Universidade de Trás-os-Montes e Alto Douro
Effect of substrate composition on productive performances
and body composition of Tenebrio molitor larvae
Dissertação de Mestrado em Engenharia Zootécnica
Raquel Patrícia Anunciação Barbarrôxa
Orientadores: Professora Ana Luísa Guimarães Dias Lourenço
Professor José Júlio Gonçalves Barros Martins
III
Universidade de Trás-os-Montes e Alto Douro
Effect of substrate composition on productive performances
and body composition of Tenebrio molitor larvae
Dissertação de Mestrado em Engenharia Zootécnica
Raquel Patrícia Anunciação Barbarrôxa
Orientadores: Professora Ana Luísa Guimarães Dias Lourenço
Professor José Júlio Gonçalves Barros Martins
Composição do Júri:
Presidente: Professora Maria José Marques Gomes
Vogais:
Professor Miguel António Machado Rodrigues
Professor Paulo José de Azevedo Pinto Rema
Professora Ana Luísa Guimarães Dias Lourenço
V Esta dissertação é submetida à Universidade de Trás-os-Montes e Alto Douro para obtenção do grau de Mestre em Engenharia Zootécnica.
O conteúdo desta dissertação é da inteira responsabilidade da autora e todos os trabalhos consultados e adequadamente citados estão incluídos na bibliografia.
VII Recomeça…
Se puderes Sem angústia E sem pressa.
E os passos que deres, Nesse caminho duro Do futuro
Dá-os em liberdade. Enquanto não alcances Não descanses.
De nenhum fruto queiras só metade.
E, nunca saciado, Vai colhendo
ilusões sucessivas no pomar. Sempre a sonhar
E vendo, Acordado,
O logro da aventura.
És homem, não te esqueças! Só é tua a loucura
Onde, com lucidez, te reconheças…
IX Agradecimentos
Em primeiro lugar, referir que este é um agradecimento feito pelo percurso de cinco anos neste nobre curso, Engenharia Zootécnica, e nesta bela academia, UTAD.
A Vila Real, cidade que escolhi e que me acolheu, e que, agora, tenho como minha para onde quer que vá. Agradeço a esta cidade pelas tantas histórias que aprendi e por outras tantas que vivi, todas as ruas e caminhos que descobri, e até por me ter descoberto a mim própria. A ti, simples e majestosa, “ó que linda és”.
À UTAD, e mais do que isso, aos meus professores, por me transmitirem o conhecimento e por terem paciência sempre que eu abria discussão sobre um tópico na sala de aula. Eu sei que não é fácil cumprir conteúdos programáticos…desculpem. E obrigada por serem os meus mestres. Sei que poderei sempre voltar para questionar e contar com vocês para me ajudarem no meu percurso vindouro. Continuar-nos-emos a encontrar. Em especial, à Prof. Ana Luísa Lourenço, por me permitir o desenvolvimento do tema que escolhi para a dissertação de mestrado e todo o acompanhamento, nem sempre fácil. Ao Prof. José Júlio Martins por todo o auxílio prestado para tornar possível este trabalho. Ao Prof. Divanildo Monteiro, ao Prof. Miguel Rodrigues e ao Prof. Luís Mendes por me auxiliarem sempre que foi necessário no desenvolvimento deste trabalho.
À AAUTAD, e a toda equipa que eu acompanhei, sei, hoje, que teve um papel preponderante no meu crescimento, como pessoa e como profissional. Deu-me ferramentas que em nenhum outro lado eu as conseguiria, para hoje saber lidar com as demais situações com uma postura muito diferente de quando cheguei (vocês sabem, perfeitamente, do que estou a falar). Tenho em pleno dentro de mim que é um facto que “A AAUTAD dar-te-á muito mais, do que algum dia lhe poderás dar”.
Ao TUTRA, e a todas as pessoas que fizeram parte deste maravilhoso percurso, por termos aprendido e ensinado juntos, por todos os limites testados, e depois quebrados, por fazer de mim mais do que eu era, sem dúvida que expressão dramática antes de formar atores forma pessoas.
Aos meus pais, aos mais importantes, sem os quais nada deste percurso e deste crescimento teria sido possível. Eu sei que sou o vosso maior investimento e irei honrar com coragem esse compromisso. Eu sei que não tem sido fácil ver-me esticar as asas e fazer planos, já, de como será o grande voo. Mas sei também, que vocês sabem que faz parte, e a grande viagem aguarda-me. Obrigada por me ajudarem a tornar numa grande mulher e obrigada por toda a paciência.
X
Aos meus amigos, e são tantos, obrigada por fazerem de mim uma pessoa muito mais feliz, muito mais rica, muito mais do que eu própria. Obrigada por todas as conversas, abraços, gargalhadas, choros e discussões. Sei que sou muito o que sou hoje, porque me rodeei de vocês. A alguns um especial obrigado, por me acompanharem sempre. A ti, Cristiana, por teres sempre um discurso apaziguador e tentares mostrar o melhor caminho, o da alegria. A ti, Cristina, pelo espírito brincalhão e a forma leve de ver a vida. A ti, Mariana, por todas as chamadas de atenção e por todas as gargalhadas entusiasmadas. A ti, Miranda, por seres a alegria da festa e o ombro conselheiro. A ti, Renata, por apreciares a vida com uma simplicidade muito particular, que nem todos entenderão, mas eu sim, e por seres como uma irmã. A ti, Telma, por esclareceres as confusões da minha cabeça e pelo abraço calmante. A ti, Xana, por me fortaleceres e mostrares que tudo é mais fácil do que parece. A vocês, por me verem tal como eu sou e me aceitarem, mais do que isso, por me apreciarem.
Ao Nuno, por nada o fazer prever e, ao mesmo tempo, ser tão natural acontecer. Obrigada pela reentrada no meu percurso e por todo o apoio nesta fase final. Obrigada por toda a partilha e companheirismo. Obrigada por toda a paciência e compreensão. Obrigada pelo teu espírito acriançado e a tua revolta com as injustiças no mundo, parece-me que os dois extremos se ligam na perfeição. Há coisas que nos ultrapassam. A criação inusitada da nossa história parece-me uma delas. Obrigada por me acrescentares todos os dias.
Em tom de despedida, eu sei que aqui não é o fim, porque quem por mim passou um pedaço seu deixou, levou um pedaço de mim.
XI Abstract
The exponential growth of the world population and the growing demand for food puts high pressure on the natural resources. Thus, more than maximize production it is important to maximize efficiency, reduce environmental impact and guarantee food security. Edible insects present a well-balanced nutrient content and a high feed conversion efficiency. Their production demands less land, less water, and generates less greenhouse gas emissions. Thus, insects can become an interesting alternative to the conventional food sources. In addition, they use organic products with low nutritive value as feed sources.
The goal of this dissertation was to compare the growth performance (g) and nutritional composition of Tenebrio molitor larvae produced with different substrates of low nutritional value, namely: wheat bran, soybean hulls, guar churi and styrofoam (polystyrene).
The dissertation involved three different studies:
Study 0: larvae purchased from a supplier were grown on wheat bran to complete their life cycle in order to produce the progenitors used in Study 1. The progenitors were successfully obtained, and it was concluded that the substrate should be provided finely grinded to allow the isolation of the larvae from the substrate.
Study 1: the beetles obtained in Study 1 were used to colonize and lay eggs on the four studied substrates and the larvae production was evaluated. The amount of larvae produced was significantly different between substrates (P<0.001). The best production was obtained with the wheat bran treatment (9.87g), with the soy hulls, guar churi and styrofoam the amounts obtained were 1.15g, 0.45g, 0.10g, respectively.
Study 2: the larvae from the treatment with the best production in Study 1 ( wheat bran substrate), were used to colonize the four substrates (wheat bran, soybean hulls, guar churi and styrofoam) to evaluate and compare their production and nutritional composition at the end of larval stage. The final production (in weight, g) was significantly different between substrates (P<0.001) and the best production was obtained with the wheat bran treatment (57.44g) , with the soy hulls, guar churi and styrofoam the obtained values were 9.93g, 1.60g, 2.77g, respectively . The dry matter (P<0.001; 34.59g, 17.27g, 17.05g, 18.77g), organic matter (P<0.001; 95.96g, 92.72g, 88.09g, 86.86g), ashes (P <0.001; 4.04g, 7.28g, 11.91g, 13.14g) and crude protein (P <0.01; 47.07g, 64.89g, 57.61g, 54.89g) were significantly different between the wheat bran, soy hulls, guar churi and styrofoam substrates, respectively. There were no differences (P>0.05) in the crude fat or crude fibre contents of larvae among treatments. The
XII
differences in the chemical composition of larvae between treatments could be explained by different rhythms of larval maturation.
It was concluded that, compared to the other substrates studied, wheat bran provides conditions for higher growth rate when provided as substrate from hatching to the end of the larval stage of the Tenebrio molitor and possibly a higher development rate.
Keywords: Tenebrio molitor, substrate, insect production, by-products, chemical composition.
XIII Resumo
O crescimento exponencial da população global e o aumento da demanda de alimentos pressionam os recursos naturais. Desta forma, mais do que maximizar a produção é importante maximizar a eficiência, reduzindo o impacto ambiental para atender às perspetivas de segurança alimentar. Os insetos comestíveis apresentam um conteúdo de nutrientes equilibrado, uma alta eficiência de conversão alimentar, ocupam pouco espaço, utilizam pouca água e emitem menos gases de efeito de estufa, revelando-se alternativas interessantes às fontes de alimento convencionais. Adicionalmente, utilizam como fonte alimentar produtos orgânicos pouco nutritivos.
Esta dissertação teve como objetivo comparar o desempenho produtivo (g) e a composição nutricional de larvas de Tenebrio molitor produzidas com diferentes substratos, nomeadamente: farelo de trigo, casca de soja, guar churi e esferovite (polistireno).
A dissertação envolveu três estudos distintos:
Estudo 0: larvas adquiridas a um fornecedor foram criadas em farelo de trigo até completarem o seu ciclo de vida com o objetivo de produzir os progenitores usados no Estudo 1. Os progenitores foram obtidos com sucesso e concluiu-se que o substrato deve ser fornecido moído finamente para facilitar a separação das larvas do substrato.
Estudo 1: os escaravelhos obtidos no Estudo 0 colonizaram e fizeram posturas nos quatro substratos estudados e avaliou-se a produção de larvas resultantes dessas posturas. A produção de larvas foi significativamente diferente entre substratos (P <0,001). A maior produção foi obtida com o tratamento de farelo de trigo (9,87g), com a casca de soja, o guar churi e a esferovite obtiveram-se os valores de 1,15g, 0,45g, 0,10g, respectivamente.
Estudo 2: as larvas resultantes do tratamento com maior produção no Estudo 1 (substrato de farelo de trigo), foram utilizadas para colonizar os quatro substratos estudados (farelo de trigo, casca de soja, guar churi e esferovite) com o objetivo de testar a sua produção e composição nutricional no final do estado larval. A produção final em peso (g) foi significativamente diferente entre substratos (P < 0,001) e a maior produção foi obtida com o tratamento de farelo de trigo (57,44g), com a casca de soja, o guar churi e a esferovite obtiveram-se os valores de 9,93g, 1,60g, 2,77g, respetivamente. Os teores em matéria seca (P < 0,001; 34,59g, 17,27g, 17,05g, 18,77g), matéria orgânica (P < 0,001; 95,96g, 92,72g, 88,09g, 86,86g), cinzas (P < 0,001; 4,04g, 7,28g, 11,91g, 13,14g) e proteína bruta (P < 0,01; 47,07g, 64,89g, 57,61g, 54,89g) foram significativamente diferentes entre os substratos de farelo de trigo, casca de soja, guar churi e esferovite, respetivamente. Não se observaram diferenças
XIV
(P>0.05) nos teores de gordura e fibra brutas das larvas entre tratamentos. As diferenças na composição química das larvas entre tratamentos, poderia ser explicada por ritmos diferentes de maturação das larvas proporcionados pelos substratos.
Dos estudos efetuados conclui-se que, em comparação com os restantes substratos estudados, o farelo de trigo proporciona melhores condições de produção desde a eclosão até ao final do estado larval do Tenebrio molitor, e, possivelmente, uma taxa de desenvolvimento superior.
Palavra chave: Tenebrio molitor, substrato, produção insetos, co-produtos, composição química.
XV Index Agradecimentos ____________________________________________________________ IX Abstract __________________________________________________________________ XI Resumo ________________________________________________________________ XIII Index ___________________________________________________________________ XV Figure Index ____________________________________________________________ XVII Table Index _____________________________________________________________ XXI Abbreviations __________________________________________________________ XXIII 1. General Introduction ____________________________________________________ 1 2. State of Art ____________________________________________________________ 5 2.1. Edible Insects ______________________________________________________ 5 2.2. Tenebrio molitor ____________________________________________________ 9 3. Practical Studies _______________________________________________________ 27 3.1. Study 0 __________________________________________________________ 27 3.1.1. Objective ____________________________________________________ 27 3.1.2. Material and Methods __________________________________________ 27 3.1.3. Results and Discussion _________________________________________ 30 3.1.4. Conclusion ___________________________________________________ 41 3.2. Study 1 __________________________________________________________ 43 3.2.1. Objective ____________________________________________________ 43 3.2.2. Material and Methods __________________________________________ 43 3.2.3. Results and Discussion _________________________________________ 46 3.2.4. Conclusion ___________________________________________________ 52 3.3. Study 2 __________________________________________________________ 53 3.3.1. Objective ____________________________________________________ 53 3.3.2. Material and Methods __________________________________________ 53 3.3.3. Results and Discussion _________________________________________ 55
XVI
3.3.4. Conclusion ___________________________________________________ 66 4. General Conclusion ____________________________________________________ 67 5. Bibliography _________________________________________________________ 69
XVII Figure Index
Figure 1 – Beetle; elytra protecting the hindwings and body (left), elytra, hindwings and body exposed (right). ... 9
Figure 2 – Beetle of Tenebrio spp. (a) T. molitor (b) T. obscurus (Adapted from Robinson (2005)). ... 10
Figure 3 – Life cycle of the Tenebrio molitor; egg and larva, larva, pupa, and beetle (left to right). ... 11
Figure 4 – Tenebrio molitor copulation, the female extrudes the ovipositor and the male, on the female’s back, extrudes the aedeagus (adapted from Font & Desfilis (2003)). ... 12
Figure 5 – Eggs of Tenebrio molitor (left); egg and larvae of T. molitor (right). ... 13 Figure 6 – Larvae of T. molitor; freshly moulted (left) and “mature” (right). ... 13 Figure 7 – Pupae of Tenebrio molitor; emerging from larvae (left); newly emerged (centre); “mature” (right). ... 14
Figure 8 – Beetle of T. molitor maturation (left to right); newly emerged (left) shiny black beetle (right). ... 14
Figure 9 – Reception of parental larvae ... 27 Figure 10 – Study timeline of T. molitor production. Larvae (0 to 21st day), pupae (2nd to 29th day) and beetle (from 10th day onwards) stages (arrows representing the beginning of the stage transitions and the removal of the parental larvae); (orange and black indexs: carrot and wheat bran supplementation, respectively). ... 28
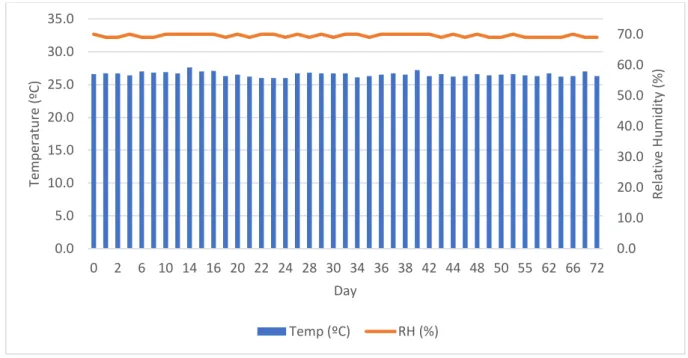
Figure 11 – Temperature (Temp, ºC) and relative humidity (RH, %) during the entire duration of study 0. ... 30
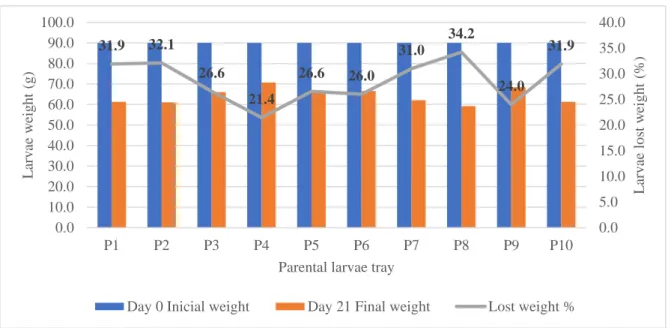
Figure 12 – Parental larvae trays incubated. ... 31 Figure 13 – Parental larvae and resulting pupae. ... 31 Figure 14 – Parental larvae weight loss in 21 days per tray, and the representing percentage. ... 32
Figure 15 – Average number of pupae collected per day and standard error bars from the 10 parental larvae trays during study 0. ... 32
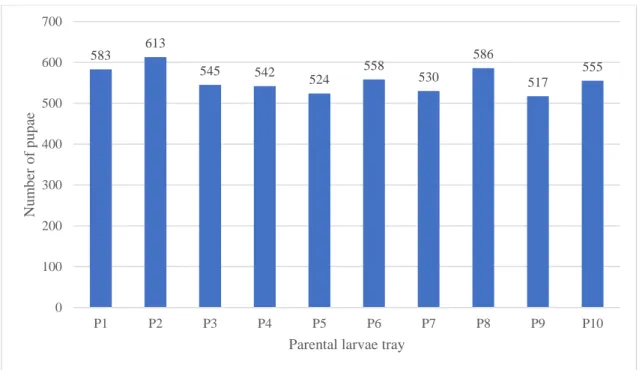
Figure 16 – Total number of pupae collected per parental larvae tray (P1- P10) during the 21 days. ... 33
Figure 17 – Number of pupae distributed per tray (Pupae 1 – Pupae 10). ... 33 Figure 18 – Pupae tray with the collected pupae from the parental larvae trays. ... 34
XVIII
Figure 19 – Pupae collected for the trays (Pupae 1 – Pupae 10) and the emergence of those pupae into beetles by tray (represented by the number of the pupae tray). ... 34
Figure 20 – Beetle emergence in the pupae trays. ... 35 Figure 21 – Temperature (ºC) and relative humidity (%) during pupae incubation and beetle emergence. ... 37
Figure 22 – Parental beetles’ tray with the collected beetles from the pupae trays. .... 37 Figure 23 – Number of beetles distributed per tray (Beetle 1 – Beetle 12)... 38 Figure 24 – Beetles collected to the trays (Beetle 1 – Beetle 12) from the 1st egg laying
period. The transfer of these beetles to a 2nd laying period trays and the removal of the beetles to obtain the offspring (same numbers represent the beetles from the same trays). ... 38
Figure 25 –Tenebrio molitor eggs deposited at the bottom of the trays and adhered to each other (left); eggs exhibited approximately 2 mm length (right). ... 39
Figure 26 – Rare, tiny larvae of T. molitor at the trays after the egg laying periods. .. 40 Figure 27 – Larvae from the 1st egg laying period at the 52th day, showing considerable size differences. ... 40
Figure 28 – The result of sifting the larvae from the substrate. ... 41 Figure 29 – Study timeline of T. molitor rearing. Beetles laying eggs from day 0 to day 7; egg hatched and larvae growth from day 7 until the end of the study at day 67. ... 44
Figure 30 – Egg laying by T. molitor beetles at day 1 (A – Wheat bran, B – Soy hulls, C – Guar churi, D – Polystyrene) ... 46
Figure 31 – Egg laying by T. molitor beetles at day 4 (A – Wheat bran, B – Soy hulls, C – Guar churi, D – Polystyrene). ... 47
Figure 32 – Eggs of T. molitor reared with wheat bran substrate (left) and no egg laying in the polystyrene substrate at the 4th day (right). ... 47
Figure 33 – Egg laying of T. molitor beetles at the polystyrene trays one day after the supplementation with the wheat bran. ... 47
Figure 34 – Evidence of hatched larvae at day 7 in the guar churi trays. ... 48 Figure 35 – Eggs (left) and evidence of hatched larvae (right) at day 11 in the polystyrene trays (7days after wheat bran supplementation). ... 48
Figure 36 – T. molitor larvae obtained at day 67 of the study... 49 Figure 37 – Tenebrio molitor larvae at day 22; wheat bran, soy hulls, guar churi and polystyrene treatment (left to right); guar churi shows evidence of darkening. ... 56
XIX Figure 38 – Dead larvae (dark larvae) in the guar churi treatment at day 22 (left) and 25 (right). ... 56
Figure 39 – Darkening of the guar churi treatment at day 22(left), 25(centre-left), 32 (centre-right), and 36 (right). ... 57
Figure 40 – Darkening of the guar churi treatment at day 38 (left), 43 (centre), and 45 (right). ... 57
XXI Table Index
Table 1 –Tenebrio sp. taxonomic classification (Bousquet, 1990; Bousquet & Campbell, 1861; Capinera, 2008; Dunford et al., 2005; Robinson, 2005) ... 9
Table 2 – Tenebrio molitor life cycle duration under optimum conditions (sources are shown in table). ... 11
Table 3 – Chemical composition of the T. molitor larvae (sources are shown in table). ... 18 Table 4 – Life cycle assessment of T. molitor larvae, mealworms and livestock products production on a cradle-to-farm-gate approach, and T. molitor meal from cradle to mill gate (sources are shown in table). ... 20
Table 5 – Replacement of soybean and fish meals in poultry, pig, fish, and shrimp diets (sources are shown in the table). ... 23
Table 6 – Number of pupae, number of beetles emerged, and emergence rate per tray of pupae. ... 36
Table 7 – By-products substrates chemical composition (DM basis). ... 49 Table 8 – By-products substrate chemical composition (on as is basis) and recommendations for optimum larval growth (Fraenkel, 1950; Martin & Hare, 1942). ... 50
Table 9 – Growth performance of T. molitor at different substrates ... 51 Table 10 – By-products substrates chemical composition (DM basis). ... 58 Table 11 – By-products substrate chemical composition (on as is basis) and recommendations for optimum larval growth (Fraenkel, 1950; Martin & Hare, 1942). ... 58
Table 12 – Growth performance of the T. molitor larvae ... 59 Table 13 – Comparison of the body chemical composition of Tenebrio molitor larvae from coffee mill and 1mm mill grinding samples. ... 61
Table 14 – Crude fat composition of Tenebrio molitor larvae analysed using samples with different size. ... 62
Table 15 – Crude protein and crude fibre composition of Tenebrio molitor larvae analysed from original and defatted samples. ... 62
XXIII Abbreviations
ADF – Acid Detergent Fibre C – Carbon
CH4 – Methane
CO2 – Carbon dioxide
DM – Dry Matter EU – Energy Use
FCR – Food Conversion Ratio GWP – Global Warming Potential K – Potassium
LU – Land Use N – Nitrogen
N2O – Nitrous oxide
NDF – Neutral Detergent Fibre NFE – Nitrogen Free Extract NH3 – Ammonia
P – Phosphorous
SD – Standard deviation SME – Standard Mean Error WF – Water Footprint
1
1. General Introduction
The human population passed 1 billion around 1800 years d.C, and in only 200 years it raised to 7 billion (Haub, 2011). The exponential growth of the world population, and therefore the increasing demand for food, puts pressure on natural resources and results in global resources scarcity and environmental degradation (Schneider et al., 2011; Tilman et al., 2002). The increasing demand for food is also due to a growth in the per capita income, which results in diet changes, in particularly, an increase in the demand of livestock products (Rask & Rask, 2011; van Beek et al., 2010).
The technological advances allowed a raise in productivity, which was needed to face the increase in food demand (Tilman et al., 2002). As countries continue to develop, the diet changes will continue to pressure land resources and the environmental impact of the climate change will continue to raise, increasing the importance of effective mitigation measures (Nardone et al., 2010; Rask & Rask, 2011; van Beek et al., 2010).
The world needs to produce more food with fewer resources (Schneider et al., 2011). However, one third of the food produced for human consumption is lost or wasted (Gustavsson et al., 2011). This represents a lot of resources wasted and purposeless greenhouse gas emissions and emphasizes the need to increase efficiency (Gustavsson et al., 2011).
In low-income countries waste or loss occurs at the beginning or in the middle of the food supply system due to financial, infrastructure or technological limitations (Gustavsson et al., 2011). However, in medium- and high-income countries, the loss and waste occur at the consumption level and is due to the seller and the consumer behaviour (Gustavsson et al., 2011). The industrialized countries waste more food than the developing countries on a per capita basis (Gustavsson et al., 2011). Furthermore, major losses occur from consumption of food in excess of the human nutritional requirements and in the livestock production (Alexander et al., 2017b).
The environmental impact of the conventional animal production can be explained mainly by three factors: utilization of nutrients and energy in feed, differences in enteric CH4
emission, and differences in reproduction rates (de Vries & de Boer, 2010). Beef protein has the highest impact on land and energy use, and global warming potential (de Vries & de Boer, 2010). It is followed by pig protein, and chicken protein, eggs and milk have the lowest impact (de Vries & de Boer, 2010).
To meet environmental and food security goals, the challenge is not only to maximize production, but also to optimize it through efficiency and sustainability (Godfray et al., 2010;
2
Rask & Rask, 2011; Tilman et al., 2002). The agricultural efficiency, the production practices, and the consumer’s behaviour have a major impact on the overall food supply system (Alexander et al., 2017b; Clark & Tilman, 2017; Nardone et al., 2010; Rask & Rask, 2011).
It is important to notice that hunger and malnutrition are not just a matter of food scarcity but an unequal distribution of wealth, where starvation results from the choices made by governments and policy makers (Gilland, 2002). The resources of the less-developed countries are exploited by the developed ones and, due to corruption, the economic outcome is not invested in the development of the resource supplier (nation or people) but goes to a small elite (Gilland, 2002). Nevertheless, the reduction of food losses and waste and the increment of the food chain efficiency is a priority, since the world natural resources are limited (land, water, energy, fertilizer) (Gustavsson et al., 2011).
Edible insects are a promising alternative to the conventional food and feed sources and serve environmental and food security purposes (DeFoliart, 1992; Ghosh et al., 2017). They have a high edible portion with a well-balanced nutrient content and are a more efficient protein source that the conventional food and feed sources (Ghosh et al. 2017; Oonincx & De Boer, 2012; Rumpold & Schlüter, 2013a, 2013b, Zielińska et al., 2015). The latter is due to their high reproduction rate, high feed conversion efficiency, the use of waste biomass as feed and the reduced land use and greenhouse gas emissions of their production (Oonincx & De Boer, 2012; Oonincx et al., 2010; Ramos-Elorduy et al., 2002). Overall, the edible insects offer an economical and sustainable animal protein source (Oonincx & De Boer, 2012; Ramos-Elorduy et al., 2002; Rumpold & Schlüter, 2013a, 2013b; Zielińska et al., 2015).
The transition to a healthy and sustainable diet can only be achieved by a joint effort of the government, industries and environmental organizations to inform and educate the public (de Boer & Aiking, 2017). The consumer awareness is essential to change its diet according to the current world situation, i.e., towards the reduction of the meat consumption and the use of more sustainable food sources (Alexander et al. 2017a; Castañé & Antón, 2017; de Boer & Aiking, 2017; Looy et al., 2014; van de Kamp et al., 2017).
A healthy and sustainable diet would be the one that meets the nutrient requirements through a balance between animal and plant sources, where those with the smaller environmental impact are chosen, it would include the more efficient conventional animal products (e.g. chicken, eggs and milk) or alternative protein sources such as meat surrogates, lentils and other pulses, or edible insects (Alexander et al., 2017a; Castañé & Antón, 2017; de Vries & de Boer, 2010; Schosler et al., 2012).
3 The animal diets must also change towards sustainability, this can be achieved by the replacement of conventional feeds with over demand, like soybean and fish meal and oil, by more sustainable alternatives (Ng et al., 2001; Ramos-Elorduy et al., 2002, van Huis, 2013). The edible insects are an alternative with high potential to replace these conventional feeds (Barroso et al., 2014; Ng et al., 2001; Ramos-Elorduy et al., 2002; Rumpold & Schlüter, 2013b; van Huis et al., 2013).
As a future prospect, it would be important to wide spread the inclusion of edible insects into animal feed and human diets (Glover & Sexton, 2015). This would contribute to alleviate food and feed insecurity and to reduce the environmental challenges (Glover & Sexton, 2015). However, edible insects must not raise the world inequalities by becoming a more affordable meat than conventional meat and fish that become a scarce luxury item only available to a small elite (Glover & Sexton, 2015). This puts forth the role of the present and future government policies, since undernutrition is a consequence of poverty, where the increasing cost of livestock makes these food source inaccessible to a large part of the human population, in particular, in the developing and underdeveloped countries (Glover & Sexton, 2015).
5
2. State of Art
2.1.
Edible Insects
Insects are a class of invertebrate animals within the Arthropoda phylum, characterized by a chitinous exoskeleton, a three-part body (head, thorax and abdomen), three pairs of jointed legs, compound eyes and one pair of antennae (van Huis et al., 2013).
All around the world there is a diverse use of insects as human food that contributes to the nutritional enrichment of the diets (DeFoliart, 1992, 1995, 1999; Looy et al., 2014; Ramos-Elorduy et al., 1997). They are used not only as delicacies, but also as high-quality nutritional supplements, which are normally seasonally available (DeFoliart, 1995; Looy et al., 2014). Additionally, their harvesting and trade also serves to raise the family and local income (DeFoliart, 1995). Thus, insects play an important role fighting malnutrition but also increasing family sustainability (DeFoliart, 1995; Looy et al., 2014).
Not all insects are safe for consumption, some species present as edible and can be consumed by humans without any additional hazard in comparison to other animal products (Belluco et al., 2013; Finke et al., 2015, Rumpold & Schlüter, 2013b). In fact, allergenic, microbial, parasitical and chemical hazards, also present in other animal products, can be surpassed by controlled farming and dietary conditions, correct labelling (allergen), hygienic handling and correct storage and consumption (Belluco et al., 2013; Eilenberg et al., 2015; Finke et al., 2015).
Entomophagy is the consumption of insects by humans and is estimated to be practiced by 2 billion people all around the world (DeFoliart, 1995, 1999; van Huis et al., 2013). However, there has been a reduction of insect use due to acculturation of western lifestyles and international crop production policies with intensive implementation of pesticides, which reduces the safety of insects as food (DeFoliart, Looy et al., 2014). These policies have minimal economic benefit for the farmers but a high dietary cost, increasing protein-energy malnutrition (Looy et al., 2014). Thus, western attitude towards insect use is important, because it is essential that insects become widely acceptable as a food resource (DeFoliart, 1999; Looy et al., 2014). The protection of natural habitats and the non-use of pesticides would benefit the insect catching and consumption, which in turn would have economic and social implications, e.g. preserving the cultural identity and richness of insect consuming practices (DeFoliart, 1992, 1999; Deroy et al., 2015; Looy et al., 2014).
6
The insect acceptance as human food faces barriers, mainly of disgust (Looy et al., 2014). This occurs despite the human consumption of other organisms associated with decay, as fungi and marine scavengers (Looy et al., 2014). Insects remain as object of repulsion and the western thought that “only the primitive and desperate eat them” reveal disrespect and intolerance towards other classes, races and cultures (Deroy et al., 2015; Looy et al., 2014). This attitude leads to the destruction of diversity and this needs to be fully understood before it can be changed (DeFoliart, 1999; Deroy et al., 2015; Looy et al., 2014).
The edible insects include a wide range of nutrient content that varies with species, development stage and feed source (Barker et al.,1998; Finke, 2002; Ghosh et al., 2017; Ramos-Elorduy et al., 1997; Rumpold & Schlüter, 2013a, 2013b; van Huis, 2013; Vrabec et al., 2015). Although much is known, it is important to mention that there are still significant gaps in the data available on the nutritional content of the major edible insect species (Payne et al., 2016). Nevertheless, they are a good source of protein and fat, and present a well-balanced nutrient content with a high content of essential amino acids, monounsaturated and polyunsaturated fatty acids and other micronutrients (Ghosh et al., 2017; Rumpold & Schlüter, 2013a, 2013b; Veldkamp et al., 2012, Vrabec et al., 2015; Zielińska et al., 2015). These nutritional qualities can still be further improved by selective breeding and nutrient enrichment (Ghosh et al., 2017). Furthermore, the nutritional content diversity among different species of insects allows the possibility to mixture different species to obtain a better nutritional product, turning insects into an alternative to the conventional sources of food and feed (Ghosh et al.,2017).
The protein content of insect is involved in controversy because insects exoskeleton is primarily chitin, thus contain nitrogen, and crude protein is usually estimated by the following formula: Nitrogen content x 6.25 (Jonas-Levi & Martinez, 2017) . This assumes the nitrogen content is all in protein and nitrogen content of the protein is 16% (Jonas-Levi & Martinez, 2017). According to some authors this assumptions cannot be made for insects and this formula has been incorrectly used to calculate the protein content of the insects (Jonas-Levi & Martinez, 2017). Other authors argue that the value of chitin can be determined through the ADF content, and 16% is a reasonable nitrogen content assumption for the majority of the insect species (Finke, 2007), or that chitin contains about 7% of nitrogen, thus each 1% of ADF (presumed to be chitin) is equivalent to 0.4% of crude protein (1 x 0.07 x 6.25) (Bernard et al., 1997). In fact, Marono et al. (2015) shows that crude protein digestibility is negatively correlated to ADF and chitin values.
7 Insects are mostly valued as a source of protein, but many species are rich in oils that can be directly or indirectly used to improve the texture, the flavour and the digestibility of a food (Ghosh et al., 2017).
Insects production is more efficient that the conventional animal productions, because they have high feed conversion efficiency, high edible portion, produce less greenhouse gas emissions, require less land, can use low nutritive waste as feed, have short life cycles, and high reproduction rates, and so they offer an economical and sustainable food and feed source alternative (Ghosh et al., 2017; Oonincx & De Boer, 2012; Oonincx et al., 2010; Ramos-Elorduy et al., 2002; Rumpold & Schlüter, 2013a, 2013b; Zielińska et al., 2015).
Edible insects have been mainly harvested from the wild or harvested from crops, serving also as pest control (Cerritos & Cano-Santana, 2008; DeFoliart, 1992, 1995; van Huis, 2013). In other situations, semi-cultivation happens, i.e. intentional creating additional breading sites (DeFoliart, 1995; van Huis, 2013). In all these situations overexploitation is possible as a result from higher demands (DeFoliart, 1995; van Huis, 2013). Thus, mass-production of insects is essential to make the transition from conventional food and feed sources to the alternative insect source (van Huis, 2013).
Mass-rearing insects has been happening for centuries with Apis melifera L. and Bombyx mori L. for food, for honey, for silk production, and for pollination (Dossey et al., 2016; Rumpold & Schlüter, 2013b; van Huis, 2013). Later the fish bait market led to mass-production of crickets, waxworms and mealworms (Dossey et al., 2016; Rumpold & Schlüter, 2013b; van Huis, 2013). More recently the exotic pet market demanded more live feed insects, since pet owners, pet shops and these pet breeders needed a regular supply of clean, safe and easy to access live insects (Dossey et al., 2016; Rumpold & Schlüter, 2013b). Now, insect farming finds a more impactful market, oriented to human food and to animal feed, and faces major challenges, e.g. to develop mass-rearing techniques and diets, to assure safe products with consistent quality (nutrition, hygiene and sanitation) at a cost-effective value (Dossey et al., 2016; Rumpold & Schlüter, 2013b; van Huis, 2013).
Insects are poikilothermic, i.e. do not spend energy to maintain their body temperature above ambient temperature (Oonincx & De Boer, 2012; Oonincx et al., 2010; van Huis, 2013). This characteristic allows them to have a higher feed conversion efficiency but also makes their growth and development a function of the ambient temperature (Oonincx & De Boer, 2012; Oonincx et al., 2010; van Huis, 2013). Thus, the energy input required by mass-production of
8
insects can also have a significant environmental impact that needs to be evaluated and taken into account (Deroy et al., 2015; Oonincx & De Boer, 2012).
As is many others animal production systems, to have successful large scale insect production the reproduction rate, the feed conversion ratio, the daily weight gain, the resistance to diseases, the automation of the production system, the selection of high quality strains and the supply of organic waste must be considered (van Huis, 2013).
Insects can use a variety of low nutritive products as feed source (Ramos-Elorduy et al., 2002; Oonincx et al., 2015), and so can contribute to value and reduce waste, which is globally estimated to be 1.3 billion tons per year (Gustavsson et al., 2011). Insects can then be used as feed and food, valuing these low nutritive products into a high nutritive biomass (Ramos-Elorduy et al., 2002; Oonincx et al., 2015; van Broekhoven et al., 2015; Veldkamp et al., 2012; Vrabec et al., 2015).
The high protein and fat contents of the insects are well within the range of widely used feedstuffs, like soy bean and fish meal, and have been shown to be a suitable replacement for these feedstuffs in pig, poultry and fish diets (Allegretti et al., 2018; Barroso et al., 2014; Cannella et al., 2016; De Marco et al., 2015; Veldkamp et al., 2012). However, at the moment, the European legislation only allows the use of insects in feed for pet foods, their use as feed for animals used as human food is prohibited under Regulation EC 999/2001 (EC, 2001) and EC Directive 2002/32 (EC, 2002). The exception is aquaculture, where the use of non-ruminant protein is allowed since July, 2017 (EU 2017/893) (Finke et al., 2015; Marberg et al., 2017).
The widespread of the insect consumption in the western must surpass the bias mentioned before. The introduction of entomophagy as a normal practice and the increase of familiarity with the cooking process will help to overcome the emotional barrier and will increase the willingness to consume insects (Caparros Megido et al., 2014; Gmuer et al., 2016; Hamerman, 2016). A big gap in all the research being done is that, despite all the nutritional and environmental advantages, no firm conclusions can be made on the impact of insect consumption on human health (Payne et al., 2016).
The insect species identified as being the most promising for large scale production are the larvae of the Black soldier fly (Hermetia illucens), the Common housefly (Musca domestica), and the Yellow Mealworm (Tenebrio molitor) (Veldkamp et al., 2012).
9
2.2.
Tenebrio molitor
Tenebrio sp. is a darkling beetle (Tenebrionidae family) that belongs to the order Coleoptera within the Insecta class (Table 1). Coleoptera includes all beetles. They are easily distinguishable by the typically elytra, a pair of hardened forewings, that serve as wing cases, hiding and protecting the body and the hindwings (Figure 1) (Bousquet, 1990; Robinson, 2005).
Figure 1 – Beetle; elytra protecting the hindwings and body (left), elytra, hindwings and body exposed (right).
Table 1 –Tenebrio sp. taxonomic classification (Bousquet, 1990; Bousquet & Campbell, 1861; Capinera, 2008; Dunford et al., 2005; Robinson, 2005)
Kingdom Animalia Phylum Arthropoda Superclass Hexapoda Class Insecta Order Coleoptera Suborder Polyphaga Superfamily Tenebrionoidea Family Tenebrionidae Subfamily Tenebrioninae Tribe Tebrionini Genus Tenebrio
10
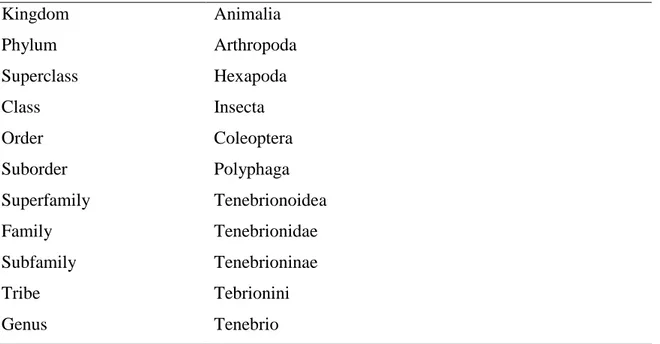
The Tenebrio spp. belongs to the family Tenebrionidae, which include beetles that are nocturnal and prefer dark and moist habitats. This genus is summed up to two species of darkling beetles, which are similar in colour, size, and form of larvae, pupae and adult stages (Figure 2), and are commonly known as mealworms: the yellow mealworm, Tenebrio molitor, and the dark mealworm, Tenebrio obscurus (Lord, 2008; Robinson, 2005).
Figure 2 – Beetle of Tenebrio spp. (a) T. molitor (b) T. obscurus (Adapted from Robinson (2005)).
These beetles are the largest insects found in stored cereal grains and by-products, but they are not serious pests because under natural conditions they only complete one generation cycle per year (Applebaum, 1964; Balfour & Carmichael, 1928; Daggy, 1946; Fraenkel, 1950; Lord, 2008).
Widely reared and sold as feed for captive animals as reptile, fish and avian, T. molitor is a promising alternative to protein-rich animal feed, and also fit for human consumption (Bernard et al., 1997; Finke, 2002; van Huis et al., 2013).
Life Cycle
The Tenebrio molitor, as a member of the Coleoptera order, is an holometabolous insect, undergoing a complete metamorphosis, comprising four life stages: egg, larva, pupa, and adult (beetle) (Figure 3) (Bousquet, 1990; Font & Desfilis, 2003; Robinson, 2005; Willis & Willis, 2008).
11
Figure 3 – Life cycle of the Tenebrio molitor; egg and larva, larva, pupa, and beetle (left to right).
The duration of the life cycle is variable according to the production conditions (temperature, humidity, diet), with wide time ranges for each stage in the literature (Table 2). Thus, the life cycle of T. molitor is variable in length, and varies according to the literature source. Makkar et al. (2014) state that it takes from 280 to 630 days to complete the life cycle. Robinson (2005) states that the same period takes from 30 to 649 days. Friederich & Volland (2004) cited by Oonincx & De Boer (2012) states that it reaches adulthood in 10 weeks.
Table 2 – Tenebrio molitor life cycle duration under optimum conditions (sources are shown in table).
~: authors do not provide the information; d– day, w – week, m – month
The reproduction of the T. molitor occurs at the adult stage of the life cycle, beetles have little sexual dimorphism and are sexual mature between the 5th and 8th day of this stage (Bousquet, 1990; Happ, 1970). The intra-species recognition of the male and the female occurs by the presence of sexual pheromones and begins with the courtship, that starts with antennation, where the male touches the female with the antennae (Font & Desfilis, 2003). Then, the male moves to the tip of the female’s abdomen, when the female stops, the male climbs onto her back and extrudes his copulatory organ (aedeagus) until it contacts the tip of the female’s abdomen (Font & Desfilis, 2003). If the female is sexually receptive, she will raise
Life Cycle Stage
Egg Larva Pupa Beetle
(Font & Desfilis, 2003) 1w 3m 1-2w 2-3m
(Ghaly & Alkoaik, 2009) 2w 6-8m 6d 37-96d
(Makkar et al., 2014) 10-12d 3-4m 7-9d 2-3m
12
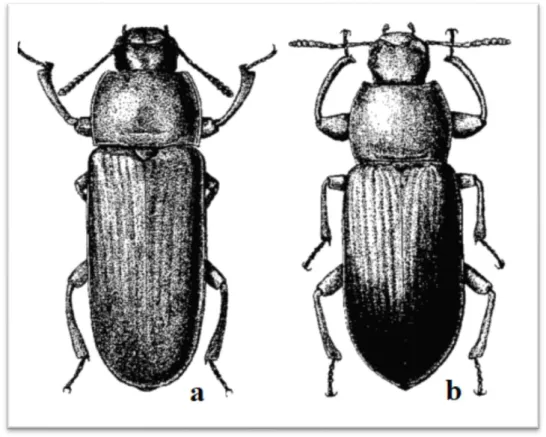
the tip of the abdomen and extrude the ovipositor, allowing the intromission of the aedeagus (Figure 4) (Font & Desfilis, 2003). Copulation usually takes between 1-2 minutes, in which the male transfers his sperm via spermatophore into the female (Font & Desfilis, 2003).
Figure 4 – Tenebrio molitor copulation, the female extrudes the ovipositor and the male, on the female’s back, extrudes the aedeagus (adapted from Font & Desfilis (2003)).
The T. molitor female beetle then lays the eggs, the number of eggs laid varies with the literature source, Robinson (2005) states that each female lays 40 eggs per day, in a total of 276 eggs and Ghaly & Alkoaik (2009) states an average of 400-500 eggs in total.
The eggs are white, bean shaped, and measure approximately 1mm length (Balfour & Carmichael, 1928; Font & Desfilis, 2003; Robinson, 2005). They are covered with a secretion that causes its adherence to the substrate or the surfaces of where they are deposited (Font & Desfilis, 2003; Robinson, 2005) (Figure 5). The eggs take 6 to 8 days to hatch, depending on the temperature (Font & Desfilis, 2003; Robinson, 2005). Parental age has no effect on the egg weight or the incubation time, but is negatively correlated with the hatchability (Ludwig & Fiore, 1960).
The newly hatched larvae are white and tiny, not easily discernible (Font & Desfilis, 2003).
13
Figure 5 – Eggs of Tenebrio molitor (left); egg and larvae of T. molitor (right).
The larvae are yellow, waxy and slender, and moult as they grow (Balfour & Carmichael, 1928). The number of moults depend on the substrate and production conditions (Font & Desfilis, 2003; Ludwig & Fiore, 1960). The larvae from parents older than 8 or 9 weeks grow at a faster rate than the ones from younger parents (Font & Desfilis, 2003; Ludwig & Fiore, 1960). Newly moulted larvae are distinguishable by the white appearance and soft body (Figure 6) (Balfour & Carmichael, 1928; Font & Desfilis, 2003).
Figure 6 – Larvae of T. molitor; freshly moulted (left) and “mature” (right).
The larvae exhibit cannibalistic behaviour, preying on freshly moulted and quiescent larvae and on pupae (Weaver & McFarlane, 1990).
The full-grown larvae length is about 25 mm, and become quiet and adopt a C – shape posture when in transition to pupae (Balfour & Carmichael, 1928; Font & Desfilis, 2003; Robinson, 2005).
The pupae are white or light brown, do not move unless disturbed (Font & Desfilis, 2003). In nature, the pupae stage or the full-grown larvae goes through winter with little change and emerge as beetles in the next spring (Figure 7) (Font & Desfilis, 2003; Robinson, 2005).
14
Figure 7 – Pupae of Tenebrio molitor; emerging from larvae (left); newly emerged (centre); “mature” (right).
The newly emerged beetles are white but their colour turns into light brown, reddish-brown, and to shiny black after a few days (Figure 8) (Balfour & Carmichael, 1928; Font & Desfilis, 2003). They measure in average 1.5 cm in length, the sizes is not directly related to age because there is no continuous growth once the adult stage is reached (Balfour & Carmichael, 1928; Font & Desfilis, 2003).
Figure 8 – Beetle of T. molitor maturation (left to right); newly emerged (left) shiny black beetle (right).
Tenebrio molitor growth is modified by changes in ambient temperature and humidity, age, and nutrition status of the larvae (Martin & Hare, 1942). A diet that was submitted to high temperature or to chemical manipulation, its fat, carbohydrate, or protein composition and its contamination with yeast or bacteria can also affect the growth of the larvae (Martin & Hare, 1942).
15 Diet
The T. molitor can survive for long periods without feed or moisture (Robinson, 2005). It feeds on a wide variety of materials and is naturally found in stored cereal grains and its by-products (Applebaum, 1964; Bousquet, 1990; Robinson, 2005).
The T. molitor, like any other flour insect, grows well in a powdery medium with a water content up to 10% (Fraenkel, 1950). When produced under artificial conditions the T. molitor is usually fed with a mixed grain feed of flour or bran (e.g. wheat, oats, soy, rye or corn) that can be supplemented with brewer’s yeast and the water is usually supplied with soaked sponges, or with moisturized cotton on glass plates, or with vegetables (e.g. carrot, cabbage) (Connat et al., 1991; Ghosh et al., 2017; Martin & Hare, 1942; Oonincx & De Boer, 2012; Oonincx et al., 2010). When there is water available, the larvae grow larger, develop faster, and weigh more before pupation, they also contain a higher concentration of total lipids (mainly triglycerides) (Urs & Hopkins, 1973a, 1973b). The pupae and adults from these larvae also tend to weigh more (Urs & Hopkins, 1973a, 1973b).
For optimum larval growth, the diet must contain within 50% to 80% of carbohydrate and between 15-25% of protein (Fraenkel, 1950; Martin & Hare, 1942). A fat content above 3% inhibits growth (Fraenkel, 1950; Martin & Hare, 1942). The lifespan of the beetle is shorter when the dietary protein:carbohydrate ratio (P:C) is higher, and the highest fecundity rate is achieved with a P:C of 1:1 (Rho & Lee, 2016). Furthermore, a high protein diet at larval stage leads to higher survival, shorter development time, and a higher food conversion efficiency (van Broekhoven et al., 2015).
The development of the larvae is superior on starch when compared to other carbohydrates (Fraenkel, 1955). High lipid content compromises the immune response of the T. molitor larvae (Morales-Ramos et al., 2013). The complex B vitamins are essential for optimal growth. The thiamine, the riboflavin, the nicotinic acid, the pyridoxin and the pantothenic acid are limiting growth factors, and the riboflavin and the pantothenic acid increase the survival rate (Fraenkel, 1950; Martin & Hare, 1942). A sterol (such as cholesterol), yeast and rat liver provided as supplements were also shown to increase the growth rate (Fraenkel, 1950; Martin & Hare, 1942). Regardless, wheat whole flour and 5 % yeast is considered an optimum diet, despite the incongruity of a riboflavin deficiency (Fraenkel, 1950). The α-amylase inhibitor and β-amylase naturally present in the wheat bran seem to have a major role on the suitability of this bran for the T. molitor production (Applebaum, 1964). The α-amylase inhibitor inhibits the Tenebrio amylase and impacts the larval development and
16
increases mortality, but the presence of the β-amylase completely reverses its effect allowing a better larval development (Applebaum, 1964). On the contrary, the larvae of T. molitor undergo growth inhibition when whole ground corn or unheated corn germ is included in their diet, despite the supplementation with vitamins and amino acids known to be limiting in maize (Lipke & Fraenkel, 1955).
The studies of Ramos-Elorduy et al. (2002), Oonincx et al. (2015) and van Broekhoven et al. (2015) show that T. molitor can be successfully produced with diets composed of a mix of organic by-products and waste. Oonincx et al. (2015) and van Broekhoven et al. (2015) tested different levels of protein, fat and starch in diets of food manufacturing waste products such as: beet molasses, spelt grain and beer yeast, potato steam peelings, bread crumbles, cookie crumbles, and maize distillers’ dried grains. Ramos-Elorduy et al. (2002) tested diets with different levels of incorporation of waste of different plant sources (fruits, legumes and cereals), both raw and cooked.
Ideal Environmental Conditions
The insects are poikilothermic, not spending energy to maintain their body temperature above the ambient temperature, thus they depend on suitable ambient temperatures to grow and develop (Oonincx & De Boer, 2012; Oonincx et al., 2010; van Huis, 2013).
The optimum ambient temperature is advocated to range between 22ºC and 28ºC (Punzo & Mutchmor, 1980). Although the ideal production temperature varies in the literature between 25ºC (Connat et al., 1991; Fraenkel, 1950; Oonincx et al., 2010; Rho & Lee, 2016), 26,7 ºC (Urs & Hopkins, 1973a), 27ºC (Morales-Ramos et al., 2013), 28ºC (Thévenot et al., 2018; van Broekhoven et al., 2015) and 30ºC (Fraenkel, 1950; Weaver & McFarlane, 1990).
At 30ºC the T. molitor presents a shorter egg stage (6 days), larvae have more moults, and the pupal stage is reduced (6 days) (Ludwig & Fiore, 1960). However, the larvae stage is longer and the adults do not live as long as when the ambient temperature is maintained at 25ºC (Ludwig & Fiore, 1960). Thus, 30ºC seems to be above the optimum ambient temperature for T. molitor production (Ludwig & Fiore, 1960), nevertheless the larvae double their weight at 30ºC when compared to 25ºC (Fraenkel, 1950).
The optimum ambient relative humidity for T. molitor production varies, according to the literature, between 50% (Urs & Hopkins, 1973a), 55% (Weaver & McFarlane, 1990), 65% (Morales-Ramos et al., 2013; Thévenot et al., 2018; van Broekhoven et al., 2015), 70% (Connat et al., 1991; Fraenkel, 1950) and 80% (Oonincx et al., 2010). However, the optimum relative
17 humidity seems to be somewhere within 70 and 75%, since a humidity increase up to 85% increases the growth rate but favours the contamination by mould and other microorganisms, relative humidity of 12% leads to excessive water loss (Fraenkel, 1950; Punzo & Huff, 1989; Punzo & Mutchmor, 1980; Robinson, 2005).
The T. molitor exhibits negative phototaxis, preferring dark environments (Balfour & Carmichael, 1928; Cloudsley‐Thompson, 1953; Ghosh et al., 2017). However, the literature provides photoperiods that vary between 0 hours (Morales-Ramos et al., 2013; Oonincx et al., 2010) till 12 hours (Rho & Lee, 2016; van Broekhoven et al., 2015), 14h of light (Weaver & McFarlane, 1990) and 16 hours of light (Urs & Hopkins, 1973a).
The ventilation is also a factor to be considered in T. molitor production. Oonincx et al. (2010) advises a ventilation rate of 6.82 L/min.
The above literature review led us to conclude that the ideal environmental conditions are: a photoperiod of 0 hours light, an ambient temperature within 25 and 30ºC and an ambient relative humidity within 70 and 75%.
Chemical composition
The chemical composition of the T. molitor larvae is given in Table 3. On a dry matter basis, the protein content of the T. molitor is around 50% and its fat content is around 30%, (Table 3). However, by comparing the values obtained in different studies, it is possible to conclude that the nutritional content varies with the production conditions (Vrabec et al., 2015). The diet amino acids, fatty acids, vitamins and minerals content can affects the macro and micronutrients content of the insect (Oonincx et al., 2015; Ramos-Elorduy et al., 2002).
The fibre content is mostly chitin, and this indigestible component contains nitrogen, that can be estimated trough the ADF content (Bernard et al., 1997; Finke, 2007; Jonas-Levi & Martinez, 2017). The fact that this might not have been always taken into account, can explain some differences in the crude protein and fibre content reported in the literature.
18
Table 3 – Chemical composition of the T. molitor larvae (sources are shown in table).
~: authors do not provide the information * authors report crude fibre content
Chemical composition (%) Dry matter basis
Source Dry matter Crude Protein Crude Fat ADF NDF Ash
(Jones et al., 1972) ~ 52.82 35.42 ~ ~ 1.36 (Bernard et al., 1997) 37.6 52.7 32.8 5.7 3.2 (Barker et al., 1998) 38.1 51.9 31.1 0.5 2.9 4.3 (Barker et al., 1998) 41.7 48.1 40.3 0.4 11.2 3.2 (Finke, 2002) 38.1 49.08 35.17 6.56 14.96 2.36 (Finke, 2015) 31.1 59.80 26.37 7.17 ~ 3.63 (Zielińska et al., 2015) ~ 52.35 ± 1.1 24.7 ± 1.5 1.97 ± 0.3* 3.62 ± 0.6 (Ghosh et al., 2017) ~ 53.22 ± 0.32 34.54 ± 0.87 6.26 ± 0.03* 4.04 ± 0.13 (Thévenot et al., 2018) 93 50.7 28.2 ~ ~ 3.2
19 When produced with waste by-products the nutrient composition of the T. molitor can be similar to the composition obtained in the commercially produced larvae (Oonincx et al., 2
2015; Ramos-Elorduy et al., 2002; van Broekhoven et al., 2015). 4
Environmental Impact
The environmental impact of the conventional animal production can be explained 6
mainly by three factors: reproduction rates, enteric CH4 production, and feed conversion
efficiency (de Vries & de Boer, 2010). 8
The T. molitor do not produce CH4 or NH3, butproduces 1.5 mg of N2O per kg of body
mass per day (Oonincx et al., 2010). It has a higher daily gain (7.3 ± 2.5 %) than conventional 10
livestock, and a higher feed conversion efficiency, while its CO2 production is comparable or
lower (1,031± 349 g/kg mass gain) (Oonincx et al., 2010). Thus, the T. molitor production is 12
expected to have a smaller environmental impact.
When analysing T. molitor production per kg of edible protein, global warming potential 14
(GWP) and land use (LU) is low compared to other conventional animal products, and energy use (EU) is lower than beef, similar to pork, and higher than chicken, milk or eggs (Table 4). 16
The GWP and EU values of T. molitor production resulted mainly from the grains, gas, electricity and carrots, and the LU value from grains and carrots (Oonincx & De Boer, 2012). 18
The T. molitor meal also presents a competitive GWP and LU, with lower values when compared to conventional proteins. However, its EU is higher than for chicken meat, eggs and 20
milk and similar to pork meat production (Table 4). The diet of the T. molitor is a major contributor to the environmental impact of the larvae and meal production (Thévenot et al., 22
2018). Since land scarcity is a concern and the other factors can be mitigated by improved practices and technology, the T. molitor should be considered as a more sustainable alternative 24
to these products (Oonincx & De Boer, 2012).
Water footprint (WF) also represents a concern, insect production has three mainly 26
contributors: water used for growing the feed, water necessary for the animals’ growth, and water used on the farm for cleaning (Miglietta et al., 2015). Miglietta et al. (2015) estimates a 28
water consumption of 361,148.2 m3 to produce 83.2 ton of mealworms per year, revealing a water footprint of 4341 m3/ton, that is dominated by the WF of the diet, where the mixed grains 30
20
Table 4 – Life cycle assessment of T. molitor larvae, mealworms and livestock products production on a cradle-to-farm-gate approach, and T. molitor meal from cradle to mill gate (sources are shown in table).
Source (Thévenot et al., 2018) (Oonincx & De
Boer, 2012) (de Vries & de Boer, 2010)
T. molitor meal
T. molitor
larvae Mealworms* Pork Chicken Beef Milk Egg
GWP (kg of CO2-eq) kg of fresh 3.75 0.99 2.7 3.9–10 3.7–6.9 14–32 0.84–1.3 3.9–4.9 kg of edible protein 5.77 ~ 14 21–53 18–36 75–170 24–38 30–38 EU (MJ) kg of fresh 141.29 24.29 34 18–34 15–29 34–52 >10 >20 kg of edible protein 217.37 ~ 173 95–236 80–152 177–273 37–144 87–107 LU (m2 per year) kg of fresh 4.13 1.60 3.6 8.9–12.1 8.1–9.9 27–49 1.1–2.0 4.5–6.2 kg of edible protein 6.35 ~ 18 47– 48 42 – 52 144 – 258 33–59 35 – 48
*Tenebrio molitor and Zophobas morio ~: authors do not provide the information
GWP- Global Warming Potential; EU – Energy Use; LU – Land Use.
21 Mealworms production has the lowest annual water footprint per standard animal (0.003 m3/year/animal) compared to the conventional livestock meat (Miglietta et al., 2015). When referring to water footprint per edible ton, mealworms (4341 m3/ton) are comparable to chicken meat (4325 m3/ton) and lower than the other conventional meats, i.e. pig (5988 m3/ton) and beef meat (15,415 m3/ton) (Miglietta et al., 2015). Thus, from the water consumption
perspective, the production of T. molitor is more efficient.
Commercially produced T. molitor diet contains a mix of flours that can be used directly as livestock feed, thus T. molitor meal inclusion in poultry or fish diets decreases the efficiency of these productions, this is the reason why this practice does not decrease the environmental impact of these livestock productions (Thévenot et al., 2018).
Feed conversion ratio, weigh of feed consumed per live weight gained, of commercially produced yellow mealworm is around 2. This value varies in the literature between 2.2 (Oonincx & De Boer, 2012) and 1.8 (Thévenot et al., 2018), and when produced with diets composed of by-products is around 3 (van Broekhoven et al., 2015), and between 1.8 (high protein and fat diet) and 3.1 (high protein and low fat diet) with carrot supplementation (Oonincx et al., 2015). Diets high in protein result in higher feed conversion efficiency (Oonincx et al., 2015; van Broekhoven et al., 2015).
However, when fed on high protein diets the larvae excreted more acid uric trough faeces, indicating that they might use this strategy to cope with excess dietary protein (van Broekhoven et al., 2015). As so, mealworm manure, normally referred for insects as frass, can be collected and used for other applications. Thévenot et al. (2018) suggests it could be useful as organic fertilizer due to its C:N ratio (13.8) and NPK contents (29‰/34‰/22‰, respectively), similar to poultry manure. Since it presents less than 10% of moisture it would ease transport and spreading. Dossey et al. (2016) suggests it could be composted by adding some carbon rich feedstock as sawdust or straw, and then moistened and the pH adjusted to be around 7.5, then piled and turned every 48 h until the temperature of the pile would start to decrease, then left to rest until the pile does not heat up anymore, and then shipped in big bags. Furthermore, DeFoliart (1995) suggests the use of frass as fish feed.
The ability to convert low nutritive wastes in protein and fat, with a two-fold increase in protein, and a five- to six-fold in fat (Ramos-Elorduy et al., 2002); the feed conversion efficiency comparable to poultry and pigs (van Broekhoven et al., 2015); the 100% edible portion (van Broekhoven et al., 2015); the lower GWP and LU (Oonincx & De Boer, 2012); the low water footprint (Miglietta et al., 2015); the potential use of organic wastes as feed and
22
the potential to mass production (Ramos-Elorduy et al., 2002) makes T. molitor a sustainable alternative to the current feed and food products.
Tenebrio molitor Usage Feed
The usage of T. molitor larvae as feed has a long existence, used mostly for captive insectivorous animals, such as fish, amphibians, reptiles, birds, small mammals (Bernard et al., 1997) and primates (Jones et al., 1972). This species is included in the zoo feeding programs (Barker et al., 1998). At first, mealworm mass-production was for fishing bait, then the exotic pet market increased and demanded a bigger supply of live insects (Dossey et al., 2016). The T. molitor is also used for in vivo mass production of entomopathogenic nematodes (Morales-Ramos et al., 2011).
More recently, T. molitor appears as a potential alternative to the conventional fish meal and soybean meal due to the higher content of crude protein, and its potential use of organic waste as feed (Azagoh et al., 2016; Bußler et al., 2016; De Marco et al., 2015; Ng et al., 2001; Ramos-Elorduy et al., 2002). The results of the replacement of the conventional soybean and fish meal for T. molitor meal in poultry, weaning pigs, fish and shrimp diets are shown in (Table 5). The inclusion of the T. molitor meal at different levels in the diet of various species had no negative effects in their growth performances (Table 5).
The T. molitor meal has been proving to be an alternative to the conventional soybean meal for chickens, with protein quality similar to soybean meal, and a good source of metabolizable energy and digestible amino acids (De Marco et al., 2015; Ramos-Elorduy et al., 2002). The amino acid content reported in the literature shows some discrepancies, De Marco et al. (2015) states that T. molitor meal is rich in methionine, lysine, and threonine and Ramos-Elorduy et al. (2002) and Bovera et al. (2015) state that methionine and lysine content were not adequate in this meal.
Tenebrio molitor has been also proving to be an alternative to conventional fish meal to produce fish and shrimp, although methionine is a limiting amino acid for the shrimp production (Panini et al., 2017a). The inclusion of T. molitor meal modifies the fatty acid profile of the fish (e.g. European sea bass) and of the shrimp (e.g. Pacific white) (Gasco et al., 2016; Panini et al., 2017b).
23 The use of T. molitor meal in species other than insectivores must take into account the value of chitin, since insectivores produce an intestinal chitinase or host gut microorganisms that synthesise chitinase(s) (Bernard et al., 1997). The partridge seems to have a higher chitin digestibility than the broiler, probably due to a higher production of chitinase in the stomach, that increases the diet digestibility when the insect meal is included (Loponte et al., 2017).
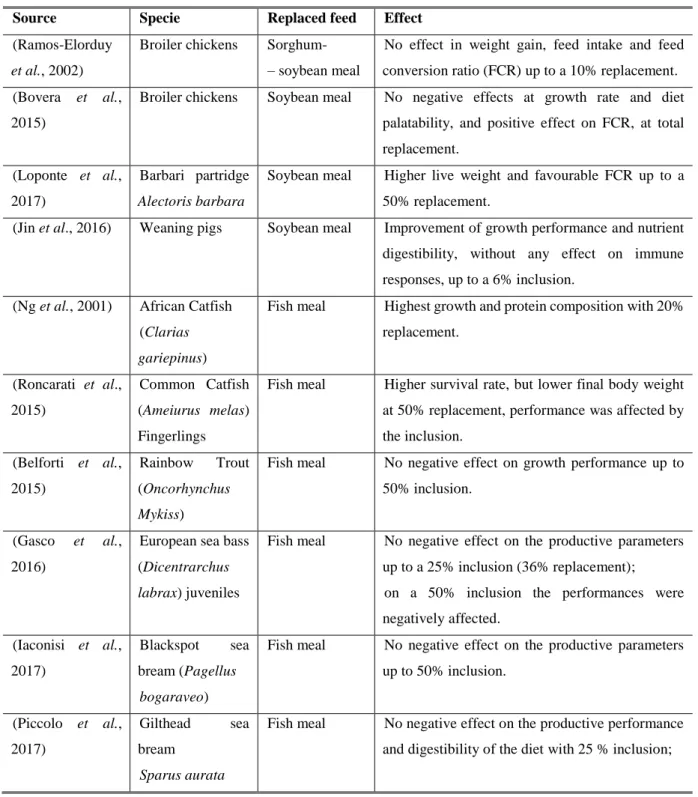
Table 5 – Replacement of soybean and fish meals in poultry, pig, fish, and shrimp diets (sources are shown in the table).
Source Specie Replaced feed Effect
(Ramos-Elorduy
et al., 2002)
Broiler chickens Sorghum- – soybean meal
No effect in weight gain, feed intake and feed conversion ratio (FCR) up to a 10% replacement. (Bovera et al.,
2015)
Broiler chickens Soybean meal No negative effects at growth rate and diet palatability, and positive effect on FCR, at total replacement.
(Loponte et al., 2017)
Barbari partridge
Alectoris barbara
Soybean meal Higher live weight and favourable FCR up to a 50% replacement.
(Jin et al., 2016) Weaning pigs Soybean meal Improvement of growth performance and nutrient digestibility, without any effect on immune responses, up to a 6% inclusion.
(Ng et al., 2001) African Catfish (Clarias
gariepinus)
Fish meal Highest growth and protein composition with 20% replacement. (Roncarati et al., 2015) Common Catfish (Ameiurus melas) Fingerlings
Fish meal Higher survival rate, but lower final body weight at 50% replacement, performance was affected by the inclusion. (Belforti et al., 2015) Rainbow Trout (Oncorhynchus Mykiss)
Fish meal No negative effect on growth performance up to 50% inclusion.
(Gasco et al.,
2016)
European sea bass (Dicentrarchus
labrax) juveniles
Fish meal No negative effect on the productive parameters up to a 25% inclusion (36% replacement); on a 50% inclusion the performances were negatively affected. (Iaconisi et al., 2017) Blackspot sea bream (Pagellus bogaraveo)
Fish meal No negative effect on the productive parameters up to 50% inclusion. (Piccolo et al., 2017) Gilthead sea bream Sparus aurata
Fish meal No negative effect on the productive performance and digestibility of the diet with 25 % inclusion;