The Endocannabinoid System: A New Perspective
for Cardiometabolic Risk Control
Emilio Antonio Francischetti and Virginia Genelhu de Abreu
Hypertension Clinic, Laboratory of Clinical and Experimental Pathophysiology, Rio de Janeiro State University - Rio de Janeiro, RJ - Brazil
Mailing address: emilio Antonio Francischetti •
The prevalence of many cardiovascular risk factors has been significantly reduced in the last forty years1. Therapeutic advance gained from lipid-lowering agents, anti-hypertensive drugs, and anti-diabetic oral agents have acted as invaluable tools to reduce the heavy burden imposed by cardiovascular and metabolic risk factors on public health. Despite this evidence, cardiovascular diseases still remain a major cause of death in many countries in the Western World - whether due to inappropriate control of diseases such as diabetes mellitus and hypertension, and the smoking habit1, or by the emergence of new risk factors such as abdominal obesity2,reduced levels of HDL-C, hypertriglyceridemia and higher proportion of small and dense LDL particles3, all recognized to act as contributory elements for cardiovascular risk as a whole.
Although clinical trials have demonstrated significant reduction in the number of events by the use of highly effective therapeutic regimens, a major residual risk still remains, leaving a considerable number of treated patients vulnerable to cardiovascular and metabolic morbidity4-6. This is particularly alarming for individuals reporting multiple risk factors.
Obesity – especially visceral adiposity – is an ongoing pandemic affecting the populations of both developed and developing countries, as Brazil7,8, in a similar way. In our days, visceral adipose tissue is seen as a potentially diabetogenic, pro-hypertensive, pro-inflammatory, and pro-atherosclerosis endocrine organ9. Changes in the expression and secretion of adipocytokines and inflammatory mediators explain the association of abdominal adiposity to insulin resistance, atherogenic dyslipidemia, and hypertension. These factors are included as syndrome components, in the different definitions, of Metabolic Syndrome — ATP III11, World Health
Organization12 and International Diabetes Federation (IDF)13. Recent studies have identified the molecular basis, the neuronal circuits, and the metabolic pathways involved in food intake regulation. A considerable number of neuropeptides has been characterized in distinctive hypothalamic nuclei as interacting with signals originated at peripheral organs, which suggests that there is a complex network participating not only in appetite and satiety control, but also in energy balance modulation and body constitution14.
The endocannabinoid system is an endogenous signaling system with physiological action on energy homeostasis regulation as well as on lipids and carbo hydrates metabolism15. Endocannabinoid system hyperactivation not only results in body weight increase15 but can also induces dyslipidemic and dysglycemic phenotypes16. A number of clinical and experimental studies have shown that pharmacological
intervention in the system has proven to be a promising therapeutic perspective to control obesity, dyslipidemia, insulin resistance and atherosclerosis17,18.
The endocannabinoid system
History
Cannabis Sativa (marijuana) is the most widely consumed illegal drug in the world as of the 1960’s19. Having been cultivated for over five thousand years for the fibers it provides for materials manufacturing process, Cannabis had been prescribed by the Chinese as from 2600 BC to treat cramps, rheumatic and menstrual pain20. However, not until 1964 was
its active ingredient ∆9-tetrahydrocannabinol(THC) isolated and its chemical structure characterized21. In our days, a considerable number of Cannabis Sativa analogues have been prescribed as antiemetic and appetite stimulants to oncology patients on chemotherapy. Dronabinol – a THC synthetic compound – was approved by the FDA over fifteen years ago as ancillary management for advanced stages of AIDS and cancer patients that develop anorexia and cachexia22-24.
The first cannabinoid receptor was identified25 in 1988. In 1993, that receptor was called CB1, after that same year a second receptor had been characterized and named CB226. Both receptors are coupled to Gi/o proteins and belong to a wide and diverse family of proteins coupled to the cellular membrane. Tissue distribution in those structures explains most of the psychotropic effects of THC accounted to CB127 receptors. The effects of CB2 peripheral receptors are more closely associated to immune response28.
The first cannabinoid receptors endogenous ligands – the endocannabinoids – were isolated in 199229.Presently, anandamide (N-arachidonoyl ethanolamine) and 2-araquido-noil glycerol (2-AG) are the most extensively studied among all endogenous cannabinoids. “Ananda” comes from Sanscrit and means serene happiness or eternal happiness20. Both endocannabinoids are CB1 and CB2 receptors agonists. 2-AG cell and tissue levels are higher than those of anandamide as a result of their higher involvement in different metabolic pathways. Cannabinoid receptors, endocannabinoids, as well as the enzymes that catalyze their biosynthesis and degradation constitute the endocannabinoid system.
CB1 and CB2 Receptors
inhibit) and MAPK (Mitogen-Activated Protein Kinases) – which they stimulate. As for CB1 receptors, specifically, modulation is carried out on voltage activated Ca2+ channels (which they inhibit) and K+ channels (which they stimulate)30. The primary function of those receptors is the transduction of extra cellular stimuli in intracellular signals.
Among the G Protein-coupled membrane receptors, the CB1 are the more abundant so far identified in the central nervous system, although they are also found in peripheral nervous system31.Through their receptors, major actions can be seen by endogenous cannabinoids on central nervous system, such as cognitive and emotional function regulation at neuronal circuits in the cortex, in the hippocampus and amygdala, and in boosting substances effects that lead to chemical dependence in the mesolimbic system: cocaine32, heroin33, anphetamine34 and alcohol35.
Some studies have shown the important role played by the endocannabinoid system in modulating nicotine dependence. In CB1-/- animals, nicotine rewarding effects are abolished36 and the administration of a selective CB1 antagonist – rimonabant – reduces their search for the alkaloid37.
CB2 receptors are located in structures associated to immune system and hematopoiesis modulation. Stimulation of those structures by ∆9-tetrahydrocannabinol results in an immunosuppressant phenotype38.
Formation and inactivation of endocannabinoids. Retrograde Neurotransmission Process
Most endocannabinoids identified so far are by-products of PUFAs –long chain polyunsaturated fatty acids, especifically arachidonic acid (Figure 1). Therefore, anandamide and
Fig. 1 - Most endocannabinoids are long-chain polyunsaturaded fatty acids by-products. Anandamide and 2-arachidonoyl glycerol (2-AG) are produced from the remodeling of phospholipids through pathways that use NAPE-PLD (N-acylphosphatidylethanolamine-seletive phospholipase D) and DAG (Diacylglycerol) lipase synthesis enzymes. They are rapidly metabolized and hydrolized by FAAH (Fatty Acid Amide Hydrolase) and MAG L (Monoacyl Gycerol Lipase) enzymes.
Endocannabinoids have local action and are produced on demand. Adapted from: Di Marzo V et al.52
Endocannabinoids Formation
and Inactivation
N H
O O
P O
O-O
O-R2
R1O
O O
CH O-R3
OH
N H
OH O
O O
CH OH
OH
NAPE-PLD DAG Lipase
Phospholipids-derived Precursors
Endocannabinoids
2-Arachidonoyl glycerol
Anandamide
Phospholipids remodeling
O
OH
H2N OH HO CH
OH
OH
FAAH MAG Lipase
2-AG are formed by phospholipid independent pathways; the synthesis enzymes are N-acylphosphatidylethanolamine-selective phospholipase D (NAPE-PLD) and diacylglycerol lipase (DAG Lipase), respectively39,40.
Although most endocannabinoids act under demand or need, in response both to physiological (neuronal depolarization) and pathological15 stimuli, evidence has shown that their primary activation takes place in some areas in the brain where energy balance is controlled, thus suggesting an ongoing tonus that favors energy intake and storage41.
Both anandamide and 2-AG have their action interrupted by a neuronal reuptake process, followed by their metabolism. This stage seems to take place by mere diffusion and/or through a process facilitated by a carrier protein. Both endocannabinoids are quickly metabolized and hydrolized by FAAH (Fatty Acid Amide Hydrolase)and MAG lipase (Monoacyl Glycerol), respectively, in inactive compounds16,42.
The multiple functions of the
endocannabinoid system
Fig. 2 - 2-AG levels measured directly at the hypothalamus and in limbic
forebrain show different values in food-deprived animals, whose levels are significantly higher when compared to animals being fed. No change was
observed in satiated animals. Adapted from: Kirkham TC et al.55
2
-ar
ac
hi
do
no
yl
g
ly
ce
ro
l
(n
m
ol
/g
ti
ss
ue
w
ei
gh
t)
0
5
10
15
20
Control Eating Satiated Deprived
Hypothalamus Limbig forebrain
*
* *p<0.05
**p<0.01 **
**
relaxation9; 7) antitumoral activity50; 8) neuroprotection against trauma and hypoxia conditions51; 9) food intake modulation due to its effects on the release of peptides and hypothalamic hormones and to their regulation by steroids31. All of those pleiotropic effects have been summarized by Di Marzoand cols52in one statement: “The endocannabinoid system reduces the feeling of pain; controls movement, memory, sleep, and appetite; and is protective.”
The tonic activation of cardiac and vascular CB1 receptors seems to limit blood pressure increase. Recently, Kunos and cols53 have observed that when spontaneously hypertensive rats (SHR) were treated with an anandamide degradation inhibitor their hypertension condition was controlled. Such effect was reversed by the administration of CB1 antagonists. In addition to reducing SHR blood pressure, endocannabinoids inactivation blocking concurrently reduced left ventricle contractile performance, although the same parameters were not shown to have been affected in normal animals53.
Another extremely intriguing observation – this time in regard to CB2 receptors function – is that their immunosuppressant properties would have a beneficial, protective effect on the inflammatory milieu of the atherosclerotic lesions. While working with apoliprotein E receptors knock-out mice fed with a cholesterol-rich diet, Stefens and cols54 have observed significant regression of the atherogenic plaques that are peculiar to that model when the animals were treated with small oral doses of THC. A plausible explanation would be that CB2 receptors expressed in the atherosclerotic lesions, but not in normal arteries, would be activated by THC.
Food Intake Regulation by Endocannabinoids
Central effects of CB1 receptors activation are ultimately reflected on energy balance modulation and appetite control. A body of evidence from experimental studies with obese animals (ob/ob and db/db mice and obese Zuker rats) and normal murine models has shown that: 1) the activation of CB1 receptors by endogenous cannabinoids or THC and the injection of endocannabinoid directly in the hypothalamus or in the mesolimbic region stimulate food intake55,56; 2) in contrast, animals whose CB1 receptors genes had been suppressed (CB1-/-) have lower food intake and exhibit a slim phenotype resistant to diet-induced body weight increase57; 3) under normal conditions, the intake of nutrients reduces endocannabinoids levels in the hypothalamus and in limbic forebrain, while fasting has the opposite effect, significantly increasing them55.
Figure 2 shows that food deprived rats have consistently increased 2-AG tissue levels both in the limbic forebrain and in hypothalamus, which are areas strongly associated to motivation and the pleasure of eating55. The 2-AG levels are shown to decrease when animals are being fed.
In another experiment, when anandamide was administered to mice, food intake increased 44% and was shown to be significantly associated to hypothalamic concentrations of norepinephrine, dopamine, and serotonin58.
In 2003, Cota and cols38 demonstrated that CB
1-/- animals – although slimmer – did not report any change in their locomotor activity, body temperature or energy expenditure when compared to wild akin. Such data show that the decrease
in central orexigenic stimulus, as a result of the absence of the CB1 receptor, explains the differences between those animals rather than the changes in their locomotor activity or their energy expenditure.
The administration of rimonabant – the first CB1 selective antagonist, described in 1994 by Rinaldi-Carmona and cols59 – to diet induced obese mice, led to sustained body weight reduction when compared to control animals. CB1 blocker persistent effects on body weight reduction in these animals – in contrast with transitory decrease in food intake – suggest that other mechanisms would contribute for rimonabant long-lasting effects in addition to caloric intake60 (Figure 3).
These data suggest that the endocannabinoid system controls energy intake at two levels. Firstly, by tonically accentuating and incentivating motivation for food search and intake, possibly from interacting with the mesolimbic pathways (nucleus accumbens) involved in reward mechanisms. Secondly, the system is activated on demand in the hypothalamus – after a short period of food deprivation – to then transiently modulate the levels and/or the action of other orexigenic and anorexigenic mediators for the purpose of appetite induction. The assumption of a dual action in the mesolimbic and hypothalamic regions has been proven by the demonstration that food intake in rodents42 is stimulated when endocannabinoids are injected in those encephalic areas.
In the hypothalamus, endocannabinoid levels changes are inversely correlated to leptin plasma concentrations, the hormone secreted by the adipocyte which plays a key role in food intake and energy expenditure regulation. Leptin reduces endocannabinoid levels in the hypothalamus, similarly to what it does to other orexigenic mediators. Additionally, obese mice genetically defficient in leptin signalling pathways exhibit increased concentrations of endocannabinoids in the hypothalamus57.
Co-expression of CB1 receptors with anorexigenic and orexigenic mediators
Fig. 3 - Effects of rimonabant on food intake (to the left) and obese mice weight (to the right) after fat rich diet intake. It should be pointed out that sustained effects
on drug induced weight loss contrast with lower intake as observed only in the first treatment week. Adapted from: Ravinet Trillou C et al.60 Daily Average of Food Intake
-Fat-rich Diet (FRD)
Vehicle 3 mg/kg rimonabant 10 mg/kg rimonabant Treatment Time (days)
Da
ily
in
ta
ke
(k
ca
l)
0
5
10
15
20
Days
0 8 16 24 32 40
W
ei
gh
t(g
)
26 28 30 32 34 36 38 40 42
0-8
** **
9-16 17-24 25-32 33-40
FRD + 3 mg rimonabantFRD + Vehicle
FRD + 10 mg rimonabant
* * ** ** **
**
** ** ** **
(-18 %)
Standard diet/Vehicle Standard diet/10 mg
have shown that CB1 receptors are co-expressed: 1) at the paraventricular nucleus with the anorexigenic mediator CRH (Corticotropin Release Hormone)61, where the endocannabinoids act in a retrograde way reducing the glutamatergic transmission of pre-synaptic neurons, thus attenuating CRH release62; 2) at hypothalamus lateral nucleus with orexigenic mediator MCH (Melanin-ConcentratingHormone)61; 3) at the arcuate nucleus with CART (Cocaine Amphetamine Regulated Transcript)61 expressing cells; and 4) at ventromedial hypothalamus with prepro-orexin61. The genetic deletion of CB
1 receptors increases CRH expression, thus reflecting the tonic inhibition of that mediator by endocannabinoids63.
A positive and direct correlation can be observed between endocannabinoid system tonus and ghrelin circulating levels after food deprivation. That peptide – secreted by the digestive tract – acts locally and interacts with endocannabinoids at vagal afferent terminations, thus increasing food intake. Those effects are blocked by rimonabant64.
As for the mesolimbic system, there is evidence showing that endocannabinoids would increase the yearning for food while inducing higher dopamine release at the nucleus accumbens or by synergic action with opioids through yet unkonwn mechanisms65.
Another important aspect in satiety control is the relationship between endocannabinoid system and vagal terminations connecting the gastrointestinal tract to the medulla and brainstem nuclei area. Endocannabinoids reduce satiety through their action on the vagus. Such effects may be reversed by the destruction of capsaicin-sensitive vagal terminations that modulate the effects of cholecystokinin on satiety66. On the other hand, cholecystokinin inhibits the expression of CB1 receptors through vagal afferent neurons67.
Those data suggest that reduced endocannabinoid activity may mediate the induction of satiety activity by cholecystokinin. As a counterpart, fasting overcomes satiety by stimulating the secretion of endocannabinoids in the small intestine, thus
releasing vagal CB1 from cholecystokinin inhibition.
Peripheral Effects of CB1 Receptors Activation
The endocannabinoid system plays an effective role in lipogenesis modulation. When stimulated, CB1 receptors increase lipoprotein lipase expression and reduce adiponectin44. As a counterpart, CB1 receptor blocking has resulted in increased expression of adiponectin – both in vitro and in vivo. Adiponectin has a crucial role in reducing the expression of enzymes involved in lipogenesis69,70, with potential major role in atherogenic dyslipidemia and disglycemia. Additionally, the activation of CB1 receptors in hepatocytes is translated into increased de novo synthesis of fatty acids by these cells, as a result of the higher genic expression of the lipogenic transcription factor SREBP-1c (Sterol Regulatory
Element-Binding Protein 1c), as well as of associated enzymes: FAS (Fatty-Acid Synthase) and Acyl-CoA C1 (Acetyl-CoA
Carboxilase-1). Contrarily, CB1-/- mice are resistant to those changes and to the development of hepatic steatosis71.
As for glycemic homeostasis, CB1-/- mice shows lower glucose levels after insulin intraperitoneal administration when feeding a fat-rich diet, as compared with wild animals72. They also exhibited a reduction in leptin and insulin plasma concentrations, thus suggesting higher sensitivity to these two hormones.
It has recently been demonstrated that rimonabant increases oxygen consumption and glucose uptake by the solear muscle of ob/ob73 mice, thus showing the favorable effects of the drug on thermogenesis and insulin sensitivity.
The pathophysiologic consequences
of endocannabinoid system hyperactivity
increasing lipogenesis and reducing energy expenditure42. This is consistent with the concept that increased levels of endocannabionoids – inevitable in the presence of stimuli associated to stress – would act as a strategy in helping superior organisms for the purpose of homeostasis retrieval.
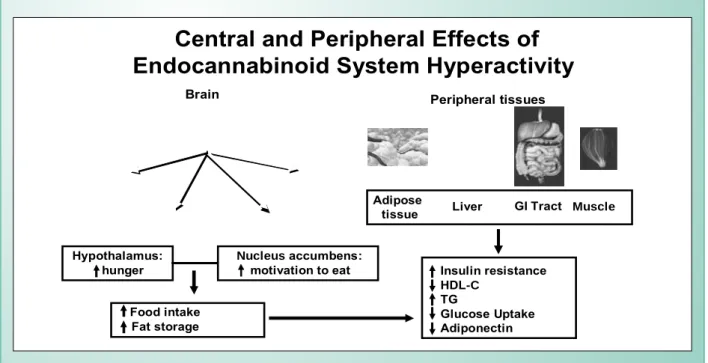
As a counterpart, results from pre-clinical and clinical trials have clearly indicated that the system also contributes for the modulation of conditions accompanied by hyperphagia and adipose mass accumulation, and that its pharmacological blocking would reverse this scenario. Sustained hyperactivity of the system in tissues that control energy balance would, therefore, play a key role not only in the development of obesity but in the emergence of the consortium of cardiometabolic risk factors15 (Figure 4).
The question that must be asked is: Which causal factors would be involved in the changes from a system that acts “on demand” to a sustained hyperactivity system? Apparently, such hyperactivity would be associated to fat-rich diets that make polyunsaturated fatty acids available for the biosynthesis of the endocannabinoids42. Additionally, obese rats’ adipocyte has shown higher expression of CB1 receptor when compared to the adipocyte of slim rats or immature adipocytes70. A fat-rich diet also results in higher anandamide synthesis by the hepatocytes with higher expression of CB1 receptors71
. Against to this scenario, openly pro-orexigenic, significant resistance to leptin anoretic actions would take place57.
A missense polymorphism in homozygote genotype FAAH 385 A/A has recently been identified in overweight and obese individuals, showing potentially inadequate functioning of one of the key enzymes in endocannabinoids74 degradation pathways, which would be an additional explanation for system hyperactivity.
Indeed, the assumption of endocannabinoid system
Fig. 4 - Repercussions of endocannabinoid system hyperactivity at central sites responsible for hunger and motivation for food, as well as in peripheral tissues. Sustained hyperactivity contributes for the development of overweight and the emergence of cardiometabolic risks that are aggregated under the metabolic syndrome
denomination. Adapted from: Di Marzo V, Matias I 15, Pagotto U et al.16
Adipose
tissue Liver GI Tract Muscle
Food intake Fat storage
Insulin resistance
HDL-C
TG
Glucose Uptake Adiponectin Hypothalamus:
hunger Nucleus motivation to eataccumbens:
Brain Peripheral tissues
Central and Peripheral Effects of
Endocannabinoid System Hyperactivity
sustained hyperactivity in certain circumstances has been the
leitmotiv to sponsor the indication of CB1 selective antagonists for the management of obesity and its consequences.
Effects of CB
1receptors selective
blocking on cardiovascular risk factors.
The RIO (
Rimonabant In Obesity
) Program
The blocking of CB1 receptors with selective antagonists, as rimonabant, seems to be a promising perspective for the reduction of cardiovascular diseases that persist even after therapeutic regimens, considered highly effective, have been unfavorable.
Rimonabant pharmacokinetic studies have shown that the drug has rapid oral absorption, with a terminal half-life of 9 days in healthy individuals, and of 16 days in obese individuals. It is metabolized by the CYP3A and amidohydrolase, and eliminated by biliary pathways, with insignificant renal excretion. No rimonabant dosage adjustments are required for mild to moderate renal and hepatic failure patients. Co-administration of rimonabant with food or orlistat showed to be of minimal impact on the drug pharmacokinetics75.
Dyslipidemia rate ranged from 55.7% in the RIO-Diabetes to 100% in the RIO-Lipids. Metabolic syndrome rate ranged from 34.7% in the North America to 79.3% in the RIO-Diabetes. Hypertension percentage ranged from 61.2% in the RIO-Diabetes to 27.2% in the RIO-Lipids. The trials were carried out in the United States, Canada and Europe.
RIO-Lipids trial included 1,033 patients who were overweight or obese, and had non-treated dyslipidemia. Diabetics were excluded. The trial was one-year long17. The RIO-Europe trial included 1,507 overweight or obese individuals, with or without comorbidities. Diabetics were also excluded in this two-year long76 trial. The RIO-North America trial included 3,040 obese or overweight individuals, with or without associated comorbidities. The study also excluded diabetics and had two phases: the first was 12-month long; the second, with patients who had been on rimonabant and randomized to an arm that used placebo and another that kept the same dosing for rimonabant18. The RIO-Diabetes randomized 1,047 patients – all of them overweight, obese, and with type 2 diabetes. This trial last one-year77.
Significant waist circumference (-8,5cm) and body weight (-8,6kg) reduction could be seen after one year in the three studies that were published, with rimonabant, 20mg/ day (Figure 5). Prevention against weight and abdominal circumference regain was evaluated in RIO-North America patients, who were re-randomized to the 20mg/20mg rimonabant arm.
As for rimonabant effect on cardiometabolic risk factors, the following results were observed:
1) HDL-C, triglycerides, small and dense LDL, and LDL-C levels
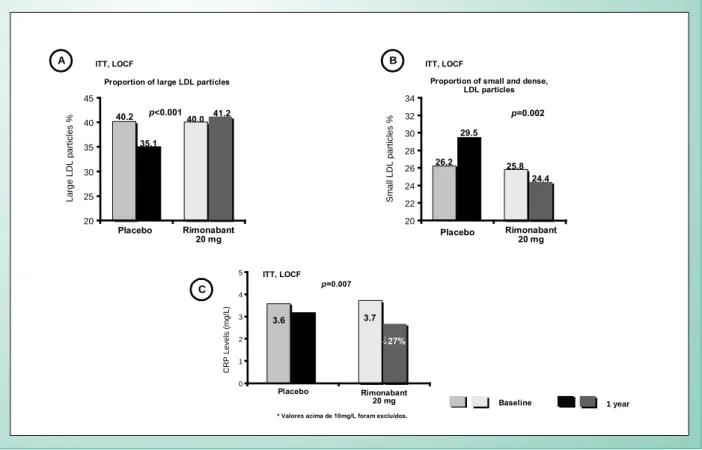
Rio-Europe reported significant changes in triglycerides (-6,8%) and HDL-C (22.3%) concentrations after one year under treatment (vs placebo) in the group on 20mg rimonabant. The changes in those two parameters were very similar in RIO-Lipids and were maintained after two years on the drug in RIO-North America. Rimonabant had no appreciable effect on cholesterol and LDL-C levels in any of the three studies. In RIO-Lipids, there was a significant reduction in the proportion of LDL small and dense particles, in the Rimonabant 20 mg group (Figure 6), when compared to placebo. Logistic regression models and/ or ANCOVA using body weight loss as covariable, showed that after 20mg of rimonabant, both HDL-C and triglycerides reported changes partially independent of weight loss (Figure 7).
2) Changes in glycemic parameters.
An analysis of the three clinical trials that were published characterized a subgroup of pre-diabetic patients (n=1,290) whose glucose fasting levels ranged between equal to or higher than 100mg/dl and lower than 126mg/dl. The results showed that 46.5% of pre-diabetic patients who were administered 20mg/day of rimonabant for one year had fasting glucose levels back to normal values (under 100mg/dl).
As for drug effects on glycosylated hemoglobin values, RIO-Diabetes showed that 43% of patients on 20mg of rimonabant had this parameter reversed to normal values (under 6.5% after one year of treatment, when compared to the placebo group, where that change was reported for 21% of patients).
Significant improvement was also reported in insulin fasting concentration, as well as in insulin resistance calculated by HOMA, when results were compared with the placebo group. After one year of treatment with placebo, and 5mg and 20mg/ day of rimonabant, metabolic syndrome prevalence in those groups was 48.1%, 46.4% and 32.3% (p=0.30 and p<0.001
vs placebo, respectively).
3) RIO-Lipids showed that adinopectin levels increased by 57.7% with the administration of 20mg of rimonabant. That difference was significant when compared to the placebo group (Figure 8). It is important to point out that over 50% of such increase happened irrespective of body weight loss. Additionally, adiponectin levels had a positive and significant correlation with changes in HDL-C and Apo-A1. The same trial showed that leptin levels significantly decreased with the administration of rimonabant - both at 5mg and 20mg regime. Plasma concentrations of C-reactive protein were significantly reduced in the rimonabant group, thus showing favorable drug interference in that inflammatory marker (Figure 6). Systolic and diastolic pressure were significantly reduced (-2.1mmHg and -1.7mmHg, respectively). Hypertensive patients showed a higher reduction.
4) Drug primary efficacy analysis was applied to the ITT (Intention-To-Treat) population and based on LOCF (Last Observation Carried Forward). As general, 20mg dosing of rimonabant was well tolerated, and adverse effects – mild to moderate – were mostly limited to depression episodes [2.9% vs 0.6% (placebo)], anxiety [1.7% vs 0.6% (placebo)] and nausea [1.2% vs 0% (placebo)]. Serious adverse effects that led to study discontinuation were reported by 5.2%, 4.0% and 2.3% among patients who were on 20mg, 5mg and pla-cebo, respectively17, for one year. Patients receiving the same treatment for two years reported comparable discontinuation rate due to adverse effects (4% placebo, 6.3% 5mg, 4.2% 20mg), thus suggesting that they occur early, and that 5mg and 20mg of rimonabant exhibit tolerability and safety profile similar to placebo18.
5) Based on RIO-Program, no relevant interaction was reported between anti- hypertensive agents, vastatins, oral anti-diabetic drugs, fibrates and rimonabant17,18,76,77.
In addition to the clinical trials included in the RIO-Program, other studies are being carried out to investigate whether the improvement in cardiometabolic risk profile by the administration of rimonabant is translated by changes in coronary atherosclerotic plaque volume, as measured by intravascular ultrasound (STRADIVARIUS Study)79. The possible benefits of rimonabant on fatal and non-fatal outcomes, as a result of acute myocardial infarction episodes and stroke, are being evaluated in a prospective, randomized, and controlled trial (CRESCENDO Study), currently in patient recruiting phase.
Fig. 5 - Effects of rimonabant (20 mg/day) on body weight (A) and waist circumference (B) after one year of treatment. Data show patients who have completed the three studies: RIO-Lipids, RIO-North America and RIO-Europe. Adapted from: Després JP et al 17, Pi-Sunyer FX et al 18, Van Gaal LF et al. 75
-10 -8 -6 -4 -2 0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 LOCF
Body weight change (kg)
Weeks
-8.6 kg* -2.8 kg*
-10 -8 -6 -4 -2 0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 LOCF
Waist circumference change (cm)
Weeks
-8.5 cm* -3.9 cm*
RIO-Europe
Placebo
R 20 mg*
RIO-Lipids
Placebo
R 20 mg*
RIO-North America
Placebo
R 20 mg*
A B
Fig. 6 - In the RIO-Lipids, LDL particle distribution showed that the larger ones were found in the group that received 20mg of rimonabant (A), when compared to placebo. This resulted in significant 4.7% reduction in the proportion of small and dense LDL particles (B). Significant reduction was also shown (0.6 mg/L) in ultrasensitive CRP (C). P values refer to the differences between the 20mg rimonabant group vs placebo group. Adapted from: Després JP et al.17
20 22 24 26 28 30 32 34
20 25 30 35 40 45
Small LDL particles %
p=0.002
p<0.001
Large LDL particles %
Proportion of small and dense, LDL particles Proportion of large LDL particles
Placebo Rimonabant 20 mg Placebo Rimonabant
20 mg
1 year
Baseline ITT, LOCF
26.2 29.5
25.8 24.4 40.2
35.1
40.0 41.2
ITT, LOCF
0 1 2 3 4 5
CRP Levels (mg/L)
* Valoresacima de 10mg/L foram excluídos.
Placebo Rimonabant
20 mg
p=0.007
3.6
3.2 3.7
↓27%
ITT, LOCF
A B
C
* Data show patients that completed the 3 studies p<0.001.
References
1. Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA 2005;293:1868-1874.
2. Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among US adults. Obes Res 2003;11:1223-1231.
3. Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation 2004;109:III2-III7.
4. Heart Protection Study Collaborative Group. MRC; BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo controlled trial. Lancet 2002;360:7-22.
5. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events
in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:145-153.
6. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifatorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383-393.
7. Instituto Brasileiro de Geografia e Estatística – IBGE. Pesquisa de orçamentos familiares 2002-2003. Rio de Janeiro 2004, 80p.
8. WHO MONICA. Project Geographical variation in the major risk factors of coronary heart disease in men and women aged 35-64years. World Health Statistics Quaterly 1988;41:115-140.
9. Barroso SG; Abreu VGA, Francischetti EA. A participação do tecido adiposo visceral na gênese da hipertensão e doença cardiovascular aterogênica. Um Data analysis of these clinical trials shows that pharmacological intervention on endocannabinoid system is not only an innovative alternative, but also very promising for the treatment of cardiometabolic risk factors associated to abdominal obesity, and possibly a truly potential tool for the prevention of atherosclerosis and its consequences. It also points towards a 2-year extension period for these effects, while emphasizing that the metabolic profile improvement was partially independent from body weight loss.
The treatment of obesity with drugs that antagonize the CB1 receptors goes beyond body weight loss or merely aesthetic purposes. It is meant for high risk patients, most exhibiting excessive intra-abdominal fat associated to cardiovascular and metabolic risk factors. Actions aiming at changes in lifestyle must always be implemented. Additionally, identifying the dyslipidemic and dysglycemic phenotypes, as mentioned earlier, by measuring abdominal waist circunfrrence, may change the residual risk that is still reported by a significant number of patients.
Fig. 7 - Changes observed in HDL-c concentrations and triglycerides with the administration of rimonabant 20 mg were partially independent of weight loss. Data
from RIO-Europe. Logistic regression models were used, using weight loss as co-variable. Adapted from: Van Gaal LF et al.75
20 mg vsPlacebo
P=0.005
-14 -10 -6 -2
2
6
10
14
Total Triglycerides reduction:
-15.3 %
Total HDL-c
increase: +9.3 %
20 mg vsPlacebo
P=0.005
C
ha
ng
e
%
Triglycerides
HDL-cholesterol
Effect related to weight loss
Effect related to weight loss
-6.7% -6.7% Effect not related to
weight loss -8.6% Effet related to weight loss
Effet related to weight loss
+5.1% +5.1% Effect not related to
weight loss
+4.2%
Fig. 8 - RIO-Lipids data show plasma adiponectin levels increased over 57%
with the administration of rimonabant when compared to baseline levels.
It is relevant that over 55% of such increase was independent of weight loss. Asterisk represents P<0.01 (difference between rimonabant 20mg vs placebo). Adapted from: Després JP et al.17
0 1 2 3 4 5 6 7 8 9
A
di
po
ne
cti
n
le
ve
ls
(u
g/
m
L)
Placebo Rimonabant20 mg
1 year
Baseline
5.7 6.4
5.9 8.1 ↑57.7%
↑57.7%
↑17%
conceito emergente. Arq Bras Cardiol 2002;78:618-630.
10. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548-2556.
11. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Final Report. National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health. NIH Publication No. 02-5215. September 2002.
12. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications, 1999. WHO/NCD/NCS/99.2.
13. International Diabetes Federation, http://www.idf.org.
14. Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661-671.
15. Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 2006;27:73-100.
16. Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosc 2005;8:585-589.
17. Després JP, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005;353:2121-2134.
18. Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients. JAMA 2006;295:761-775.
19. Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction 1996;91:1585-1614.
20. Mechoulam R. in Cannabis as Therapeutic Agent (ed. Mechoulam, R.) 1-19 (CRC Press Roca Ranton, 1986).
21. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 1964; 86:1646–1647.
22. Plasse TE, Gorter RW, Krasnow SH, Lane M, Shepard KV, Wadleigh RG. Recent clinical experience with dronabinol. Pharmacol Biochem Behav 1991;40:695-700.
23. Beal JE, Olson R, Laubenstein I, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Sympton Manage 1995;10:89-97.
24. Volicer I, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 1997;12:913-919.
25. Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 1988:34:605–613.
26. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993;365,61-65.
27. Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol 1999;58:315-348.
28. Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Ther 2001;90:45-60.
29. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992;258:1946-1949.
30. McAllister SD, Glass M. CB1 and CB2 receptor mediated signalling: a focus on endocannabinoids. Prostaglandins Leukot. Essent. Fatty Acids 2002;66:161-171.
31. Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploration. Nature Rev 2004;3:771-784.
32. De Vries TJ, Shaham Y, Homberg JR, et al. A cannabinoid mechanisms in relapse to cocaine seeking. Nat Med 2001;7:1099-1100.
33. Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci 2003;17:1723-1726.
34. Anggadiredja K, Nakamichi M, Hiranita T, et al. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology 2004;29:1470-1478.
35. Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats, following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol 1999;370:233-240.
36. Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology 2002;43:857-867.
37. LeFoll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport 2004;15:2139-2143.
38. Cota D, Marsicano G, Tschop M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 2003;112:423-431.
39. Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamine and its congeners. J Biol Chem 2004;279:5298-5305.
40. Bisogno T, Howell F, Williams G, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 2003;163:463-468.
41. De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. British J Pharmacol 2004;141:765-774.
42. Bisogno T, De Petrocellis L, Di Marzo V. Fatty acid amide hydrolase, an enzyme with many bioactive substrates. Possible therapeutic implications. Curr Pharm Des. 2002;8:533-547.
43. Lutz B. Molecular biology of cannabinoid receptors. Prostaglandins Leukot Essent Fatty Acids 2002;66:123-142.
44. Pagotto U, Marsicano G, Fezza E, et al. Normal human pituitary adenomas express cannabinoid receptor type 1 and synthesize endogenous cannabinoid: first evidence for a direct role of cannabinoids on hormone modulation at the human pituitary level. J Clin Endocrinol Metab 2001;86:2687-2696.
45. Navarro M, Hernandez E, Munoz RM, et al. Acute administration of the CB1 cannabinoid receptor antagonist SR141716A induces anxiety-like responses in the rat. Neuroreport 1997;8:491-496.
46. De Petrocelli L, Melck D, Bisogno T, Di Marzo V. Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem Phys Lipids 2000;108:191-209.
47. Calignano A, Katona I, Desarnaud F, et al. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature 2000;408:96-101.
48. Wagner JA, Jarai Z, Batkai S, Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB1 receptors. Eur J Pharmacol 2001;423:203-210.
49. Wenger T, Ledent C, Csernus V, Gerendal I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem Biophys Res Commun 2001;284:363-368.
50. Bifulco M, Di Marzo V. Targetting the endocannabinoid system in cancer therapy: a call for further research. Nat Med 2002;8:547-550.
51. Panikashvili D, Simeonidou C, Ben Shabat S, Hanus I, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 2001;413:527-531.
52. Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoid: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci 1998;21:521-528.
53. Bátkai S, Pacher P, Osei-Hyiaman D, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004;110:1996-2002.
54. Steffens S, Veillard NR, Arnaud C, et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005;434:782-786.
56. Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 2001;134:1151-1154.
57. Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001;410:822-825.
58. Hao S, Avraham Y, Mechoulam R, Berry EM. Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur J Pharmacol 2000;392:147-156.
59. Rinaldi-Carmona M, Barth F, Heauline M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 1994;350:240-244.
60. Ravinet Trillou C, Arnone M, Delgorge C, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Comp Physiol 2003;284:R345-R353.
61. Pagotto U, Cervino C, Vicennati V, Marsicano G, Lutz B, Pasquali R. How many sites of action for endocannabinoids to control energy metabolism? Int J Obes 2006;30:S39-S43.
62. Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosc 2003;23:4850-4857.
63. Horvath TL. Endocannabinoids and the regulation of body fat: the smoke is clearing. J Clin Invest 2003;112:323-326.
64. Tucci SA, Rogers EK, Korbonits M, Kirkham TC. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol 2004;143:520-533.
65. Kirkham TC, Williams CM. Synergistic effects of opioid and cannabinoid antagonists on food intake. Psychopharmacology 2001;153:267-270.
66. Gomez R, Navarro M, Ferrer B, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosc 2002;22:9612-9617.
67. Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 2004;24:2708-2715.
68. Cota D, Marsicano G, Lutz B, et al. Endogenous cannabinoid system as a modulator of food intake. Int J Obes 2003;27:289-301.
69. Poirier B, Bidouard JP, Cadrouvele C, et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab 2005;7:65-72.
70. Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in culture adipocyte cells. Mol Pharmacol 2003;63:908-914.
71. Osei-Hyiaman D, DePetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005;115:1298-1305.
72. Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistanse to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 2004;28:640-648.
73. Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes Relat Metab Disord 2005;29:183-187.
74. Sipe JC, Waalen J, Gerber A, Beutler E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int J Obes 2005;29:755-759.
75. Clinical Investigator Brochure. Sanofi-Synthelabo Recherche SR141716, Rimonabant. 2004; pp. 35-38.
76. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005;365:1389-1387.
77. Scheen AJ, et al. Oral presentation: American Diabetes Association 65th Annual Scientific Sessions, San Diego, CA, June 12, 2005.
78. Padwal R, Li S, Lau D. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomised controlled trials. Int J Obes Relat Metab Disord 2003;27:1437-1446.
79. STRADIVARIUS (Strategy to Reduce Atherosclerosis Development Involving Administration of Rimonabant - the intravascular ultrasound study). http://www. clinicaltrials.gov / ct/gui/show/ NCT00 124332. Accessed in 5/06/2006.
80. CRESCENDO Comprehensive Rimonabant Evaluation Study of Cardiovascular. ENDpoints and Outcomes. http://www.clinicaltrials.gov/ ct/gui/show/NCT00263042. Accessed em 15/06/2006.