of the haemocyte population of Carcinus maenas and other marine decapods and prophenoloxidase distribution. Dev. Comp. Immunol., v. 7, p. 229–239, 1983.
WABETE, N.; CHIM, L.; PHAM, D.; LEMAIRE, P.; MASSABUAU, J. C. A soft technology to improve survival and reproductive performance of Litopenaeus stylirostris by counterbalancing physiological disturbances associated with handling stress. Aquaculture, v. 260, p. 181–193, 2006.
XIE, S. W.; TIAN, L. X.; JIN, Y.; YANG, H. J.; LIANG, G. Y.; LIU, Y. J. Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile Pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture, v. 418-419, p. 159–164, 2014.
YE, L.; JIANG, S.; ZHU, X.; YANG, Q.; WEN, W.; WU, K. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture, v. 290, p. 140–144, 2009.
ZHOU, X. X.; ZHANG, J. Y.; LIU, S. L.; DING, Y. T. Supplementation of sodium chloride in diets to improve the meat quality of Pacific white shrimp, Litopenaeus vannamei, reared in low-salinity water. Aquaculture Research, v. 45, p. 1187–1195, 2014.