REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
PublicaçãoOficialdaSociedadeBrasileiradeAnestesiologiawww.sba.com.br
SCIENTIFIC
ARTICLE
Isoflurane
provides
neuroprotection
in
neonatal
hypoxic
ischemic
brain
injury
by
suppressing
apoptosis
De-An
Zhao
∗,
Ling-Yun
Bi,
Qian
Huang,
Fang-Min
Zhang,
Zi-Ming
Han
TheFirstAffiliatedHospitalofXinxiangMedicalUniversity,DepartmentofPediatrics,Weihui,China
Received16February2015;accepted22April2015 Availableonline30May2016
KEYWORDS Isoflurane; Hippocampus; Braininjury; Neuroprotection; Apoptosis
Abstract
Backgroundandobjectives: Isoflurane is halogenated volatile ether used for inhalational anesthesia.Itiswidelyusedinclinicsasaninhalationalanesthetic.Neonatalhypoxicischemia injury ensuesintheimmaturebrainthatresultsindelayedcelldeathviaexcitotoxicityand oxidativestress.Isofluranehasshownneuroprotectivepropertiesthatmakeabeneficialbasis ofusingisofluraneinbothcellcultureandanimalmodels,includingvariousmodelsofbrain injury.Weaimedtodeterminetheneuroprotectiveeffectofisofluraneonhypoxicbraininjury andelucidatedtheunderlyingmechanism.
Methods:A hippocampal slice, in artificial cerebrospinal fluid with glucose and oxygen deprivation, was used as anin vitro model for brain hypoxia. The orthodromic population spike and hypoxic injury potential were recorded in theCA1 andCA3 regions. Amino acid neurotransmittersconcentrationinperfusionsolutionofhippocampalsliceswasmeasured.
Results:Isofluranetreatment caused delayedelimination ofpopulationspike andimproved the recovery of population spike; decreased frequency of hypoxic injury potential, post-poned the onset of hypoxic injury potential and increased the duration of hypoxic injury potential. Isoflurane treatment also decreased the hypoxia-induced release of amino acid neurotransmitterssuchasaspartate,glutamateandglycineinducedbyhypoxia,butthelevels of␥-aminobutyricacidwereelevated.Morphologicalstudiesshowedthatisofluranetreatment attenuatededemaofpyramidneuronsintheCA1region.Italsoreducedapoptosisasevident byloweredexpressionofcaspase-3andPARPgenes.
Conclusions: Isofluraneshowedaneuro-protectiveeffectonhippocampalneuroninjuryinduced byhypoxiathroughsuppressionofapoptosis.
©2016SociedadeBrasileiradeAnestesiologia.Publishedby ElsevierEditoraLtda.Thisisan openaccessarticleundertheCCBY-NC-NDlicense( http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗Correspondingauthor.
E-mail:zhaodean17@gmail.com(D-A.Zhao).
http://dx.doi.org/10.1016/j.bjane.2015.04.008
PALAVRAS-CHAVE Isoflurano;
Hipocampo; Lesãocerebral; Neuroprotec¸ão; Apoptose
Isofluranoforneceneuroprotec¸ãoemlesãocerebralhipóxico-isquêmicaneonatal porinibic¸ãodaapoptose
Resumo
Justificativaeobjetivos: Isoflurano é um éter volátil halogenado usado para anestesia por viainalatória.Éamplamenteutilizadonaclínicacomoumanestésicopara inalac¸ão.A lesão hipóxico-isquêmicaneonatalocorrenocérebroimaturoeresultaemmortecelulartardiavia excitotoxicidadeeestresseoxidativo.Isofluranomostroupossuirpropriedadesneuroprotetoras queformamumabasebenéficaparaoseuusotantoemculturadecélulasquantoemmodelos animais,incluindovários modelosde lesãocerebral. Nossoobjetivo foideterminaroefeito neuroprotetordeisofluranoemhipóxiacerebraleelucidaromecanismosubjacente.
Métodos: Fatiasdehipocampo,emfluidocerebrospinalartificial(CSFA)comglicoseeprivac¸ão deoxigênio,foramusadascomoummodeloinvitrodehipóxiacerebral.Opicodepopulac¸ão ortodrômica(PPO)eopotencialdelesãohipóxica(PLH)foramregistradosnasregiõesCA1e CA3.Aconcentrac¸ãodeneurotransmissoresdeaminoácidosnasoluc¸ãodeperfusãodasfatias dehipocampofoimedida.
Resultados: O tratamento com isoflurano retardou a eliminac¸ão do PPO e melhorou a recuperac¸ãodoPPO; diminuiuafrequênciadoPLH,retardou oiníciodoPLHeaumentoua durac¸ão doPLH. Otratamentocomisofluranotambémdiminuiu aliberac¸ão de neurotrans-missoresdeaminoácidosinduzidapelahipóxia,comoaspartato,glutamatoeglicina,masos níveisdeácido␥-aminobutírico(GABA)estavamelevados.Estudosmorfológicosmostramqueo tratamentodeedemacomisofluranoatenuouoedemadeneurôniospiramidaisnaregiãoCA1. Tambémreduziu aapoptose,como mostradopela expressãoreduzida dacaspase-3e genes PARP.
Conclusões:Isoflurano mostrou um efeito neuroprotetor na lesão neuronal no hipocampo induzidaporhipóxiaatravésdasupressãodeapoptose.
©2016SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.Este ´eum artigoOpen Accesssobumalicenc¸aCCBY-NC-ND( http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Hypoxicbraininjuries causeseveralpathologicconditions, whichmay alsooccur during neuro- andcardiac-surgeries and anesthesia. The mechanism underlying such hypoxic braininjuryisstillunclear.Howtoprotectthebrainfrom hypoxicinjuryandhowtotreathypoxicbraininjuryremains clinicallychallenging.Hypothermiaandpre-ischemia treat-mentshave been shown protective effect on the brain1,2
yetdifficulttoimplementclinically,whilepharmacological treatmentclinicallymorepractical.Duringtherecentthree decades,theneuroprotectiveeffectofanestheticdrugshas drawnhighattentionfromclinicians.
Isoflurane[2-chloro-2-[difluoromethoxy)-1; 1.1-trifluoro-ethane, CHF2-O-CHCl-CF3] is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structuralisomer ofenflurane, hencetheyhave thesame empiricalformula. It is a racemic mixture of (R) and (S) optical isomers. Its use in human medicine is now start-ingtodecline,beingreplacedwithsevoflurane,desflurane, andtheintravenous anestheticpropofol.Isofluraneis still frequently used for veterinary anesthesia. Propofol could reduce arterial blood flow in brain, intracranialpressure, andmetabolismmaintainingbloodsupplyandoxygenratio. It improved the oxygen supply during hypoxia suggesting
protective effects of propofol against hypoxic brain damage.3---5 Studies suggest that propofol plays a role
in central nervous system (CNS) protection through the modulation of Ca2+, oxygen free radicals,
␥-aminobutyric
acid (GABA) receptor and N-methyl-d-aspartate (NMDA)
receptor.6---9 Yet some data suggest that propofol had no
brain protective effect after cardiac surgery and even worsened brain hypoxia10 and that hypothermia is
neuro-protective ratherthan propofol.1 The volatile anesthetics
all differ in potency, adverse effects, and cost, and are used extensively during surgery in human neonates and during neonatalanimal research.Isofluranewas reviewed tohaveneuroprotectivefunctionsinstudieswithneonatal hypoxicischemicbraininjury.11Isofluranehasbeenstudied
in animal modelsof variousdiseases, suchas lipopolysac-charide (LPS)-induced acute inflammation of the lung,12
acutelunginjury,13glucose-inducedoxidativestress,14renal
ischemia/reperfusion injury,15 and cardiac injury.16
Isoflu-rane was shown to provide protection from injury and improve various negative functional outcomes in these models.
Neonatalhypoxia ischemiais a majorcauseof mortal-ityandneurologicaldeficitssuchascerebralpalsy,mental retardation, and epilepsy in the perinatal period.17
ananestheticinthesurgeryformodelingneonatalhypoxia ischemiahasledtospeculationsthatisofluranemaybe hav-ingprotectiveeffectsonneonates.Inparticular,isoflurane shows neuroprotection in several stroke models including subarachnoid hemorrhage (SAH),19 middle cerebral artery
occlusion (MCAO),20,21 intracerebral hemorrhage,22
trau-matic brain injury,23 and neonatal hypoxic ischemic brain
injury.24Astudyshowedthat2hexposuretoisofluranedid
notcauseneuroapoptosisinadultbrainsandsuggeststhat delayed reduction in astroglial processes after isoflurane exposure may explain why some volatile anesthetics can conferneuroprotectionafterexperimentalstroke.25
Isofluranehasbeen claimedtobebothneuroprotective andneurotoxicyettheclaimaboutisofluranecausingneural apoptosisremains controversial. In thisstudy, we investi-gatedthe effects ofisoflurane exposuresonhippocampus slicesofratbrainandassessedtheapoptoticfunction.To investigatetheeffectsandmechanismsofisoflurane, neu-rologicalchanges, degreesof neurogenesis, andapoptosis were studied inrats after exposure tovarious concentra-tionsofisoflurane.Theoverallgoalofthisstudyis tofind evidencesdemonstratingthatisofluranetreatmenthas neu-roprotectiveeffectsonbrainduringhypoxia.Similartothe resultsinseveralstudies,wefoundnoevidenceofbraincell deathor neurogenesisin neonatal hypoxic ischemic brain tissuesafterisofluraneanesthesia.
Methods
Experimentalanimals
AlltheanimalprotocolswereapprovedbytheInstitutional AnimalEthicalCommittee.Animalswerehousedand main-tainedunderdefinedprotocolwitha12hlight---darkcycle andtheroomtemperaturewasmaintainedat25±1◦C.Sixty Sprague Dawley (SD) rats at postnatal day (P) 7 (weight, 16---18g) were randomly divided into five groups (n=12). Group(i)controlreviewednormoxiatreatment.Fortyeight SDratsweresubjectedtohypoxiathen distributedin fur-therfour groups:(ii)hypoxia (Hx);(iii) Hx-isoflurane 0.5% treated; (iv) Hx-isoflurane 1.5% treated; (v) Hx-isoflurane 2.5%treated.
Neonatalhypoxia---ischemia
Ratsofgroups(ii---v)wereanesthetizedusing3%halothane followedbya3mmmidlinecervicalincisionasperstandard procedure.Theleftcarotidarterywasexposedand coagu-latedusingabipolarelectrocauteryunitfollowedbyclosure of theincision usingtopicalsurgical adhesive. Aftera 3h recoveryperiod,themicewerethen placedinachamber containing8%O2/92%N2for45min.Thetemperatureinthe chamberwasmaintainedat37◦C.Controlmice(zerotime) wereanesthetizedandunderwentexplorationofleftcarotid arterywithoutcauterization.
Isofluraneexposure
Rats were prepared for isoflurane exposure 18h after hypoxia.Ratswereplacedinplasticcontainersandexposed
toisofluranefor6hcontinuouslyusingairasacarrierwith a total gas flow of 2L/min. During the isoflurane expo-sure,containerswere maintainedto37◦C usingaheating device.The levelsof isoflurane, oxygenand carbon diox-idewere monitored in the chamber using a gas monitor. After6h,isofluraneadministrationwasterminatedandthe ratswereexposedtoairsolely.Afterobservingfree move-mentinrats,theywereplacedbackintothematernalcage. Therespiratory frequency andskin color ofthe ratswere monitoredduringisofluraneexposure.Ratswereobserved for any case of apnea or hypoxia and were immediately exposedtoairandexcluded fromtheexperiment.Ratsin thecontrolgroupwereplacedintosimilarcontainerasthe ratsintheisofluranegroup,butwereexposedtoairalone for6h.
Tissuepreparation
Rats were anesthetized with isoflurane after the recov-ery period. A transcardial perfusion was performed with buffered10%formalinphosphateaccordingtoprevious pro-tocol. The brains were extracted from the skulls through surgical procedure. Brains were stored in buffered 10% formalin for 48h. Then the tissue was frozen-sectioned into30mmslicesusingaLeicaSM2000Rmicrotome(Leica, Germany).Sectionsfromthehippocampalregionwere ran-domlyselectedforhistologicalandotherstudies.
Histopathologicalexamination
The rats exposed to isoflurane as well as control group weresacrificedat6h(n=3).Hippocampalslicesfromeach groupwere fixed in0.1% methanoland embedded inwax afterdehydration. Tissueblockswereslicedin 5mmthick sectionsand stainedwithhematoxylinandeosin (H&E) as perstandardprotocol.Theresultswereexaminedindetail underalightmicroscopesoastodeterminemorphological changesinpartsoftheCA1andCA3regions.
Extracellularpotentialrecording
as the time period when HIP is recorded after oxygen deprivation.HIPdurationis definedashowlongHIPlasts. HIPincidenceisdefinedasthepercentageofhippocampal slicesinwhichHIPwererecordedafteroxygendeprivation.
Highperformanceliquidchromatographic(HPLC)
analysis
Ratbrainhippocampalslicesfromeachgroup(n=3)were incubated in ASCF (NaCl 124Mm; KCl 3.3mM; NaH2PO4 1.24mM; MgSO4 2.4mM; NaHCO3 25.7mM; CaCl2 2.4mM) withandwithoutglucose(10.0mM)at37.5◦Cfor2---2.30h. Theflowingvolumewas200mLmin/L.TheASCFfrom15min ofperfusionfromeachgroupwascollectedandcentrifuged toclearsupernatant.Supernatant wasprocessedforHPLC analysisasperstandard protocolwithnecessary modifica-tions.The mobilephaseA wasSodiumAcetate(0.5M, pH 6.0)with0.05%THF.ThemobilephaseBwasmethanol.The followingwashinggradients(minutes,PhaseB%)wereused: 0, 30%; 7, 60%; and9, 30%; withvelocity of 0.9mL/min, temperature at 35◦C, lem at 330nm and lex at 456nm. DerivationagentusedwasOPA,2-MCE,Boricacid---sodium hydroxideandmethanol(Ph9.6).Derivationagent(25mL) andsample(25mL)wasincubatedfor 2min atroom tem-perature.Injectionvolumewas20mL.Standardcurveswere preparedusing10;5;2.5;1.25 and0.625mMofAsp,Glu, Gln,GlyorGABA(HPLCgrade).Theconcentrations(mM)of Asp,Glu,Gln,GlyandGABAinperfusionASCFwere calcu-latedbasedonstandardcurves.
Realtime-PCR(RT-PCR)
Hippocampalslicesfromeachgroup(n=3)weresnapfrozen at−80◦CinRNAsefreemicrofugetubesatthemomentof dissectionforRT-PCR.TotalRNA wasisolatedfromtissues usingTrizol reagent (Invitrogen, USA) according to manu-facturer’sinstructions.TotalRNA(1g)wasincubatedwith
200ng random hexamers, 0.5mM dNTPs, and RNase-free waterat65◦Cfor10min(allfromInvitrogen).Then,5×first strandbuffer, 5mMDTT,40URNAseA,and200Ureverse Transcriptase(SuperScriptIII)wasadded.Thereaction mix-turewasincubatedatroomtemperaturefor5minfollowed byat 50◦C for 60min,at 70◦Cfor 15min, andat 4◦C for 10min.Real-timequantitativePCRwasperformedusingan ABI7500RealTimePCRSystem(AppliedBiosystems).Assays fortarget cDNA levelswereperformed usingTaqManMGB probeslabeledwithFAMdye(AppliedBiosystems)ina20L
reaction containing 2L of cDNA, ROX passive reference
dyesandTaqMan UniversalPCR mix(Applied Biosystems). The specific primer sets usedand their part number are: Parp1poly(ADP-ribose)polymerase125591Rn00565018mL andCasp3 caspase 3, apoptosis relatedcysteine protease 25402 Rn00563902mL. Relativequantities of both mRNAs wereestablishedbynormalizingtheirlevelstothatof18S inthesamecDNA.
Statisticalanalysis
All values are expressed as mean with standard devi-ations (SD). Data were analyzed using the SPSS v.17
and Systat SigmaPlot 10.0 statistical software. Statis-tical significance was calculated by Student’s t test and one way analysis of variance (ANOVA) for multiple comparisons. p-Values<0.05 were considered statistically significant.
Results
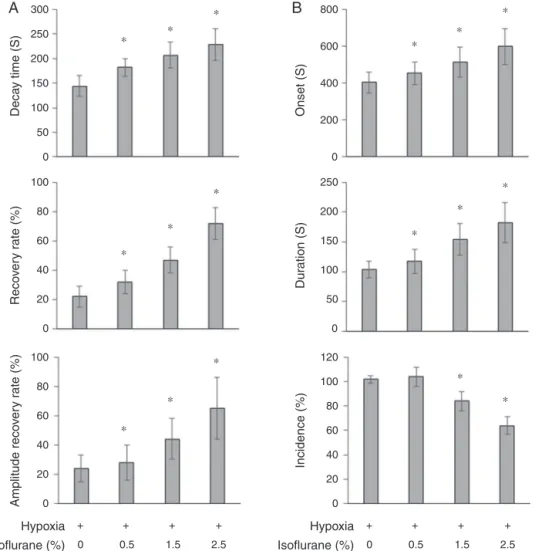
EffectofisofluranetreatmentonOPSandHIPafter
neonatal---ischemiahypoxia
OPSandHIPwererecordedattheCA1region.Inthehypoxia group, theaverage decaytimeof OPS was144±21s; the ratioofOPSrecoverywas22%andtheamplitudeof recov-erywas24±9%.In1.5and2.5%isofluraneexposuregroups, the decay time of OPS was significantly prolonged and the ratio of OPS recovery and the amplitude of recovery were increased as compared with that of hypoxia group. No significant changes of the decay time, recovery ratio andamplitudeofOPSwerefound in0.5%isoflurane expo-sure group (Fig. 1A). In the hypoxic group, the average onsettime ofHIP was403±56s; theaverage duration of HIPwas104±14sandtheincidenceofHIPisapproximate 100%.The incidenceofHIPwasreduced;theonsetof HIP was postponed and the duration of HIP was extended in 1.5and2.5%isofluraneexposuregroupsascomparedwith that of hypoxic group. The above parameters were sta-tistically not significant in 0.5%isoflurane exposure group (Fig.1B).
Effectofisofluraneexposureonreleaseofamino
acidneurotransmitters
300
A
B
∗
∗
∗ ∗
∗ ∗
∗ ∗
∗
∗
∗ ∗
∗
∗ ∗ ∗
∗
250
200
Deca
y time (S)
Onset (S)
Reco
ver
y r
ate (%)
Dur
ation (S)
Amplitude reco
ver
y r
ate (%)
Incidence (%)
150
100
50
100
80
60
40
20
0
250
200
150
100
50
0
100
80
60
40
20
0
120
100
80
60
40
20
0
+
0 +
0.5 +
1.5 +
2.5 Isoflurane (%)
0
800
600
400
200
0
Hypoxia +
0 +
0.5 +
1.5 +
2.5 Isoflurane (%)
Hypoxia
Figure1 TheeffectofisofluraneonOPSandHIP.Decaytime(s),recoveryrate(%)andamplituderecovery(%)forOPS(A);onset (s),duration(s)andincidence(%)forHIP(B)wereestimatedasinMaterialsandMethods(n=3;*p<0.05vs.hypoxicgroup).
Isofluraneimprovesmorphologicalchangesin
hypoxichippocampalslice
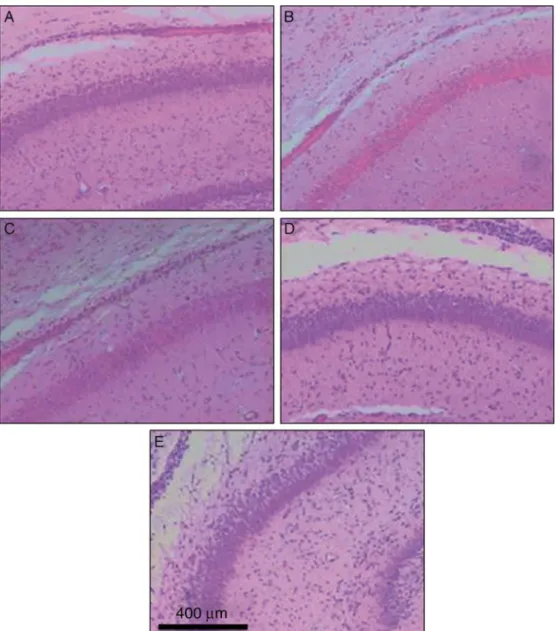
The histological studies sing HE staining (Fig. 2) showed thatpyramidneuronsintheCA1regionofthehippocampus became swollen. The boundaries of cells become blurred andthe nuclei weredark andshrunken. HEdata suggests that celldamage occurred in thehypoxic group. Further-more,isofluranetreatmentamelioratedthosemorphologic changes of pyramid neurons induced by hypoxia in dose-dependentmanner.
Isofluranesuppressedneonatalhypoxiaischemia
inducedapoptosis
We quantitated levels of caspase-3 and PARP mRNA from thehippocampususingRT-PCR.Relativequantitiesofboth mRNAswereestablishedbynormalizingtheirlevelstothat of18SinthesamecDNA.Thelogexpressionlevelsofgenes wereexpressedinterms offold-changecomparedto con-trolanimals.Fig.3showsthatcaspase-3mRNAwaselevated about2-foldafterneonatalhypoxiaischemiaascompared withnormoxia control. Increases in caspase-3 mRNA is a
Table1 Theeffectofisofluraneonreleaseofaminoacidsneurotransmittersfromhippocampalslices(pmoL/L).
Group Asp Glu Gln Gly GABA
Control 2.03±0.12 1.85±0.12 0.77±0.05 1.05±0.07 1.79±0.08 Hypoxia(Hx) 2.68±0.14a 2.35±0.09a 0.76±0.05 1.87±0.04a 2.09±0.06a Hx-ISF0.5% 2.61±0.15a 2.42±0.11a 0.75±0.04 1.78±0.07a 2.21±0.09a,b Hx-ISF1.5% 2.44±0.13a,b 2.01±0.14a,b 0.71±0.05 1.65±0.09a 2.43±0.12a,b Hx-ISF2.5% 2.41±0.14a,b 1.92±0.12b 0.72±0.04 1.57±0.07a,b 2.55±0.14a,b
ISF,isoflurane.
Figure2 HEstainingofhippocampalslices.(A)normoxiacontrol;(B)hypoxia(Hx);(C)Hx-isoflurane0.5%;(D)Hx-isoflurane1.5%; (E)Hx-isoflurane2.5%.HEstainingwasperformedasdescribedinMaterialandMethods(n=3).
directevidenceofapoptosisinductioninhippocampalslices. Apoptosisinductionrequiresactivationofcaspase-3,which cleavesnuclearsubstratePARP.AnalysisofPARPmRNAshows thatitslevelincreased1.86-foldafterhypoxiaascompared to normoxia control. Increases in PARP mRNA confirmed theapoptosisinductioninhippocampalslices.Furthermore, isofluraneexposuretothesetissuesshoweddose-dependent decrease in the expression levels of both genes. Isoflu-rane decreased the levels of caspase-3 and PARP mRNAs withstatistical significance. These results show that apo-ptosisinduced byneonatalhypoxiaischemiaissuppressed byisofluraneexposure.
Discussion
Thisstudyinvestigatedtheeffectsofthevolatileanesthetic isoflurane on possible mechanisms of anesthetic-induced neuroprotection and neurotoxicity in hippocampus of rat
brain under hypoxia ischemia. We mainly observed that isofluranedoesnotalterneurogenesis,nordoesitcause neu-ronalcelldeath,invariousformationsofthehippocampus. Ourfindings demonstratethat isofluranecauses neuropro-tectionbysuppressingapoptosisinducedbyhypoxia.
2.5
A
B
Caspase-3
PARP ∗
∗ ∗#
∗# ∗#
∗# ∗#
∗#
1.5
F
old change
F
old change
1
0.5
0 2
2.5
1.5
1
0.5
Hipoxia
Isoflurane (%) 0
–
0 0 0.5 1.5 2.5
+ + + +
2
Figure3 Quantitative real time-PCRfor caspase-3 (A) and PARP (B). Total RNA were isolated from hippocampul slices (n=3).ThecDNAwassynthesizedandqRT-PCRwasperformed asdescribedinMaterialsandMethods.
thedecaytimeofOPSafterhypoxiacanbeusedasa param-eter toevaluatetolerance ofneurons. The hypoxicinjury potential (HIP) is an indicator of the irreversible hypoxic damageinduced in hippocampalslices.27,28 The onset and
duration of HIP is correlated with hypoxic damage. The longerHIP appearancecorrelateswitheasier recoveryfor hippocampalsliceafterrestorationofoxygensupply.Ifonset of HIP is postponed or extended, neuron damage will be delayed.
Weinvestigatedhowisofluraneaffectstheevoked poten-tialsof hypoxichippocampal slice.In hypoxic groups, the average decay time of OPS was about 144s, the recov-ery ratio of OPS and amplitude of OPS were both small after restoration of oxygen and glucose supply (Fig. 1A). It indicated the hypoxic damage of synaptic transmission betweenhippocampalneurons.Theexposuretoisoflurane caused dose-dependent increase in recovery ratio of OPS andamplitudeofOPS.Itsuggeststhatisofluranetreatment increasedthehypoxictolerance ofthehippocampalslice. The delayofHIP onset,theextensionof HIPdurationand decreased incidence of HIP in different dosage of isoflu-ranealsodemonstratedthatisofluraneexposurepostpones theoccurrenceofirreversiblehypoxicdamageandincreases durationofreversibledamage.Collectively,itsuggeststhat
isofluranetreatmentreducesthehypoxicdamageofneurons andprotectsthesynapticfunctionsfromhypoxicdamage.
The protective effect of isoflurane may result from inhibitionofneurotransmitteraminoacidlikeGlu,amajor neurotransmitter in the CA1 region of the hippocampus. Reportssuggestthatexcitatoryaminoacids(EAA),including Gluand Asp,playa keyroleinthe hypoxicbraindamage mechanism.29---31NMDAreceptorisamajorreceptorofEAA
andits activation results in intracellular Ca2+ overload in hypoxia.32,33 Inhypoxiagroup,AspandGlu concentrations
in perfusion solution were increased 1.32 and 1.27-fold, respectively (Table 1). Isoflurane treatment reduced the hypoxia-induced release of Asp and Glu, thus reducing the excitatory toxicity. GABA is an inhibitory neurotrans-mitter in CNS that can protect neurons from hypoxic damage.34,35 This study showed that isoflurane treatment
canincreasetheconcentrationofGABAinperfusionfluid, which maybe a neuroprotecting mechanism of isoflurane duringhypoxia. Anotheranestheticpropofol wasreported toincreasesaffinity between GABAand itsreceptor, thus activatingtheGABAreceptordirectlytoresultinenhanced membrane current flow and GABA-induced hypopolarized inhibition.3Glycine(Gly)isanEAAmodulatorthatincreases
the sensitivity of NMDA receptor to EAA, amplifying the excitatory toxicity induced by EAA.36---38 Gly was found
increasedin the hypoxia group, which may facilitate the excitatorytoxicity(Table1).Isofluranetreatmentreversed theelevationofhypoxia-inducedGlyconcentrationin dose-dependent manner, suggesting the CNS protectingeffects ofisoflurane.Thedatashowednoneeffectofisofluraneon thereleaseofGln,whichmaynotdirectlyassociatedwith theneuroprotectivemechanismofisoflurane.Brainhypoxia maycauseATPexhaustion,influxofNa+andCl−effluxofK+ andH2Oresultingincellularedema.Incontrast,excessive release of Glu over-excites the neurons and causes an influxofCa2+,andincreaseinNMDAdependentCa2+influx. This resultsin overload ofintracellular Ca2+ that initiates enzymeactivation,proteinhydrolysis,formationofoxygen free radicals, DNA damage and neuronal cell death. Our data shows that isoflurane diminishes hypoxia-induced cellularedemaandmitochondrialdamage.Basedondata, we hypothesize that the other potential mechanisms by whichisofluraneprotectsCNSfromhypoxicdamageinclude modulation of Ca2+, clearance of oxygen free radicals, up-regulation of GABA receptor and inhibition of NMDA receptor.Furthermore,wefoundthatisofluranetreatment caused inhibition of hypoxia induced levels of mRNA of caspase-3and PARP.Apoptosis canbe executedmainlyby twopathways:caspase-9dependentmitochondrialintrinsic and caspase-8 dependent extrinsic. Both pathways lead to activation of executioner caspase-3 which ultimately causescelldeathbycleavinganumberofcytoplasmicand nuclearsubstrates like PARP.This study found that isoflu-rane suppressed the expression levels of apoptotic genes caspase-3andPARPindose-dependentmanner(Fig.3).
hypoxic damage. The basic mechanism behind neuropro-tection appears to be inhibition of apoptosis induced by hypoxia.Thisstudyshowsinsightsfor usingisofluraneasa safeanestheticinclinicalpracticesaswellasinveterinary practices.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.FeinerJR,BicklerPE,EstradaS,etal.Mildhypothermia,but not propofol,is neuroprotective inorganotypic hippocampal cultures.AnesthAnalg.2005;100:215---25.
2.KataokaK, YanaseH.Mildhypothermia--- arevived counter-measure against ischemic neuronal damages. Neurosci Res. 1998;32:103---17.
3.HaraM,KaiY,IkemotoY.PropofolactivatesGABAA receptor-chloride ionophore complex in dissociated hippocampal pyramidalneuronsoftherat.Anesthesiology.1993;79:781---8. 4.KochsE,HoffmanWE,WernerC,etal.Theeffectsofpropofol
onbrainelectricalactivity,neurologicoutcome,andneuronal damagefollowingincompleteischemiainrats.Anesthesiology. 1992;76:245---52.
5.HansP,BonhommeV,ColletteJ,etal.Propofolprotects cul-tured rat hippocampal neurons against N-methyl-d-aspartate
receptor-mediatedglutamatetoxicity.JNeurosurgAnesthesiol. 1994;6:249---53.
6.Daskalopoulos R, Korcok J, Farhangkhgoee P, et al. Propo-folprotectionofsodium---hydrogenexchangeactivitysustains glutamate uptake during oxidative stress. Anesth Analg. 2001;93:1199---204.
7.Grasshoff C,GillessenT.Theeffectofpropofolonincreased superoxideconcentrationinculturedratcerebrocortical neu-rons after stimulation of N-methyl-d-aspartate receptors.
AnesthAnalg.2002;95:920---2,tableofcontents.
8.O’SheaSM,WongLC,HarrisonNL.Propofolincreasesagonist efficacyattheGABA(A)receptor.BrainRes.2000;852:344---8. 9.YanoT,NakayamaR,UshijimaK.Intracerebroventricular
propo-folisneuroprotectiveagainsttransientglobalischemiainrats: extracellularglutamatelevelisnotamajordeterminant.Brain Res.2000;883:69---76.
10.TsaiYC,HuangSJ,LaiYY,etal.Propofoldoesnotreduceinfarct volumeinratsundergoingpermanent middlecerebralartery occlusion.ActaAnaesthesiolSin.1994;32:99---104.
11.BurchellSR,DixonBJ,TangJ,etal.Isofluraneprovides neuro-protectioninneonatalhypoxicischemicbraininjury.JInvestig Med.2013;61:1078---83.
12.ChungIS,KimJA,ChoiHS,etal.Reactiveoxygenspeciesby isofluranemediatesinhibitionofnuclearfactorkappaB activa-tioninlipopolysaccharide-induced acuteinflammationofthe lung.AnesthAnalg.2013;116:327---35.
13.HarrJN,MooreEE,StringhamJ,etal.Isofluranepreventsacute lunginjurythroughADP-mediatedplateletinhibition.Surgery. 2012;152:270---6.
14.KinoshitaH,MatsudaN,IranamiH,etal.Isoflurane pretreat-mentpreservesadenosinetriphosphate-sensitiveK(+)channel functioninthehumanarteryexposedtooxidativestresscaused byhighglucoselevels.AnesthAnalg.2012;115:54---61. 15.KimM,KimN,D’AgatiVD,etal.Isofluranemediates
protec-tion from renal ischemia---reperfusion injury via sphingosine kinaseand sphingosine-1-phosphate-dependentpathways.Am JPhysiolRenalPhysiol.2007;293:F1827---35.
16.LangXE, WangX,ZhangKR,etal.Isofluranepreconditioning confers cardioprotection by activationof ALDH2. PLoSONE. 2013;8:e52469.
17.SasaokaN,KawaguchiM,KawaraguchiY,etal.Isofluraneexerts a short-term but not a long-term preconditioning effect in neonatalratsexposedtoahypoxic---ischaemicneuronalinjury. ActaAnaesthesiolScand.2009;53:46---54.
18.Ferriero DM, Bonifacio SL. The search continues for the elusive biomarkers of neonatal brain injury. J Pediatr. 2014;164:438---40.
19.Altay O, SuzukiH, HasegawaY, et al. Isoflurane attenuates blood---brainbarrierdisruptioninipsilateralhemisphereafter subarachnoidhemorrhageinmice.Stroke.2012;43:2513---6. 20.Li H, Yin J, Li L, et al. Isoflurane postconditioning reduces
ischemia-inducednuclearfactor-kappaB activationand inter-leukin1betaproductiontoprovideneuroprotectioninratsand mice.NeurobiolDis.2013;54:216---24.
21.LiL,ZuoZ.Isofluranepostconditioninginducesneuroprotection viaAktactivationandattenuationofincreasedmitochondrial membranepermeability.Neuroscience.2011;199:44---50. 22.KhatibiNH,MaQ,Rolland W,etal.Isoflurane posttreatment
reducesbraininjuryafteranintracerebralhemorrhagicstroke inmice.AnesthAnalg.2011;113:343---8.
23.StatlerKD,AlexanderH,VagniV,etal.Isofluraneexerts neuro-protectiveactionsatornearthetimeofseveretraumaticbrain injury.BrainRes.2006;1076:216---24.
24.Zhou Y, Lekic T, Fathali N, et al. Isoflurane posttreat-ment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Aktpathway.Stroke.2010;41:1521---7.
25.DallasenRM,BowmanJD,XuY.Isofluranedoesnotcause neu-roapoptosis but reduces astroglial processes in young adult mice.MedGasRes.2011;1:27.
26.TokunagaH,HiramatsuK,SakakiT.Effectofprecedinginvivo sublethalischemiaontheevokedpotentialsduringsecondary invitrohypoxiaevaluatedwithgerbilhippocampalslices.Brain Res.1998;784:316---20.
27.Fairchild MD, Parsons JE, Wasterlain CG, et al. A hypoxic injurypotentialinthehippocampalslice.BrainRes.1988;453: 357---61.
28.SickTJ,SolowEL,Roberts EL Jr.Extracellularpotassiumion activityandelectrophysiologyinthehippocampalslice: para-doxicalrecoveryofsynaptictransmissionduringanoxia.Brain Res.1987;418:227---34.
29.Pellegrini-GiampietroDE, Gorter JA, Bennett MV, et al. The GluR2(GluR-B)hypothesis:Ca(2+)-permeableAMPAreceptors inneurologicaldisorders.TrendsNeurosci.1997;20:464---70. 30.HiroseK,ChanPH.Blockadeofglutamateexcitotoxicityandits
clinicalapplications.NeurochemRes.1993;18:479---83. 31.Kollegger H, McBean GJ, Tipton KF. Reduction of striatal
N-methyl-d-aspartatetoxicitybyinhibitionofnitricoxide
syn-thase.BiochemPharmacol.1993;45:260---4.
32.Salinska E, Pluta R, Puka M, et al. Blockade of
N-methyl-d-aspartate-sensitive excitatory amino acid receptors with
2-amino-5-phosphonovalerate reduces ischemia-evoked cal-cium redistribution in rabbit hippocampus. Exp Neurol. 1991;112:89---94.
33.XieY,ZachariasE,Hoff P,etal. Ion channelinvolvementin anoxicdepolarizationinducedbycardiacarrestinratbrain.J CerebBloodFlowMetab.1995;15:587---94.
34.Cobas A, Fairen A, Alvarez-Bolado G, et al. Prenatal devel-opment of the intrinsic neurons of the rat neocortex: a comparativestudyofthedistributionofGABA-immunoreactive cells and the GABAA receptor. Neuroscience. 1991;40: 375---97.
36.Globus MY, Busto R, Martinez E, et al. Comparative effect of transient global ischemia on extracellular levels of glu-tamate,glycine, andgamma-aminobutyric acidinvulnerable and nonvulnerable brain regions in the rat. J Neurochem. 1991;57:470---8.
37.KempJA,LeesonPD.TheglycinesiteoftheNMDAreceptor-five yearson.TrendsPharmacolSci.1993;14:20---5.