Corresponding author: Farley Liliana Romero Vega. e-mail: lilianaromerovega@gmail.com
Received 29 August 2018 Accepted 13 November 2018
Major Article
Journal of the Brazilian Society of Tropical Medicine Vol.:52:e-20180347: 2019
doi: 10.1590/0037-8682-0347-2018
Emergence of chikungunya and Zika in a municipality
endemic to dengue, Santa Luzia, MG, Brazil, 2015-2017
Farley Liliana Romero Vega
[1], Juliana Maria Trindade Bezerra
[2],
Rodrigo Fabiano de Carmo Said
[3], Aloysio Nogueira da Gama Neto
[4],
Emanuela Cardoso Cotrim
[4], Dora Mendez
[5], Frederico Figueiredo Amâncio
[6]and Mariângela Carneiro
[1],[2][1]. Universidade Federal de Minas Gerais, Faculdade de Medicina, Programa de Pós-Graduação em Ciências da Saúde: Infectologia e Medicina Tropical, Belo Horizonte, MG, Brasil.
[2].Universidade Federal de Minas Gerais, Departamento de Parasitologia, Instituto de Ciências Biológicas, Laboratório de Epidemiologia de Doenças Infecciosas e Parasitárias, Belo Horizonte, MG, Brasil. [3].Secretaria de Saúde Estadual de Minas Gerais, Subsecretaria de Vigilância e Proteção à Saúde, Programa Estadual de Controle das Doenças Transmitidas pelo Aedes,Belo Horizonte, MG, Brasil.
[4].Secretaria Municipal de Saúde de Santa Luzia, MG, Brasil.
[5]. Núcleo de Ações e Pesquisa em Apoio Diagnostico, Laboratório de Genética e Biologia Molecular, Belo Horizonte, MG, Brasil. [6]. Fundação Hospitalar do Estado de Minas Gerais, Hospital João XXIII, Belo Horizonte, MG, Brasil.
Abstract
Introduction: The recent circulation of arboviruses transmitted by vectors, such as dengue, chikungunya, and Zika, is concerning due to the high morbidity rates, clinical complications, and increased demand on health services. The objective of this study was to analyze the clinical and epidemiological aspects of an epidemic caused by arboviruses in the municipality of Santa Luzia, Minas Gerais, Brazil. Methods: Longitudinal study of patients with acute febrile disease and suspected arbovirus infection
reported to Brazilian Notifiable Disease Information System (Sistema de Informação de Agravos de Notificação) from the epidemiological week 44 of 2015 to epidemiological week 52 of 2016. Patients with confirmed chikungunya were followed-up for 18 months and those with Zika for 15 months. Additionally, we analyzed and described the temporal distribution of confirmed
cases of these arboviruses in this municipality. Results: Overall 3,531 arboviruses cases, including 3,481 (98.7%) cases of
dengue, 38 (1.0%) cases of chikungunya, and 12 (0.3%) cases of Zika were confirmed. The highest incidence of arbovirus infection occurred in the first quarter of 2016 (epidemiological week 7 to 14). The most frequent symptoms were for dengue,
which included fever, headache, retro-orbital pain, and exanthema. Chikungunya infection was associated with fever, myalgia, arthralgia, and rash while Zika infection with pruritus and rash. Conclusions: Given the similarities in the initial clinical profiles of these arboviruses, it is important to perform a detailed clinical analysis, laboratory diagnosis, and patient follow-up.
Keywords: Dengue. Diagnosis. Chikungunya. Outbreak. Symptoms. Zika.
INTRODUCTION
Vector-borne infectious diseases account for more than
700.000 deaths per year worldwide and have become a concern
for public health systems due to their increasing incidence in recent decades1. Dengue, chikungunya, and Zika show high
morbidity rates, severe clinical forms, and lead to increased
demand for health and other support services. Moreover, the increasing number of epidemic regions showing co-circulation of the respective viruses DENV, CHIKV, and ZIKV is alarming1,2.
The high transmissibility and the changes in ecosystems associated with human occupation3,4 have made these diseases
a challenge to public health. The entry of the arboviruses, CHIKV and ZIKV, into countries endemic to dengue, such as Brazil, considerably impacts the health services, thus leading to negative economic, political, and social consequences1,5.
the disease, 60.9% of the deaths reported in the Americas in 2017 occurred in Brazil7. Moreover, dengue is responsible for
considerable loss of healthy years of life in the country, as it affects people from all age groups8.
Regarding chikungunya, most of the patients do not show complete recovery after the acute phase of the disease, with symptoms taking weeks or months to start regressing, and functional limitations interfering with the patient's occupational activities9,10. In 2017, Brazil was associated with 81.8% of the cases and 92.4% of the deaths due to chikungunya in the Americas11.
Zika infection, on the other hand, can lead to the development of Guillain-Barre syndrome with autoimmune and neurological alterations12. In the Americas, 45.1% of the cases and 50% of
the deaths due to Zika were concentrated in Brazil in 201611-13.
Given the similar initial symptomatology, the clinical diagnoses of DENV, CHIKV, and ZIKV may be hindered by the co-circulation of these pathogens13-15. Laboratory diagnosis,
which is still unavailable for most patients, has the drawback of long processing time; the resultant effect of the delay is helpful for epidemiological control but of little use for the treatment11.
Therefore, a better characterization of these arboviruses is required for differential diagnoses, especially in regions endemic to dengue, with co-circulation of CHIKV and ZIKV viruses. Moreover, a clinical and epidemiological follow-up of chikungunya and Zika infections would help to better understand the morbidity patterns of these diseases according to their severity, thus leading to a more comprehensive evaluation of the magnitude of the problem.
The highest incidence of cases of dengue in Brazil in 2017
was observed in the state of Minas Gerais16,and, in this state, the municipality of Santa Luzia was the first to report cases of chikungunya (November 2015), being a region endemic to
dengue, with co-circulation of CHIKV and ZIKV viruses. To better understand the clinical patterns and the consequences of the co-occurrence of these diseases, we characterized the temporal distribution of dengue, chikungunya, and Zika infections and analyzed the clinical and epidemiological characteristics of these three arboviruses in this endemic region. Understanding these patterns may help to guide and evaluate the interventions carried out by the public health system, thus improving the management of these arboviruses in endemic regions.
METHODS
Study Area - The municipality of Santa Luzia, MG (Brazil) is located in the metropolitan mesoregion of Belo Horizonte and
had an estimated population of 218,897 inhabitants in 2017.
Santa Luzia had a demographic density of 862.38 inhabitants/
km² and a territorial area of 235,076 km² in 2015. Its climate
is tropical with monthly average temperatures ranging from 18.4 to 29.2°C and mean temperatures oscillating around 5.4°C throughout the year17.
Geographically it is subdivided into 82 neighborhoods, which
are distributed into five regions: Parte Alta (28 neighborhoods), Parte Baixa, (23 neighborhoods), the São Benedito district (20
neighborhoods), expansion zone (eight neighborhoods), and the rural zone (three neighborhoods)17. The Unified Health System
(Sistema Único de Saúde – SUS) of the municipality has 26 public basic health units (Unidades Básicas de Saúde – UBS), one early care unit (Unidade de Pronto Atendimento - UPA),
and one hospital18.
Participants and inclusion criteria - We conducted a longitudinal case series study. Data were obtained from the
Brazilian Notifiable Disease Information System (Sistema de Informação de Agravos de Notificação - SINAN). We collected
information from patients with acute febrile illness that consulted public health system with suspicion of arboviruses
infection from the epidemiological week (EW) 44 of 2015 to EW 52 of 2016. Cases classified as ‘discarded’ or ‘blank’ by the
SINAN and those in which the diagnosis was not compatible with dengue, chikungunya, or Zika were excluded. Only patients
with clinical, epidemiological, and laboratory confirmation of
arboviruses infection were included.
Clinical confirmation was based on the Pan American Health Organization (PAHO)/World Health Organization (WHO)
clinical inclusion criteria for dengue, chikungunya, and Zika19,
which were established for patients presenting with acute febrile illness and two or more of the following signs and symptoms:
• Dengue: headache, retro-ocular pain, myalgia, arthralgia or rash; persistent vomiting, drowsiness and/or irritability, postural hypotension, painful hepatomegaly >2 cm, decreased diuresis, temperature drop, mucosal bleeding,
abrupt platelet collapse (<100,000) associated with
hemoconcentration19.
• Chikungunya: severe joint pain, headache, diffuse back pain, myalgia, nausea, vomiting, polyarthritis, rash, and conjunctivitis20.
• Zika: exanthema, conjunctivitis, muscle and joint pains, malaise or a headache, which is usually mild13.
Collected blood samples were submitted for viral RNA extraction, and then a reverse transcription followed by real-time
polymerase chain reaction (qRT-PCR) was performed using the
following kits:
• Dengue: Biogene Dengue PCR Kit - Bioclin Quibasa. Registration with Anvisa: 10269360302;
• Chikungunya: Biogene Kit Chikungunya PCR - Bioclin Quibasa. Registration at Anvisa: 10269360301;
• Zika: Biogene Kit Zika PCR Virus - Bioclin Quibasa. Anvisa Registration: 10269360300.
Characterization of patients - Subsequently, we evaluated
the clinical evolution of chikungunya once confirmed in a
patient, for up to 18 months. Patients were monitored through
three stages of their clinical evolution as follows: The first
monitoring occurred between one and six months after the initial diagnosis. The second and the third monitoring occurred
between 7 and 12 months, as well as between 13, and 18 months
after the initial diagnoses, respectively.
For Zika cases, two medical evaluations were carried
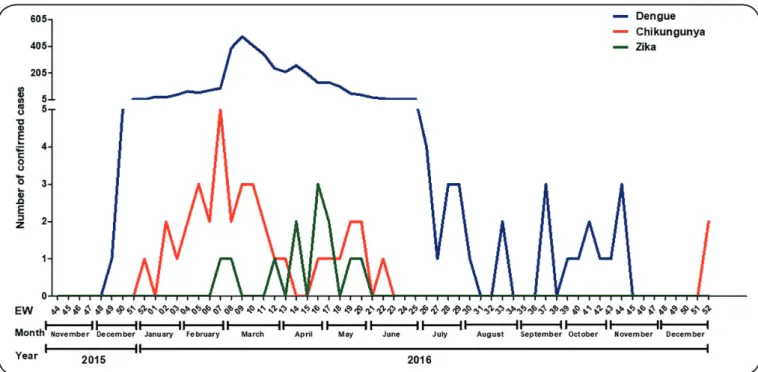
FIGURE 1: Temporal distribution of confirmed cases of arboviruses in Santa Luzia, Minas Gerais, Brazil, 2015-2016. EW: epidemiological week. monitoring), and between 12 and 15 months after diagnosis
(second monitoring).
Patients with dengue were not followed-up in the present study.
Analyses - We analyzed and described the temporal
distribution of confirmed cases of dengue, chikungunya, and
Zika in the municipality of Santa Luzia throughout the duration of the study.
The sociodemographic characteristics (age, gender, race, pregnancy, and region of origin) of the study population were
described. The clinical signs and symptoms were characterized according to the PAHO/WHO19 case definitions during the
initial phases for these three arboviruses infections. Results
were expressed as percentages and confidence intervals of 95% (95% CI).
Paired observations were evaluated to determine the occurrence of signs and symptoms in the same chikungunya patients during the acute and chronic phases of the disease.
Ethical considerations - This study was approved by the Research Ethics Committee of Hospital Foundation
of Minas Gerais State (FHEMIG), Protocol number
63286116.0.0000.5119.
RESULTS
Temporal distribution of confirmed cases
During the study period, from EW 44 of 2015 to EW 52 of
2016, 7,063 cases with International Classification of Diseases, Tenth Revision (ICD-10) diagnoses compatible with arboviruses were notified to SINAN in the municipality of Santa Luzia including 6,757 (95.7%) cases of dengue, 253 (3.5%) cases of
Zika, and 58 (0.8%) cases of chikungunya. Among those, 3,532 (3,276 of dengue, 236 of Zika, and 20 of chikungunya) were
excluded as they did not meet the clinical, epidemiological, and/or laboratory criteria. Therefore, the remaining 3,531
confirmed cases were included in the present study. These included 3,481 (98.7%) cases of dengue (with 954 (27.4%) confirmed by laboratory criteria and 2,527 (72.6%) by clinical-epidemiological criteria); 38 (1.0%) of chikungunya (confirmed by laboratory criteria); and 12 (0.3%) of Zika (confirmed by laboratory criteria).
In general, the highest incidence of arboviruses in the
municipality occurred between February (EW 7) and April (EW 14) 2016. The three diseases were observed occurring
concomitantly. However, while cases of dengue fever were
diagnosed during most of the period (November 2015 to November 2016); chikungunya cases occurred mainly between December (EW 51) 2015 and June (EW 23) 2016, with three new cases reported in December (EW 51) 2016. Confirmed
cases of Zika were restricted to the period between February
(EW 6) and May (EW 21) 2016 (Figure 1).
Demographic profile of patients with arboviruses
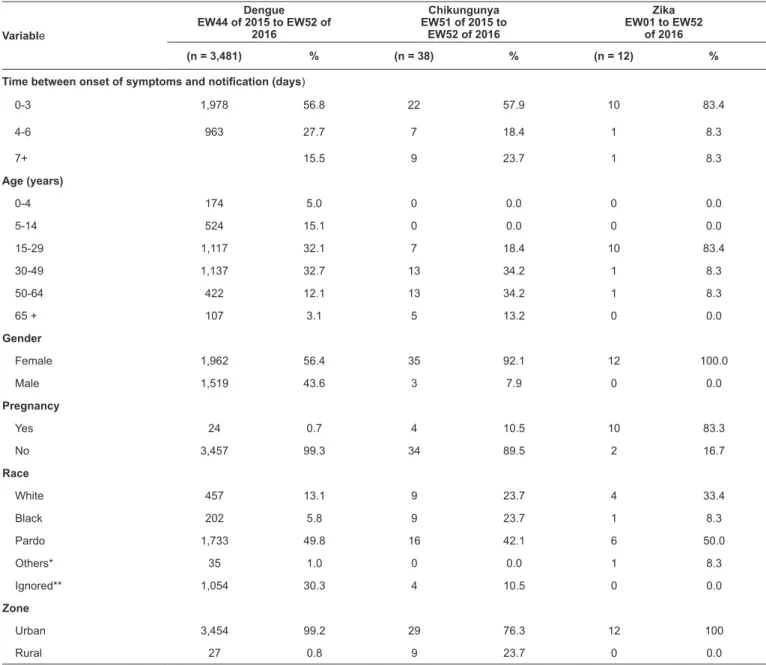
The mean age of patients with dengue was 29.4 (median, 25) years with 21.2% of patients aged between 21 to 30 years. The mean age of patients with chikungunya was 44.3 (median, 46) years, and 23.7% of patients were aged between 51 and 60 years. The mean age of patients with Zika was 29.2 (median, 26) years, with 41.7% of patients aged between 21 and 30 years. For the three diseases, most of the notifications occurred up
to three days following the onset of symptoms and the majority of the patients were females, Pardos, and urban region residents
TABLE 1: Surveillance and epidemiological characteristics of patients with arboviruses in Santa Luzia, Minas Gerais, Brazil, 2015-2016.
Variable
Dengue EW44 of 2015 to EW52 of
2016
Chikungunya EW51 of 2015 to
EW52 of 2016
Zika EW01 to EW52
of 2016
(n = 3,481) % (n = 38) % (n = 12) %
Time between onset of symptoms and notification (days)
0-3 1,978 56.8 22 57.9 10 83.4
4-6 963 27.7 7 18.4 1 8.3
7+ 15.5 9 23.7 1 8.3
Age (years)
0-4 174 5.0 0 0.0 0 0.0
5-14 524 15.1 0 0.0 0 0.0
15-29 1,117 32.1 7 18.4 10 83.4
30-49 1,137 32.7 13 34.2 1 8.3
50-64 422 12.1 13 34.2 1 8.3
65 + 107 3.1 5 13.2 0 0.0
Gender
Female 1,962 56.4 35 92.1 12 100.0
Male 1,519 43.6 3 7.9 0 0.0
Pregnancy
Yes 24 0.7 4 10.5 10 83.3
No 3,457 99.3 34 89.5 2 16.7
Race
White 457 13.1 9 23.7 4 33.4
Black 202 5.8 9 23.7 1 8.3
Pardo 1,733 49.8 16 42.1 6 50.0
Others* 35 1.0 0 0.0 1 8.3
Ignored** 1,054 30.3 4 10.5 0 0.0
Zone
Urban 3,454 99.2 29 76.3 12 100
Rural 27 0.8 9 23.7 0 0.0
*Yellow and indigenous; **Ignored: Not reported; EW: epidemiological week.
Clinical characterization
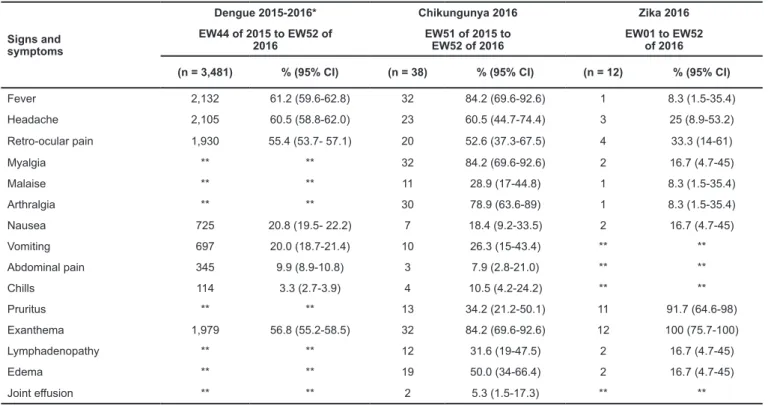
Among dengue patients, symptoms observed in the acute
phase were fever (61.2%, 95% CI 59.5-62.7), headache (60.5%, 95% CI 58.8-62.0), retro-ocular pain (55.4%, 95% CI 53.7-57.1), and exanthema (56.8% 95% CI 55.2-58.5). Regarding
chikungunya, the most frequent symptoms were fever and
myalgia (84.2%, 95% CI 69.6-92.6), arthralgia (78.9%, 95% CI 63.6-89.0), and exanthema (84.2% 95% CI 69.6-92.6). The
main symptoms observed among Zika patients were pruritus
(91.7%, 95% CI 64.6-98.0) and exanthema (100.0%, 95% CI 75.7-100.0) (Table 2).
The proportions of signs and symptoms observed during the clinical follow-up of chikungunya patients are presented in Figure 2. Eight (21.1%) patients showed no symptoms during
the first monitoring; 12 (31.6%) patients lacked symptoms
during the second; and 27 (73.3%) presented no symptoms
during the third evaluation.
Among the 38 cases of chikungunya confirmed by the laboratory and clinical criteria, 16 (42.1%) showed complicated
clinical evolution, presenting with myalgia, arthralgia, edema,
or hypoesthesia (Figure 2). Some additionally presented with clinical alterations involving the upper extremities, such as
functional limitation, trigger finger, tendinitis, and Raynaud's
syndrome. Eleven of these patients were being treated for
inflammatory arthropathy (comorbidity), while the other five had no history of comorbidity (Figure 2).
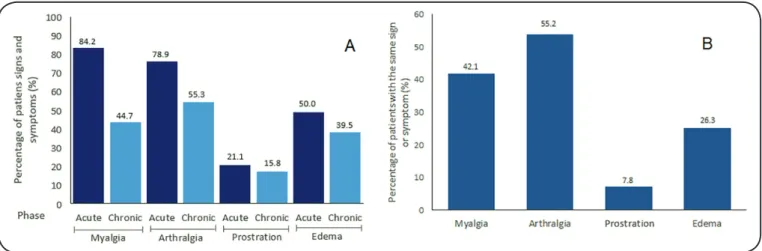
Among the signs and symptoms evaluated in the study
(myalgia, arthralgia, prostration and edema), four were verified
in patients during the acute and chronic phases. In the acute
TABLE 2: Clinical signs and symptoms of patients with arboviruses. Santa Luzia, Minas Gerais, Brazil, 2015-2016.
Signs and symptoms
Dengue 2015-2016* Chikungunya 2016 Zika 2016
EW44 of 2015 to EW52 of 2016
EW51 of 2015 to EW52 of 2016
EW01 to EW52 of 2016
(n = 3,481) % (95% CI) (n = 38) % (95% CI) (n = 12) % (95% CI)
Fever 2,132 61.2 (59.6-62.8) 32 84.2 (69.6-92.6) 1 8.3 (1.5-35.4) Headache 2,105 60.5 (58.8-62.0) 23 60.5 (44.7-74.4) 3 25 (8.9-53.2) Retro-ocular pain 1,930 55.4 (53.7- 57.1) 20 52.6 (37.3-67.5) 4 33.3 (14-61)
Myalgia ** ** 32 84.2 (69.6-92.6) 2 16.7 (4.7-45)
Malaise ** ** 11 28.9 (17-44.8) 1 8.3 (1.5-35.4)
Arthralgia ** ** 30 78.9 (63.6-89) 1 8.3 (1.5-35.4)
Nausea 725 20.8 (19.5- 22.2) 7 18.4 (9.2-33.5) 2 16.7 (4.7-45)
Vomiting 697 20.0 (18.7-21.4) 10 26.3 (15-43.4) ** **
Abdominal pain 345 9.9 (8.9-10.8) 3 7.9 (2.8-21.0) ** **
Chills 114 3.3 (2.7-3.9) 4 10.5 (4.2-24.2) ** **
Pruritus ** ** 13 34.2 (21.2-50.1) 11 91.7 (64.6-98)
Exanthema 1,979 56.8 (55.2-58.5) 32 84.2 (69.6-92.6) 12 100 (75.7-100)
Lymphadenopathy ** ** 12 31.6 (19-47.5) 2 16.7 (4.7-45)
Edema ** ** 19 50.0 (34-66.4) 2 16.7 (4.7-45)
Joint effusion ** ** 2 5.3 (1.5-17.3) ** **
*SINAN; ** Uninformed; EW: epidemiological week; 95% CI: 95% Confidence Interval.
with arthralgia, 19 (50.0%) with edema, and 8 (21.1%) with prostration. In the chronic phase, 21 (55.3%) patients had arthralgia, 17 (44.7%) had myalgia, 15 (39.5%) had edema, and 6 (15.8%) had prostration (Figure 3A). The following symptoms
presented in the same patient in both phases: arthralgia (21, [55.2%] patients), myalgia (16, [42.1%]), edema (10, [26.3%]), and prostration (3, [7.8%]), (Figure 3B).
Regarding the Zika cases, only 12 (4.8%) were confi rmed by laboratory tests, 10 (83.3%) of which were pregnant women. From the pregnant women, two, one, and seven were in the fi rst, second,
and third trimesters of gestation, respectively. These patients presented with exanthema maculopapular cases at consultation,
which declined progressively (12 cases), and pruritus (11 cases). The lesions affected the face (nine cases), upper extremities (11 cases), trunk (11 cases), abdomen (12 cases), and lower extremities (10 cases). The palms and the soles of the feet showed fewer lesions (fi ve and three cases, respectively).
During the two medical follow-ups (first and second monitoring), these Zika patients were asymptomatic. The infants
of these women were born at full term, and the initial neonatal assessments did not reveal any neurological changes until the
fi rst year of life.
DISCUSSION
In the present study, we characterized the temporal distribution and the clinical and epidemiological characteristics of arboviruses in a Brazilian municipality, endemic to dengue with co-occurrence of chikungunya and Zika. The municipality
studied was the fi rst to register the simultaneous occurrence of
these diseases in the state of Minas Gerais. A descriptive analysis of the CHIKV and ZIKV transmission scenario revealed that, in the years 2015 and 2016, cases of chikungunya were observed in 2,933 Brazilian municipalities, while Zika was reported in
2,759 municipalities16. In the same period, dengue was reported
in all federated units7.
Santa Luzia is endemic for dengue and fi rst reported cases of
chikungunya occurred in November 2015. Zika cases emerged
FIGURE 3: Signs and symptoms present (A) in the acute and chronic phase of all patients; (B) in acute and chronic phase of the same patient in Santa Luzia, Minas Gerais, Brazil, 2015-2016.
in the municipality in February of the following year. Between December 2015 and June 2016, the three diseases were observed occurring concomitantly. While dengue cases were reported throughout the study period, cases of chikungunya were
observed only from December (EW 51) 2015 to June (EW 23) 2016, and confi rmed cases of Zika were restricted to the period from February (EW 6) to May (EW 21) 2016.
The patterns of co-circulation of these three arboviruses in Santa Luzia differed compared to that observed in
another Brazilian municipality (Recife) in the Northeast
state of Pernambuco. While the three arboviruses occurred concomitantly in Santa Luzia, in Recife they occurred at different periods, with chikungunya cases being observed from October 2015 to February 2016, and Zika occurring in March and May 2016 only21. On the other hand, co-circulation of
DENV, ZIKV, and CHIKV was recorded in the municipality
of Tuparetama (also located in the state of Pernambuco), with laboratory confi rmation of two patients co-infected with DENV
and ZIKV, in April 201522.
The emergence of CHIKV and ZIKV in a region endemic for dengue creates new challenges for public health authorities,
since the initial clinical profi les of these arboviruses are very
similar1,12,23, and laboratory tests are not always available for rapid confi rmation, which makes accurate diagnosis diffi cult.
Some authors emphasize the importance of detailed clinical
analyses on the fi rst three days of acute symptoms, which
should be followed by laboratory diagnosis and follow-up of
the patients’ progression24,25.
In Santa Luzia, the highest proportion of patients with dengue
(1,394 cases, 40%) and Zika (nine cases, 75%) were between the
second and fourth decades of life. For chikungunya, the majority
of the patients were aged between 40 and 60 years (14 cases, 36.8%). Similar patterns in age distribution have been reported
in clinical follow-up surveys on the epidemiological dynamics of these three arboviruses in Brazil and other countries26,27.
documented in other studies, which reported the history of musculoskeletal system disorders28,29.
Ecological, climatological, and epidemiological conditions facilitate the propagation of mosquitoes transmitting pathogens30.
In this sense, Zika and chikungunya outbreaks in Brazil have
been most commonly reported in urban areas, thus confirming that the characteristics of the landscape play a significant role
in the distribution of the diseases, especially with regards to the life cycle of the vector Aedes aegypti31. The increased
population concentration, precarious basic sanitation, and poor health education of the population in Santa Luzia may explain the high density of the vector in this region.
The temporal distribution of arboviruses provides a useful tool that may help the public health system in making more informed decisions to reduce the occurrence and consequent burden of these diseases in the population32,33. Investments in
preparation for rapid response for dengue, chikungunya, and Zika, including surveillance and diagnosis, behavioral and social interventions, and communication, are essential not only for early detection but also for the management of acute events14.
The most frequent symptoms observed among dengue patients were fever, headache, retro orbital pain, and exanthema. Similar symptoms including myalgia, retro orbital pain, nausea, and arthralgia have been described in the literature as being consistently associated with adults, while vomiting and rash are the most common symptoms in children34. Other authors
have shown that the most frequent manifestations in dengue patients in the acute phase were fever, myalgia, headache, and arthralgia35.
The predominance of pregnant women among Zika patients is explained by the protocol adopted by the government,
which establishes that laboratorial confirmatory tests should
be performed only for this group of patients7. Among these patients, exanthema and pruritus were the most frequent clinical manifestations in Santa Luzia. Given the similarity of these initial symptoms to those of other arboviruses, we highlight the importance of correlating the epidemiological context and the evolution of individual symptoms. Similarly, in Guadeloupe, France, the dermatological characteristics at
the time of appearance of Zika in 60 confirmed cases were
exanthema associated with pruritus36,37. Moreover, certain clinical characteristics of the exanthematous lesions strongly point towards the viral etiology. This happens because the rash associated with the Zika virus infection is generally morbilliform, separates the palms and the plants, and begins on the face and extends cephalocaudally38.
In Brazil, especially in the North, Northeast, and Southeast regions, ZIKV outbreaks have been a cause of concern for pregnant women as the intrauterine infection is associated with multiple cerebral malformations and microcephaly39. A study on
newborns, from the Brazilian states of Pernambuco and Ceará, whose mothers had Zika infection during pregnancy revealed that, despite the absence of microcephaly at birth, 11 out of 13 babies presented with brain abnormalities associated with
congenital Zika syndrome at the age of five months40. In our
study, children delivered by the ten pregnant patients who had
a positive diagnosis for ZIKV infection did not present with neurological sequelae when evaluated at birth. Although it was not in the scope of our study, we highlight that it is important to ensure the clinical follow-up of these newborns, according to the recommendations of the Ministry of Health7.
For chikungunya, the most frequent symptoms were fever
(84.2%), myalgia (84.2%), and arthralgia (78.9%). This is in
agreement with a previous prospective cohort study showing
fever (89.7%) and arthralgia (87.6%) to be the most common symptoms among the 97 chikungunya patients; arthralgia
persisted for up to six weeks, especially among women41.
Our chikungunya patients were clinically evaluated over
a period of up to 18 months from the date of confirmation
of infection. Sixteen patients presented with complicated clinical evolution, including clinical manifestations such as arthropathy, arthralgia, myalgia, edema, or hypoesthesia, as well
as functional limitation, trigger finger, tendinitis, and Raynaud's syndrome. These findings are consistent with an evaluation in
50 patients with chronic musculoskeletal symptoms secondary to chikungunya fever during the 2016 outbreak that occurred
in Rio de Janeiro, (Brazil), which revealed joint synovitis and
tenosynovitis symptoms42.
Among the signs and symptoms that were present in the
three clinical phases of evolution of chikungunya (acute, subacute, and chronic), we observed that prostration and edema
improved over time, in contrast to the accentuation of myalgia and arthralgia. Similarly, a randomly sampled survey of 1,094 patients with IgG positive for chikungunya 18 months after the
outbreak that occurred in Isla Reunion revealed that 45% of
them still presented with musculoskeletal pain43.
Likewise, a prospective cohort study with 500 patients who
were clinically diagnosed and serologically confirmed with
chikungunya during the 2014-2015 epidemic in Colombia estimated that, after 20 months, a quarter of the participants
presented with persistent joint pain, and 16% of them reported
interference with their daily activities, documenting that they were missing school or work44. Therefore, it is noteworthy
that the clinical follow-up assessments should focus on these signs and symptoms to optimize the treatment according to the
individuals’ comorbidities.
One could argue that the main limitation of this study is the quality of the data obtained retrospectively from the SINAN database. In addition, the completion of clinical data during epidemics is only mandatory for patients with severe dengue, as it is assumed that dengue without warning signs usually presents with the classical symptomatology. Moreover, the Zika cases included only pregnant women who were laboratory-tested for the disease. Nevertheless, our study provides a long-term clinical evaluation of patients during an important outbreak of chikungunya and Zika in a region with high incidence of dengue, thus representing a relevant contribution to the understanding of the co-occurrence of these arboviruses.
monitoring of the geographical distribution and temporal trend of the infection, to characterize the presentation of the disease, and to identify serious complications13,14.
The public health system of countries endemic to these arboviruses should be aware of the clinical and laboratory
indicators verified at the time of diagnosis, as well as being
vigilant for the Ae. aegypti vector, responsible for the transmission of these and other viral diseases, such as yellow fever. The entomological research, together with the knowledge of the temporal distribution of these arboviruses, must also be taken into account to better prevent the spread of these diseases.
Acknowledgments: We thank all the staff of the Secretaria de Saúde do Estado de Minas Gerais. Sub-secretaria da Vigilância e Proteção à Saúde da Superintendência de Vigilância Epidemiológica, Ambiental e do Trabalho do Programa Estadual de Controle de Doenças Transmitida por Aedes and
Secretaria de Saúde da Cidade de Santa Luzia.
Conflict of interest: The authors declare that there is no conflict of interest.
Financial support: This study was supported by the Ministry of Health of Brazil, Fundo Nacional de Saúde. (Grant No.
25000.214371/2012-4). FLRV thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil), Post-Graduate Program of Infectology and
Tropical Medicine, Universidade Federal de Minas Gerais for the Doctoral schoralship. JMTB thanks to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PNPD/CAPES-Brazil), Post-Graduation Program
in Parasitology, Universidade Federal de Minas Gerais for the Postdoctoral fellowship. MC would like to thank to the Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG - Brazil) for the grant of the Programa Pesquisador Mineiro (PPM-2016) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) for the research fellowship.
REFERENCES
1. Paixão ES, Teixeira MG, Rodrigues LC. Zika, chikungunya and dengue: the causes and threats of new and reemerging arboviral
diseases. BMJ Glob Health. 2018;3(Suppl 1):e000530.
2. Rodriguez A, Villamil-Gómez WE, Franco Paredes C. The arboviral burden of disease caused by cocirculation and co-infection of dengue, chikungunya and Zika in the Americas. Travel Med Infect
Dis.2016;14 (3):177-9.
3. Fauci AS & Morens DM. The perpetual challenge of infectious
diseases. N Engl J Med. 2012;366(5):454-61.
4. Fuller TL, Calvet G, Genaro EC, Angelo R, Abiodum GJ, Halaí UA, et al. Behavioral, climatic, and environmental risk factors for Zika and Chikungunya virus infections in Rio de Janeiro, Brazil,
2015-16. PLoS One. 2017;16(11):12.
5. Montibeler EE, Oliveira DR. Dengue endemic and its impact
on the gross national product of Brazilian’s economy. Acta Trop. 2018;(178):318-26.
6. Bhatt S, Gething WP, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature.
2013;(496):504-7.
7. Ministério de Saúde (MS) Brasil [Internet]. Secretaria de vigilância em saúde. Boletim Epidemiológico 35; 2017 [updated 2017 Sept 15; cited 2017 Out 31]. Available from: http://portalarquivos-.saude.gov.
br/images/pdf/2017/setembro/15/2017-028-8. Miranda de Araújo E, Bezerra JM, Figueiredo F, de Azeredo Passos VM, Carneiro, M. Increase in the burden of dengue in Brazil and federated units, 2000 and 2015: analysis of the Global Burden of
Disease Study 2015. Rev Bras Epidemiol. 2017;20(l):205-14.
9. Schawartz O, Matthew A. Biology and pathogenesis of chikungunya
virus. Nat Microbiol. 2010;(8):497-500.
10. Sutaria RB, Amaral JK, Schoen RT. Emergence and treatment
of chikungunya arthritis. Curr Opin Rheumatol. 2018;(3):256-63.
11. Pan American Health Organization [Internet]. Chikungunya: Data,
Maps and Statics; 2017 [updated 2017 March 27; cited 2017 Oct 15].
Available from: https://www.paho.org/hq/index.php?option=com_ topics&view=readall&cid=5932&Itemid=40931&lang=en
12. Proenca-Modena JL, Milanez GP, Costa ML, Judice CC, Maranhão C. Zika virus: lessons learned in Brazil.
Microbes Infect. 2018;(18):30052.
13. Wold Health Organization (WHO) [Internet]. Zika cases and congenital syndrome associado with Zika virus reported by
countries and territories in the Americas; 2016 [updated 2017 Jan 18; cited 2017 Oct 15]. Available from: http://www.paho-.org/hq/ index.php?option=com_docman&task=doc_view&Itemid=270&gi d=37582&lang=en.
14. Wold Health Organization (WHO). Global Strategy for dengue prevention and control 2012-2020. 4th ed. Geneva; 2012. 22-4 p.
15. Pan American Health Organization (PAHO) [Internet]. Dengue:
Data, Maps and Statistics; 2017 [updated 2017 Jan 26; cited 2017 Out 31]. Available from: https://www.paho.org/hq/index. php?option=com_topics&view=readall&cid=3273&lang=en
16. Secretaria de Saúde Minas Gerais [Internet]. Boletim epidemiológico de monitoramento dos casos de dengue, chikungunya e zika vírus;
2017 [updated 2017 Dez 18; cited 2018 Feb 11]. Available from: http://www.saude.mg.gov.br/component/search/?all=boletim%20 epidemiologico%20dengue&exact=&any=&none=&created=&mo dified=&from=8&area=stories
17. Instituto brasileiro de geografia e estatística [Internet]. Santa Luzia-MG; 2017 [updated 2017 Jun 1; cited 2018 Feb 3]. Available from:
https://cidades.ibge.gov.br/brasil/mg/santa-luzia/panorama 18. Prefeitura Municipal do município de Santa Luzia [Internet]. Plano
municipal de saneamento básico; 2014 [updated 2014 Jul 23; cited 2018 april 12]. Available from: http://www.santaluzia.mg.gov.br/
images/leis/2014/Decretos/2967.pdf
19. Pan American Health Organization. Dengue guidelines for patient care in the region of the Americas. Second edition. Washington
D.C. Pan American Sanitary Bureau. Regional Office of the World
Health Organization; 2016; 10-16 p.
20. Center for Disease Control and Prevention [Internet]. About the Division of Vector-Borne Diseases. [updated 2018 May 1; cited 2018
Jul 7]. Available from: https://www.cdc.gov/ncezid/dvbd/index.html
21. Magalhaes T, Braga C, Cordeiro MT, Oliveira A, Castanha P, Maciel AP, et al. Zika virus displacement by a chikungunya outbreak in
Recife, Brazil. PLoS Negl Trop Dis. 2017; (11):11.
22. Pessôa R, Patriota JV, de Souza L, Felix AC, Mamede N, Sanabani SS. Investigation Into an Outbreak of Dengue-like Illness in Pernambuco, Brazil, Revealed a Cocirculation of Zika, Chikungunya
and Dengue Virus Type 1. Medicine.2016;95(12):3201.
23. Bautista-Reyes E, Núñez-Avellaneda D, Alonso-Palomares LA, Salazar MI. Chikungunya: Molecular Aspects, Clinical Outcomes
and Pathogenesis. Rev Invest Clin. 2017; 69(6):299-07.
24. Cardoso CW, Paploski M, Kikuti M, Rodrigues MS, Silva MM, Campo GS, et al. Outbreak of exanthematous illness associated with zika, chikungunya, and dengue viruses, Salvador,Brazil, J Emerg.
Infect. Dis. 2015;21(12):2274-6.
25. Colombo T, Estofolete C, Reis AFN, Da Silva NS, Aguiar ML, Cabrera EMS, et al. Clinical, laboratory and virological data from suspected ZIKV patients in an endemic arbovirus area. J Clin Virol.
26. Rezza, G., El-Sawaf G, Faggioni, G, Vescio F, Al Ameri R, De Santis R, et al. Co-circulation of Dengue and Chikungunya Viruses,
Al Hudaydah, Yemen, 2012. J Emerg Infect Dis. 2014;20(8):1351-4. 27. Silva MM, Rodrigues MS, Paploski IA, Kikuti M, Kasper AM, Cruz JS, et al. Accuracy of Dengue Reporting by National Surveillance
System, Brazil. J Emerg Infect Dis. 2016;22(2):336-9.
28. Javelle E, Ribera A, Degasne I, Gaüzère B-A, Marimoutou C,
Simon F. Specific Management of Post-Chikungunya Rheumatic
Disorders: A Retrospective Study of 159 Cases in Reunion Island
from 2006-2012. PLoS Negl Trop Dis. 2015:9(3):e0003603.
29. Gérardin P, Fianu A, Michault A, Mussard C, Boussaïd K, Rollot O, et al. Predictors of Chikungunya rheumatism: a prognostic survey ancillary to the TELECHIK cohort study. Arthritis Res Ther.
2013:15(1):R9.
30. Baylis M. Potential impact of climate change on emerging
vector-borne and other infections in the UK. Environ Health. 2017;16(1):112.
31. Aguiar S, Lorenz C, Virginio F, Suesdek L, Chiaravalloti-NF. Potential Risks of Zika and Chikungunya Outbreaks in Brazil: a
Modelling Study. Int J Infect Dis. 2018;(18):342-3.
32. Carlson C, Dougherty E, Getz W. An ecological assessment of the
pandemic threat of Zika virus. PLoS Negl Trop Dis. 2016;10(8):1-18.
33. Messina JP, Kraemer M, Brady O, Pigott DM, Shearer FM, Weiss DJ, et al. Mapping global environmental suitability for Zika virus.
eLife. 2016;(5):1-19.
34. Souza LJ, Pessanha LB, Mansur LC, Souza LA, Ribeiro MB, Silveira Mdo V, et al. Comparison of clinical and laboratory characteristics between children and adults with dengue. Braz J
Infect Dis. 2013;(1):27-31.
35. Matta L, Barbosa MM, Morales-Plaza CD. Clinical profile of dengue in patients consulting a tertiary hospital in the city of
Cali, Colombia, 2013. Biomedica. 2016; 36(1):133-9.
36. Cordel N, Birembaux X, Chaumont H, Delion F, Chosidow O, Rressières B, et al. Main Characteristics of Zika Virus Exanthema
in Guadeloupe. JAMA Dermatol. 2017;153(4):326-8.
37. Pinheiros T, Guimarães LF, Silva MT, Soares CN. Neurological manifestations of chikungunya and zika infections.
Arq Neuropsiquiatr. 2016;74(11):937-43.
38. Korman AM, Alikhan A, Kaffenberger B. Viral exanthems: An update on laboratory testing of the adult patient. J Am Acad
Dermatol. 2017;3(76):538-50.
39. Mendelski PA, Maia DL, Werner H, Daltro P, Fazecas T, Guedes B, et al. Zika virus and pregnancy in Brazil: What happened?
J Turk Ger Gynecol Assoc. 2018;19(1):39-47.
40. Linden V, Pessoa A, Dobyns W, Barkovich AJ, Júnior HV, Filho EL,
et al. Description of 13 Infants Born During October 2015–January
2016 With Congenital Zika Virus Infection Without Microcephaly
at Birth-Brazil. Morbidity and Mortality Weekly Report (MMWR). Center for Disease Control and Prevention. 2016;65(47): 1343-8.
41. Win M, Chow A, Dimatatac F, Go CL, Leo YS. Chikungunya fever in Singapore: acute clinical and laboratory features, and factors
associated with persistent arthralgia. J Clin Virol 2010;49(2):111-4.
42. Mogami R, Pereira JL, Chagas Y, Torezani RS, Vieira A, Koifman AC, et al. Ultrasonography of Hands and Wrists in the Diagnosis of Complications of Chikungunya Fever. J Ultrasound Med.
2017;50(2):71-5.
43. Gérardin P, Fianu A, Malvy D, Mussard C, Boussaid K, Rollot O, et al. Perceived morbidity and community burden after a Chikungunya outbreak: the Telechik survey, a population-based cohort study.
2011. BMC Med. 2011;9(5):1-11.
44. Chang A. Encinales L, Firestein GS, Simon GL, Bethony JM. Frequency of Chronic Joint Pain Following Chikungunya Virus Infection: A Colombian Cohort Study. J Arthritis Rheumatol.