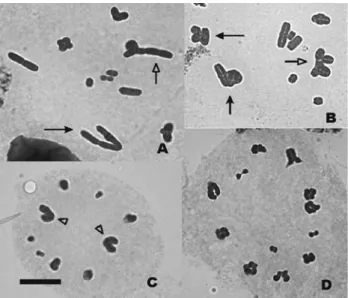
Metaphase I orientation of Robertsonian trivalents in the water-hyacinth
grasshopper,
Cornops aquaticum
(Acrididae, Orthoptera)
Pablo César Colombo
Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales,
Universidad de Buenos Aires, Ciudad Universitaria, Buenos Aires, Argentina.
Abstract
Trivalents resulting from polymorphic Robertsonian rearrangements must have a regular orientation in metaphase I if the polymorphisms are to be maintained. It has been argued that redistribution of proximal and interstitial chiasmata to more distal positions is necessary for a convergent orientation, the only one that produces viable ga-metes.Cornops aquaticum is a South-American grasshopper that lives and feeds on water-hyacinths, and has three polymorphic Robertsonian rearrangements in its southernmost distribution area in Central Argentina and Uruguay. The orientation of trivalents in metaphase I, the formation of abnormal spermatids and the frequency and position of chiasmata in the trivalents, was analysed in a polymorphic population ofC. aquaticus. In this study we observed a correlation between the number of trivalents with the frequency of abnormal spermatids; additionally, the number of chiasmata, especially proximal and interstitial ones, was strongly correlated with the frequency of the linear orienta-tion. Therefore we confirmed our previous assumption, based on other evidence, that the chiasmata redistribution in fusion carriers is essential to the maintenance of the polymorphisms.
Key words: Cornops aquaticum, Robertsonian rearrangements, trivalents, metaphase I orientation.
Received: February 28, 2008; Accepted: August 11, 2008.
Robertsonian translocations (also called centric fu-sions) are frequently involved in the evolutionary diver-gence of plants and animals (Capanna, 1982; Baker and Bickham, 1986; King, 1993). When hybrids are formed be-tween species or chromosomal races differing in one or several Robertsonian rearrangements the behaviour of the resulting trivalent(s) is frequently erratic, leading to linear configurations in metaphase I that cause the formation of imbalanced gametes. These heterozygotes for Robertso-nian rearrangements present negative heterosis, as their fer-tility is reduced due to the non-disjunctional orientation in metaphase I. Robertsonian rearrangements were thus in-cluded in models of chromosomal speciation, as negative heterosis is a necessary step in these models (White, 1978; King, 1993). No polymorphism can be maintained with negative heterosis on fitness (Hedrick, 1983), but Robertsonian rearrangements are frequently polymorphic (Bidau, 1990; Colombo, 1989; Fan and Fox, 1991; Mayret al., 1984; Narain and Fredga, 1998; Pascoeet al., 1996, to mention only a few). In these cases, the depressing effects of structural heterozygosis on the fitness of heterozygotes are suppressed. In fact, in most studied cases of
polymor-phic centric fusions, trivalents showed convergent orienta-tion, linear orientation being the exception to the rule (Bidau and Mirol, 1988; Mirol and Bidau, 1991, 1992).
How do trivalents manage to succeed? One possibil-ity is preadaptation. Robertsonian translocations between similar sized acrocentrics are more likely to do well in metaphase I. Likewise, distal chiasmata increase the proba-bility of convergent orientation, given that too many proxi-mal and/or interstitial chiasmata would spatially hinder the bending of the trivalent at the centromere. However, if chiasma formation is unrestricted, Robertsonian rearrange-ments could trigger proximal and interstitial chiasmata re-distribution to more distal positions. This is actually a frequent feature of centric fusions (Bidau, 1990; Colombo, 1989, 1990, 1993, 2007; Davison and Akeson, 1993; Du-mas and Britton-Davidian, 2002; Hewitt & Schroeter, 1968; see Colombo, 2007, for a discussion).
The water-hyacinth grasshopperCornops aquaticum
Bruner is a New World acridid that lives, feeds and ovipos-its on plants of the genusEichhornia(Adis and Junk 2003, Adiset al.2004). It has been considered for release in Af-rica as a natural control of water-hyacinths (Oberholzer and Hill 2001), which have become a serious water weed (Cen-treet al., 2002).C. aquaticumnatural distribution is be-tween the south of Mexico and east-central Argentina and Uruguay (between 23° N and 35° S). Three polymorphic Robertsonian rearrangements that follow a North-South
cline were found in the southernmost extreme of C.
aquaticumgeographic distribution (Mesa, 1956; Mesa et al., 1982; Colombo, 2008). The chromosomes involved in these centric fusions have their chiasma distribution se-verely affected, shifting from proximal and interstitial to more distal positions (Colombo, 2007). In this work, we analysed the relationship between proximal and interstitial chiasma frequency and linear orientation. We also studied the correlation between non-convergent orientation and the formation of abnormal spermatids.
Twenty-one out of the 27 males used in this study were from two highly polymorphic populations ofCornops aquaticum from Zárate and Baradero, Argentina, on the Paraná River, during the Austral Summers of 2005, 2006 and 2007. Other three males from a mostly monomorphic population ofC. aquaticum(Tigre, close to Buenos Aires) were used and represented the “zero trivalent” class. The males were dissected and their testes were fixed in 3:1 etha-nol:acetic acid. Cytological preparations were made by squashing some follicles of the testis in propionic hae-matoxylin. Metaphase I plates were scored for trivalent chiasma frequency and position and for trivalent orienta-tion (linear or convergent). In order to classify chiasma po-sitioning, the arms of the trivalents were divided in three. Chiasmata located in the proximal third of the chromosome arm were called “proximal” (P), those located in the inter-mediate third were classified as “interstitial” (I) and those present in the distal third were called “distal” (D). Signs of structural tension were taken into account when analysing trivalent orientation. If no tension was observed, the cell was considered to be in prometaphase I and was not com-puted. This consideration is important because the inclu-sion of these cells in the analysis would lead to an overestimation of linear orientation. In order to assess ab-normal spermatids, we counted the number of “centriolar adjuncts” (the root of the future flagellum) establish whether a spermatid was haploid, diploid or polyploid. Sta-tistical tests were performed with the STATISTICA pack-age (StatSoftInc, Tulsa OK, USA).
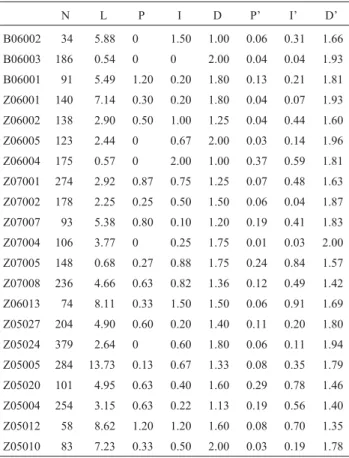
Trivalent orientation can be either convergent or lin-ear (Figures 1A and B). In each of the males we observed if there was any relationship between the frequency of linear orientation and the number and position of chiasmata (Ta-ble 1). Normality of the varia(Ta-ble “percentage of linear ori-entation” was assessed by means of a Kolmogorov-Smirnov test which was not significant (p = 0.09788), thus not ruling out normality. Total chiasma frequency among linearly and among convergently oriented trivalents was compared by means of an ANOVA and differences were highly significant (p = 0.000374). In order to check if chiasma position was relevant for metaphase I orientation, we performed a MANOVA with proximal, interstitial and distal chiasma frequencies as variables and orientation (lin-ear or convergent) as the grouping factor. The differences were highly significant (p = 0.001). As for univariate
ef-fects, differences were highly significant for proximal chiasmata (p = 0.001134) as well as for interstitial ones (p = 0.008164), whereas differences were significant for distal chiasmata (p = 0.024743). When proximal chiasmata were taken into account, the means were 0.4123 for linearly oriented trivalents and 0.1093 for convergently oriented ones. For interstitial chiasmata, the means were 0.7140 for linear trivalents and 0.3565 for convergent ones; and for distal chiasmata, means were 1.5388 for linear trivalents and 1.7247 for convergent ones. It is therefore clear that a high number of chiasmata favours linear orientation (Fig-ures 1A and B, solid arrows). Among these, proximal and interstitial chiasmata mostly precluded convergent orienta-tion, whereas distal chiasmata were associated with conver-gent orientation.
Individuals with one, two and three trivalents and without heterozygous fusions were compared with respect to the formation of diploid or tetraploid spermatids (Table 2). The variable “proportion of abnormal spermatids” did not show a normal distribution. The Kolmogorov-Smirnov test rendered non-significant values (p = 0.1024319) when the variable was transformed to log10, thus showing nor-mal distribution. The regression of “proportion of abnornor-mal spermatids (transformed)” on “number of trivalents” was significant (p = 0.019919). The regression of “percentage of abnormal spermatids (transformed)” on “percentage of linear orientation” was not significant (p = 0.591304).
Cornops aquaticumis not a favourable material for the study of aneuploidy in metaphase II, since cells in these meiotic stage are scarce and the plates are of bad quality
(Figures 1C and D). Only five out of 149 metaphase II plates analysed presented aneuploidy. Anaphase I cells were also rare but their quality was much better. A total of 53 anaphase I cells were analysed and the segregation of trivalents was always normal, with the submetacentric moving towards one pole and the two corresponding acro-centrics migrating towards the other.
Centric fusions are usually accompanied by extensive chiasma repatterning and shifting of chiasma position in acrocentric bivalents to more distal positions in Robertso-nian bivalents or trivalents (Bidau, 1990; Colombo, 1989, 1990, 1993, 2007; Davison and Akeson, 1993; Dumas and Britton-Davidian, 2002; Hewitt and Schroeter, 1968). However, the cause for chiasma redistribution in Robertso-nian bivalents may not be the same as in RobertsoRobertso-nian trivalents. When chromosome races are compared, the race with the lowest diploid number frequently has less (proxi-mal) chiasmata than the race with a higher diploid number (Davison and Akeson, 1993; Dumas and Britton-Davidian, 2002). We attribute this difference to interference across the centromere. In fact, ever since Mathers work (1938), the centromere has been considered a barrier to the operation of interference. Nevertheless, Colombo and Jones (1997)
demonstrated that interference does operate across the centromere in the grasshopper speciesLeptysma argentina
andChorthippus brunneus. The same was demonstrated in humans with an improved statistical method (Broman and Weber, 2000).
Another factor must operate in trivalents. When there is a trivalent, the synaptonemal complex (SC) is interrupted at the level of the centromere. It has been frequently sug-gested that either the operation of interference needs a com-plete SC (Sym and Roeder, 1994) or that SC formation needs the action of interference. Anyway, there is consen-sus that both SC formation and interference are related. It thus follows that interference should not operate across the centromere in trivalents.
It must be pointed out that in trivalents, it is always
polymorphic, and never polytypic or spontaneous centric
Table 1- Metaphase I orientation of trivalents and chiasma frequencyin Cornops aquaticum.
N L P I D P’ I’ D’
B06002 34 5.88 0 1.50 1.00 0.06 0.31 1.66 B06003 186 0.54 0 0 2.00 0.04 0.04 1.93
B06001 91 5.49 1.20 0.20 1.80 0.13 0.21 1.81 Z06001 140 7.14 0.30 0.20 1.80 0.04 0.07 1.93 Z06002 138 2.90 0.50 1.00 1.25 0.04 0.44 1.60
Z06005 123 2.44 0 0.67 2.00 0.03 0.14 1.96 Z06004 175 0.57 0 2.00 1.00 0.37 0.59 1.81
Z07001 274 2.92 0.87 0.75 1.25 0.07 0.48 1.63 Z07002 178 2.25 0.25 0.50 1.50 0.06 0.04 1.87 Z07007 93 5.38 0.80 0.10 1.20 0.19 0.41 1.83
Z07004 106 3.77 0 0.25 1.75 0.01 0.03 2.00 Z07005 148 0.68 0.27 0.88 1.75 0.24 0.84 1.57 Z07008 236 4.66 0.63 0.82 1.36 0.12 0.49 1.42
Z06013 74 8.11 0.33 1.50 1.50 0.06 0.91 1.69 Z05027 204 4.90 0.60 0.20 1.40 0.11 0.20 1.80
Z05024 379 2.64 0 0.60 1.80 0.06 0.11 1.94 Z05005 284 13.73 0.13 0.67 1.33 0.08 0.35 1.79 Z05020 101 4.95 0.63 0.40 1.60 0.29 0.78 1.46
Z05004 254 3.15 0.63 0.22 1.13 0.19 0.56 1.40 Z05012 58 8.62 1.20 1.20 1.60 0.08 0.70 1.35
Z05010 83 7.23 0.33 0.50 2.00 0.03 0.19 1.78
N = Total number of cells; L = percentage of linear orientation; P, I, D = proximal, interstitial and distal frequency of chiasmata in trivalents with linear orientation; P’, I’ D’ = proximal, interstitial and distal fre-quency of chiasmata in trivalents with convergent orientation.
Table 2- Metaphase I orientation of trivalents inCornops aquaticumand formation of abnormal spermatids.
n L S A N
B06002 34 5.88 nd nd 1
B06003 186 0.54 1583 1.08 2
B06001 91 5.49 986 2.60 3 Z06001 140 7.14 1962 1.08 1 Z06002 138 2.90 1059 2.32 1
Z06005 123 2.44 787 6.35 1 Z06004 175 0.57 1389 1.61 1
Z07001 274 2.92 1136 1.97 3 Z07002 178 2.25 2191 0.23 2 Z07007 93 5.38 1196 1.10 1
Z07004 106 3.77 756 3.00 2 Z07005 148 0.68 1093 2.05 2 Z07008 236 4.66 1310 1.71 2
Z06013 74 8.11 nd nd 2
Z05027 204 4.90 nd nd 2
Z05024 379 2.64 nd nd 2
Z05005 284 13.73 661 3.12 2 Z05020 101 4.95 476 5.78 3
Z05004 254 3.15 1387 2.36 3 Z05012 58 8.62 2116 2.17 1
Z05010 83 7.23 1896 0.90 1
Z06003 - - 967 1.63 0
Z06007 - - 962 0.93 0
Z06006 - - 1623 0.67 0
Ti06017 - - 1198 0.92 0
Ti06015 - - 1553 0.39 0
Ti06016 - - 1235 0.64 0
Total 3359 21984
fusions that present chiasma redistribution to more distal positions. Indeed, chiasma frequency and position are usu-ally unchanged in spontaneous centric fusions (Colombo, 1987; López-Fernández et al., 1984; Sannomiya, 1968; Southern, 1967). Concurrently, when studied, more than 36% of metaphase I plates showed linear orientation. (Co-lombo, 1987; Kayano and Nakamura, 1960; López-Fer-nándezet al., 1984; Teoh and Yong, 1983). Bidau et al.
(2001) studied chiasma frequency and distribution in a hy-brid zone of Mus musculus. In this case, the individuals studied were natural hybrids between a race with four fixed Robertsonian rearrangements and another one with all acrocentric chromosomes. The race with four submeta-centric bivalents showed a decreased number of proximal and interstitial chiasmata, as expected, but hybrids showed trivalents withincreasedproximal and interstitial chiasma frequencies. The orientation of trivalents in metaphase I was not studied.
We thus conclude that chiasma repatterning towards more distal positions in the case of Robertsonian trivalents is restricted to polymorphic conditions. The suppression of proximal and interstitial chiasmata is clearly adaptive for the maintenance of the polymorphism, given that proximal and interstital chiasmata are correlated with the frequency of linear orientation and thus with the origin of aneuploid and/or polyploid gametes (Bidau and Mirol, 1988, Mirol and Bidau, 1991, 1992). In this study, the correlation be-tween the proportion of abnormal spermatids and the per-centage of linear orientation was not significant. However, we were unable to correlate the formation ofaneuploid
spermatids and the linear orientation because we only iden-tifiedpolyploidspermatids. Nevertheless, we can assume that linear orientation leads to the formation of aneuploid sperm, even if in some cases reorientation may occur.
Whether or not the reorganisation of chiasma fre-quency and position was triggered by natural selection fa-vouring the maintenance of the polymorphism or by the rearrangement itself is largely a matter of speculation. If the latter is true, only preadapted centric fusions (i.e., those that cause chiasma repatterning) would survive in a polymor-phic state. In either case, the fact that no abnormal anaphase I and very few aneuploid metaphases II had been detected reflects the stability of the polymorphisms herein studied.
Lagging chromosomes or trivalents may result in polyploid spermatids (Johnet al., 1983). We thus studied polyploid spermatids in order to evaluate the degree of in-fertility caused by cytological heterozygosis. This ap-proach can only detect the infertility caused bypolyploid
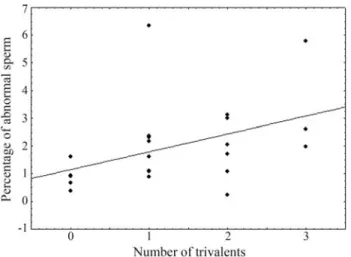
spermatids and that due toaneuploidspermatids should be studied with more sophisticated techniques (Loweet al., 1996). The analysis revealed a correlation between the per-centage of polyploid spermatids and the number of triva-lents (Figure 2), thus confirming the relationship between these two variables. This result coincides with those found by Bidau and Mirol (1988) inDichroplus pratensis. The
percentage of polyploid spermatids was extremely low, corroborating the stability of the polymorphisms herein studied.
Acknowledgments
The author wishes to thank the Club Náutico Zárate and the Prefectura Naval Argentina (Baradero) for the use of their facilities during the collection of specimens. Funding from Universidad de Buenos Aires (X-309/04) through grants to Dr. M.I. Remis is gratefully acknowl-edged.
References
Adis J and Junk W (2003) Feeding impact and bionomics of the grasshopper Cornops aquaticum on the waterhyacinth Eichhornia crassipes in Central Amazonian floodplains. Stud Neotrop Fauna Environ 38:245-249.
Adis J, Lhano M, Hill M, Junk WJ, Marques MI and Oberholzer H (2004) What determines the number of juvenile instars in the tropical grasshopper Cornops aquaticum (Leptysminae, Acrididae, Orthoptera). Stud Neotrop Fauna Environ 39:127-132.
Baker R and Bickham J (1986) Speciation by monobrachial centric fusions. Proc Nat Acad Sci USA 83:8245-8248. Bidau C (1990) The complex Robertsonian system ofDichroplus
pratensis(Melanoplinae, Acrididae). II. Effects of the fu-sion polymorphisms on chiasma frequency and distribution. Heredity 64:145-159.
Bidau C and Mirol P (1988) Orientation and segregation of Robertsonian trivalents in Dichroplus pratensis (Acrididae). Genome 30:947-955.
Bidau C, Giménez M, Palmer C and Searle J (2001) The effects of Robertsonian fusions on chiasma frequency and distribution in the house mouse (Mus musculus domesticus) from a hy-brid zone in northern Scotland. Heredity 87:305.
Börner GV, Kleckner N and Hunter N (2004) Crossover/non-crossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transi-tion of meiosis. Cell 117:29-45.
Broman K and Weber J (2000) Characterization of human cross-over interference. Am J Hum Genet 66:1911-1926. Capanna E (1982) Robertsonian numerical variation in animal
speciation:Mus musculus, an emblematic model. In: Bari-gozzi, C (ed) Mechanisms of Speciation. Alan R. Liss Inc, New York, pp 155-177.
Centre T, Hill M, Cordo H and Julien M (2002) Waterhyacinth. In: Van Driesche R, Blossey B, Hoddle M, Lyon S and Reardon R (eds) Biological Control of Invasive Plants in the Eastern United States. USDA Forest Service Publications, Washington, pp 41-64.
Colombo PC (1987) Effect of centric fusions on chiasma fre-quency and position in Leptysma argentina (Acrididae, Orthoptera). I. Spontaneous and stable polymorphic centric fusions. Genetica 72:171-179.
Colombo P (1989) Chromosome polymorphisms affecting re-combination and exophenotypic traits inLeptysma argen-tina(Orthoptera): A populational survey. Heredity 62:289-299.
Colombo P (1990) Effects of centric fusions on chiasma fre-quency and position in Leptysma argentina (Acrididae, Orthoptera). II. Intra- and interchromosome effects. Caryo-logia 43:131-147.
Colombo P (1993) A polymorphic centric fusion enhances chiasma interference in a grasshopper; a chiasma distribu-tion approach. Heredity 70:254-265.
Colombo P (2007) Effects of polymorphic Robertsonian rear-rangements on the frequency and distribution of chiasmata in the water-hyacinth grasshopper, Cornops aquaticum (Acrididae, Orthoptera). Eur J Entomol 104:653-659. Colombo P (2008). Cytogeography of three parallel Robertsonian
polymorphisms in the water-hyacinth grasshopper,Cornops aquaticum. Eur J Entomol 105:59-64.
Colombo P and Jones G (1997) Chiasma interference is blind to centromeres. Heredity 79:214-227.
Davison M and Akeson E (1993) Recombination suppression by heterozygous Robertsonian chromosomes in the mouse. Ge-netics 133:649-667.
Dumas D and Britton-Davidian J (2002) Chromosomal rearrange-ments and evolution of recombination: Comparison of chiasma distribution patterns in standard and Robertsonian populations of the house mouse. Genetics 162:1355-1366. Fan Z and Fox D (1991) Robertsonian polymorphism in plaice,
Pleuronectes platessaL., and cod,Gadus morhuaL., (Pis-ces Pleuronectiformes and Gadiformes). J Fish Biol 38:635-640.
Hedrick P (1983) Genetics of Populations. Science Books, Bos-ton, 553 pp.
Hewitt G and Schroeter G (1968) Population cytology of the ge-nusOedaleonotus.I. The karyotypic facies ofOedaleonotus enigma.Chromosoma 25:121-140.
John B, Lightfoot D and Weissman D (1983) The meiotic behav-ior of natural F1 hybrids between Trimerotropis suffusa Scudder andT. cyaneipennisBruner (Orthoptera, Oedipo-dinae). Can J Genet Cytol 25:467-477.
Kayano H and Nakamura K (1960) Chiasma studies in structural hybrids. V. Heterozygotes for a centric fusion and a trans-location in Acrida lata. Cytologia 25:476-480.
King M (1993). Species Evolution: The Role of Chromosome Change. Cambridge University Press, Cambridge, 336 pp. López-Fernández C, Rufas J, García de la Vega C and Gosálvez J
(1984) Cytogenetic studies on Chorthippus jucundus
(fisch.) (Orthoptera). III. The meiotic consequences of a spontaneous centric fusion. Genetica 63:3-7.
Lowe S, O’Hogan D, Moore I, Bishop J and Wyrobek A (1996) Aneuploid epididymal sperm detected in chromosomally normal and Robertsonian translocation-bearing mice using a
new three-chromosome FISH method. Chromosoma
105:204-210.
Mather K (1938) Crossing-over. Biol Rev 13:252-292.
Mayr L, Schwelzer D and Geber G. (1984) NOR activity, hetero-chromatin differentiation, and the Robertsonian polymor-phism inSus scrofa.J Hered75:79-80.
Mesa A (1956) Los cromosomas de algunos acridoideos uru-guayos (Orth. Caelifera. Acridoidea). Agros 141:32-45. Mesa A, Ferreira A and Carbonell C (1982) Cariología de los
acrídidos neotropicales: Estado actual de su conocimiento y nuevas contribuciones. Ann Soc Ent Fr 18:507-526. Mirol P and Bidau C (1991) Meiotic behavior of Robertsonian
heterozygotes in populations of Dichroplus pratensis (Acrididae). Genetica 84:171-178.
Mirol P and Bidau C (1992) Proximal chiasmata induce non-dis-junctional orientation of Robertsonian trivalents in a grass-hopper. Heredity 69:268-278.
Narain Y and Fredga K (1998) Spermatogenesis in common shrews,Sorex araneus, from a hybrid zone with extensive Robertsonian polymorphism. Cytogenet Res Genome 80:1-4.
Oberholzer I and Hill M (2001) How safe is the grasshopper Cornops aquaticumfor release on water hyacinth in South Africa? In: Julien M, Hill M, Centre T and Ding Jianqing (eds) Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes. Proceedings of the Second Global Working Group Meeting for the Biological and Integrated Control of Water Hyacinth 102:82-88.
Pascoe P, Patton S, Critcher R and Dixon D (1996) Robertsonian polymorphism in the marine gastropod, Nucella lapillus: Advances in karyology using rDNA loci and NORs. Chromosoma 104:455-460.
Sannomiya M (1968) Relationship between crossing-over and chiasma formation in a translocation heterozygote of Atractomorpha bedeli (Acrididae, Orthoptera). Heredity 40:305-308.
Southern D (1967) Spontaneous chromosome mutations in Tru-xaline grasshoppers. Chromosoma 22:241-257.
Sym M and Roeder G (1994) Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell 79:283-292.
Teoh S and Yong M (1983) A spontaneous fusion heterozygote in the tropical grasshopper,Valanga nigrocornis(Burmeister). Caryologia 36:165-173.
White M (1978) Modes of Speciation. W.H. Freeman and Co., San Francisco, 455 pp.
Associate Editor: Fausto Foresti