w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Treatment
of
latent
tuberculosis
in
patients
with
juvenile
rheumatic
diseases:
a
systematic
review
José
Cleosmaque
Leite
Júnior
a,
Regina
Terse
Trindade
Ramos
b,
Teresa
Cristina
Martins
Vicente
Robazzi
b,∗aUniversidadeFederaldaBahia(UFBA),FaculdadedeMedicina,Salvador,BA,Brazil bUniversidadeFederaldaBahia(UFBA),DepartamentodePediatria,Salvador,BA,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received2July2016 Accepted24November2016 Availableonline21February2017
Keywords:
Rheumaticdiseases Child
Adolescent Biologicalfactors Latenttuberculosis
a
b
s
t
r
a
c
t
Introduction:ChildrenandadolescentswithrheumaticdiseasesreceivingTNFblockersare atriskfortheactivationoflatentMycobacteriumtuberculosisinfection(LTBI).AlthoughLTBI treatmentisindicatedinthisgroup,therearedifferenttherapeuticregimensinthe litera-ture,withoutadefiniteconsensus.
Objectives: Toreviewintheliteraturetherapeuticschemesusedandindicatedforthe treat-mentofLTBIinthesepatients.
Methods:Systematicreviewoftheliterature,usinghealthdatabases,selectingstudiesthat addressedthetreatmentofLTBIinpatientswithjuvenilerheumaticdiseasesusingTNF blockers,from1990to2015.Allstudydesignswereconsidered.
Results:Atotalof162studieswereidentifiedthroughtheelectronicdatabasesandonewas foundthroughamanualsearchbytheauthor,totaling163articles.Weexcludedstudiesthat didnotmeetthementionedinclusioncriteria,andincludedaretrospectivecohortstudyand twoprospectivecohortstudies.Thethreestudiesaddressedtreatmentwithisoniazid(INH) for9monthsandoneofthemalsoaddressedINHtreatmentassociatedwithrifampicinfor 3months.
Conclusions: OnlyonecaseofLTBIactivationwasobserved;therewasgoodtreatment adher-enceandabsenceofcomplicationsduringfollow-up.Morestudiesarenecessarytoevaluate theresponsetotheotheravailabletherapeuticregimens,withbettertolerabilityassessment andalargersample.However,theresultsshowedthatINHtherapyfor9monthsandINH therapyplusrifampicinfor3monthshadalowrateofLTBIactivationandcomplications.
©2017PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:trobazzi.ufba@gmail.com(T.C.Robazzi).
http://dx.doi.org/10.1016/j.rbre.2017.01.009
Tratamento
da
tuberculose
latente
em
pacientes
com
doenc¸as
reumáticas
juvenis:
uma
revisão
sistemática
Palavras-chave: Doenc¸asreumáticas Crianc¸a
Adolescente Fatoresbiológicos Tuberculoselatente
r
e
s
u
m
o
Introduc¸ão: Crianc¸aseadolescentescomdoenc¸asreumáticasemterapiaanti-TNF-␣são
grupoderiscoparaativac¸ãodainfecc¸ãolatenteporMycobacteriumtuberculosis(ILTB).Embora otratamentodaILTBsejaindicadonessegrupo,existemdiferentesesquemasterapêuticos naliteratura,semumconsensodefinido.
Objetivos:Revisarnaliteraturaesquemasterapêuticosusadoseindicadosparaotratamento daILTBnessespacientes.
Métodos: Revisãosistemáticadaliteratura,nasbasesdedadosemsaúde,selecionaram-se estudosqueabordaramotratamentodaILTBempacientesreumáticosjuvenisemusode anti-TNF-␣,de1990a2015.Todososdesenhosdeestudoforamconsiderados.
Resultados: Foramidentificadosatravésdasbasesdedadoseletrônicas162estudoseumfoi encontradopormeiodebuscamanualdoautor,totalde163.Foramexcluídososestudos quenãoatenderamaoscritériosdeinclusãoreferidos,incluídosumestudodecoorte retro-spectivaedoisdeestudosdecoorteprospectivas.Ostrêsestudosabordaramotratamento comisoniazida(INH)pornovemeseseumdelesabordoutambémotratamentocomINH associadoarifampicinaportrêsmeses.
Conclusões: Foi observado apenas um casode ativac¸ão da ILTB; uma boa adesão ao tratamentoe ausênciadecomplicac¸õesdurante oacompanhamento.Maisestudossão necessáriosparaavaliararespostaaosoutrosesquemasterapêuticosdisponíveis,com mel-horavaliac¸ãoda tolerabilidadeemaioramostra.Porém,osresultadosmostraramquea terapiacomINHpornovemeseseaterapiacomINHmaisrifampicinaportrêsmesestêm baixoíndicedeativac¸ãoecomplicac¸ões.
©2017PublicadoporElsevierEditoraLtda.Este ´eumartigoOpenAccesssobuma licenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Even though the overall incidence of tuberculosis (TB) decreasedby18%inrelationtotheyear2000whenthe Mil-lenniumDevelopmentGoalswereestablished,TBisthefifth largestcauseofdeathworldwide,whenoneincludesdeaths fromTBinindividualsinfectedwiththehuman immunode-ficiencyvirus(HIV).However,thepossibilityofcureishigh whenearlydiagnosisandtreatmentoccur.1
Itisestimatedthat9.6millionpeoplehadthediseasein 2014,withonemillionchildren,although37%ofcaseswere notdiagnosedorreported.Additionally,TBwasresponsiblefor thedeathsof1.5millionindividualsin2014,ofwhich140,000 werechildren.2
InBrazil,theaimofreducingTBincidence,prevalence,and mortalitybyhalfin2015comparedto1990,asdefinedbythe WorldHealthOrganization(WHO)1hasbeenmet.However, thecountryremainsinthegroupof22countrieswiththe high-estratesofthedisease.BrazilhasanincidenceofTBof33.83 andamortalityof2.6per100,000inhabitants.4,5
InthestateofBahia,theincidenceofTBwas30.5newcases per100,000inhabitantsandmortalityof2.1per100,000 inha-bitantsin2014.SãoPaulo,RiodeJaneiroandBahiaarethe threestateswiththehighestnumberofTBcasesinthe coun-tryandtogethertheywereresponsiblefor44.5%ofnewcases in2013.6
TheWHO defineslatentMycobacterium tuberculosis infec-tion (LTBI)asa stateofpersistentimmune responsetoM.
tuberculosisantigenstimulationwithnoevidenceofclinical manifestations of activeTB. It is estimatedthat one-third of the world’s population isinfected by this bacterium. In Brazil,theincidenceis46per100,000individuals.Despitethe absenceofsymptoms,thereisariskthatthesepatientswill developTBdisease,especiallyinthefirsttwoyearsafterthe primaryinfection.Activationoccursin5–10%ofcases.7
RiskfactorsforthedevelopmentofactiveTBinclude:HIV infection,contactwithacaseofpulmonaryTB,therapywith TNFblockers,dialysis,organtransplantationorblood trans-fusion,andsilicosis.8
TheevaluationofcontactwithTBcasesisimportantfor thescreeningofLTBI.TheMinistryofHealthconsidersas con-tacteverypersonlivinginthesameenvironmentastheindex caseatthetimeofTBdiagnosis,andtheassessmentofthe degreeofcontactexposureshouldbeindividualized, consid-eringtheformofthedisease,theenvironment,andthetime ofexposure.Contactsundertheageoffive,peoplewith HIV-AIDSandthoseathighriskshouldbeconsideredaspriorities intheprocessofcontactevaluationandLTBItreatment.9
Thus,adequatescreeninganddiagnosisareveryimportant topreventLTBIactivation.Themostwidelyuseddiagnostic methods includethe tuberculin skintest(TST), Interferon-Gamma Release Assays (IGRAs),10 and chest X-rays.11 TST isthemostoftenusedmethod,althoughthepositiveresult can alsooccurdue toactivediseaseor previouslyresolved infection.12,13
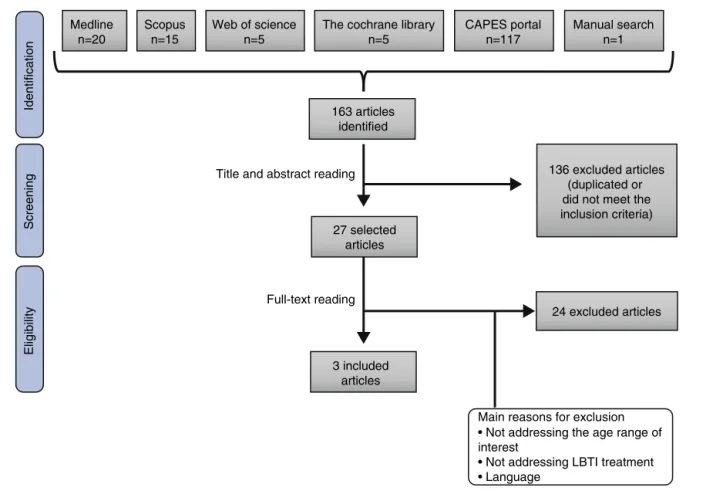
Table1–Keywordsusedinarticlesearch.
Palavras-chave Keywords
Adolescentes Adolescents
Crianc¸as Children
TuberculoseLatente LatentTuberculosis Doenc¸asreumáticas RheumaticDiseases
to illness or immunosuppressive treatment, patients with inflammatory/autoimmunediseasesusing immunosuppress-ivemedication need toundergo LTBI screening.13 Ofthese drugs,TNFblockersstandsout.TNF-␣isanessentialcytokine
formacrophageactivation,leukocyterecruitmenttothesite ofinfection andgranulomaformationandismore concen-tratedinorgansaffectedbyrheumaticdiseases.TNFblockers inhibitthiscytokineand,consequently,inhibitits inflamma-toryeffects.Thisactionofthesedrugsisveryimportantin thetreatmentofrheumaticdiseases;however,theinhibition ofTNF-␣-mediatedinflammatorypathwaysmayleaveusers
susceptibletoinfections.12,13
The Brazilian Registry of Biological Therapy Monitoring inRheumaticDiseases– BiobadaBrazil–inits 2014report, showed that the most important adverse events found in rheumatic patients submitted to biological therapies were infections and infestations. There were 19 cases of TB in the2464patientsusing biologicalagentsand controls,and only one patient was in the control group (patients with rheumatoidarthritis,juvenileidiopathicarthritis,or ankylos-ingspondylitis,nottakingbiologicalagents).Theanalysisof thereportalsoshowsthattheincidenceofTBwas101/100,000 patient-years,higherthanthatfoundintheBrazilian popula-tion,whichis37.2cases/100,000person-years.14
Additionally,itisknownthatthefrequencyofTBishigher inrheumaticpatientsthaninthegeneralpopulation,andthis riskincreaseswiththeuseofTNFblockers.13,15
Therefore,thepresentarticleaimstoreviewinthe litera-turethetherapeuticregimensusedtotreatlatenttuberculosis inpediatricpatientswithrheumaticdiseasesandtoidentify themostappropriatetherapeuticregimensforthesepatients.
Methods
Literaturesearch
This is a systematic review. The sources of information used for the literature search were health databases such as Medline (Pubmed) (www.ncbi.nlm.nih.gov/pubmed), Scopus (Elsevier) (www.scopus.com), The Cochrane Library (www.cochranelibrary.com), Web of Sci-ence (ISI) (webofknowledge.com), LILACS (Bireme) (www.bireme.br), Scielo (www.scielo.org) and CAPES Portal (www.periodicos.capes.gov.br).
ThekeywordsinPortugueseareDescriptorsinHealth Sci-ences(DeCS),aswellasthecorrespondingtermsinEnglish (Table1).English-languageanalogswereselectedfromMeSH. ThesearchwasperformedinDecember2015.
In Medline (Pubmed),the keywords and the equivalent termsintheEnglishlanguagewere cross-referencedinthe advancedsearchusingtheBooleanoperators“AND”and“OR”,
asfollows:((Latenttuberculosis)[AllFields]ANDRheumatic diseases[AllFields])AND(Children[AllFields]ORAdolescents [AllFields]).Combinedsearcheswereperformedusing(#)prior toeachsetnumberinthequery.
In the Cochrane Library database, keywords, equivalent terms,andtermswidelyusedintheEnglishlanguagewere cross-referencedintheadvancedsearchusingBoolean oper-ators“AND”and“OR”asfollows:LatentTuberculosis(Search all text) AND [Rheumatic Diseases (Search All Text)] AND [children (Search all text)OR adolescents(Search all text)]. Combinedsearcheswereperformedusing(#)priortoeachset numberinthequery.
IntheLILACSdatabase,keywords,equivalentterms,and terms widely used in the English language were cross-referencedintheadvancedsearch usingBooleanoperators “AND”and“OR”asfollows:[Latenttuberculosis(Words)]and [Rheumaticdiseases(Words)]and[children(Words)OR ado-lescents(Words)].Inthesearchsettings,theEnglishlanguage wasselectedasthe“Interfacelanguage”.
AsfortheadvancedsearchintheCAPESPortal,asthereare onlytwofieldstobefilledoutforthesearch,itwascarriedout asfollows:{[LatentTuberculosis(any/contains)]AND [Rheu-maticdiseases(any/contains)]}.Torefinethesearch,thedate ofpublicationdeterminedincludedthelast20years;forthe topic“typeofmaterial”,weselectedarticlesandthechosen languagewasEnglish.
IntheScielosite,keywords,equivalentterms,andterms widelyusedintheEnglishlanguagewerecross-referencedin theadvancedsearchusingBooleanoperators“AND”and“OR” asfollows:(LatentTuberculosis)[allindices]AND(Rheumatic diseases)[allindices]AND(Children[allindices]OR Adoles-cents[allindices]).
In the WebofSciencesite, keywords, equivalentterms, and termswidelyusedintheEnglishlanguagewere cross-referencedintheadvancedsearch usingBooleanoperators “AND”and“OR”andthe“TS”fieldlabel,whichrepresentsthe topic,andcomprisesallrecordsthatcontainthesearchterms inthefieldsofthetitle,abstract,orauthor’skeywords.The searchwascarriedoutasfollows:[TS=(LatentTuberculosis)] AND[TS=(Rheumaticdiseases)]AND[TS=(childrenOR ado-lescents)].Combinedsearcheswereperformedusing(#)prior toeachsetnumberinthequery.Sincetherewasapossibilityin thissitetorefinethesearch,studiesweresearchedbetween 1990and2015andtheselectedlanguagewasEnglish,since therewereonlytwooptions,EnglishandKoreanlanguages.
In the Scopus (Elsevier) database, keywords, equivalent terms, and terms widely used in the English language werecross-referencedintheadvancedsearchusingBoolean operators “AND” and “OR” as follows: [Latent Tuber-culosis (Article Title/Abstract/Keywords)] AND [Rheumatic Diseases (Article Title/Abstract/Keywords)] AND [children (Article Title/Abstract/Keywords) OR adolescents (Article Title/Abstract/Keywords)]. Combined searches were per-formedusing(#)priortoeachsetnumberinthequery.Studies publishedbetween1990and2015wereassessed.
Inclusionandexclusioncriteria
Medline n=20
Scopus n=15
Web of science n=5
The cochrane library n=5
CAPES portal n=117
163 articles identified
136 excluded articles (duplicated or did not meet the inclusion criteria)
24 excluded articles
Eligibility
Screening
Identification
Main reasons for exclusion • Not addressing the age range of interest
• Not addressing LBTI treatment • Language
27 selected articles
3 included articles Title and abstract reading
Full-text reading
Manual search n=1
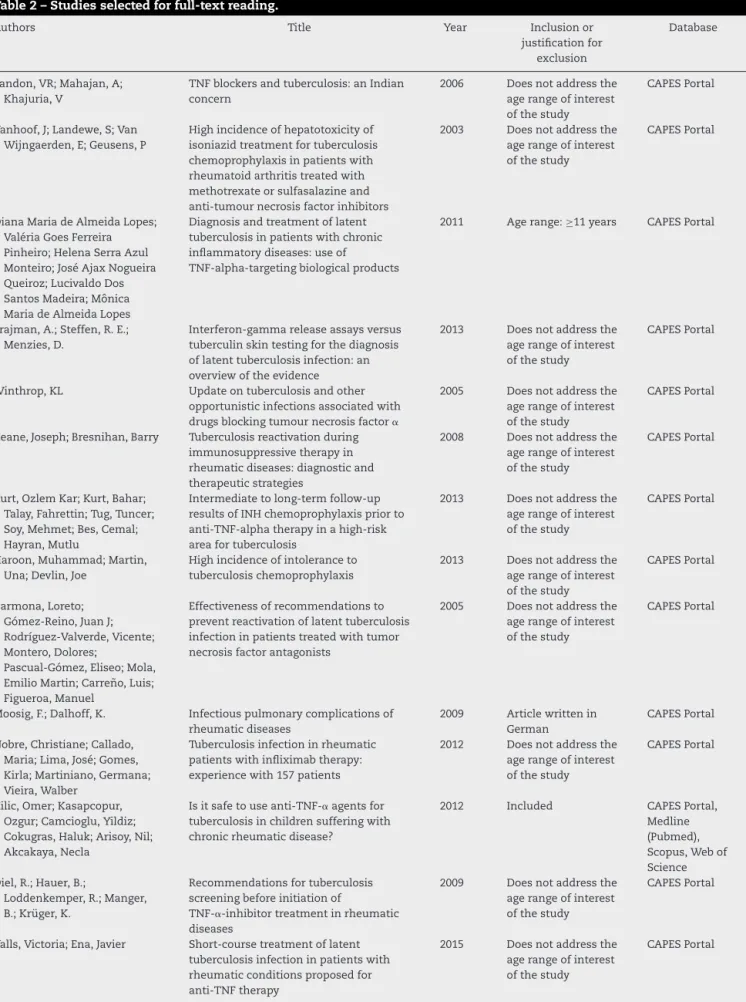
Fig.1–Flowchartofarticleidentificationandeligibilityinthesystematicreview.
adolescentsbetween1and 18years ofage withrheumatic diseases, within a publication period of 25 years (1990 to 2015) and studiespublished inEnglish and Portuguese.All studydesignswereconsidered relevant.Weexcluded stud-ies that didnot meet the previously establishedinclusion criteria.
Methodsofanalysis
Initially,all theresults,exceptduplicateswereanalyzedby readingthetitleand abstract,toselectthepossiblearticles thatwouldbeincludedinthestudy.
Afterthis previous study selection,based on the analy-sisofthe title andthe abstract,the textswere readinfull and only after that, the studies were definitivelyincluded inthe systematicreview,if they metthe previously estab-lishedinclusioncriteria.Atthismoment,whenstudieswere readinfulltodefineeligibility,theauthoralsoperformeda manualsearch ofthereferences includedin thesestudies, aimingattheidentification ofarticlesthatwere notfound duringthedatabase search,but thatcould befoundinthe references.
Asthearticlesinthereferenceswerenotidentifiedinthe resultsofthisstudy,theywereselectedforoverallreadingto determineiftheywouldbeincludedinthestudy.
Table 2 shows the authors, titles, year of publication, database, inclusion,or exclusion justificationofall studies selectedforfullreading.Furthermore,itcontainsthearticle selectedbymanualsearchperformedbytheauthor.
Results
Atotalof162studieswereinitiallyidentifiedthroughsearchin theelectronicdatabases(Medline20,Scopus15,TheCochrane Library5,WebofScience5,LILACS0,Scielo0andCAPES Por-tal117)and1wasidentifiedbymanualsearchperformedby theauthor,totaling163studies.Ofthese,136wereexcluded becausetheywereidenticalarticles,i.e.,thesamepublication wasfoundindifferentdatabases,andbecausetheydidnot meettheobjectivesand/orinclusioncriteriaofthesystematic review,basedonthereadingofthetitleandtheabstract.
The27 articlesthat were notexcluded atthescreening basedonthereadingoftitlesandabstracts,werefully eval-uatedtodetermineiftheymettheeligibilitycriteria.Ofthese, 3wereincludedinthisstudy.Fig.1showstheflowchartthat representstheselectionandeligibilityofthestudies.Table3
indicatesthearticlesincludedinthestudy highlightingthe author, year of publication,study design,sample size, age groupandLTBItreatmentused.
Studycharacteristics
Table2–Studiesselectedforfull-textreading.
Authors Title Year Inclusionor
justificationfor exclusion
Database
Tandon,VR;Mahajan,A; Khajuria,V
TNFblockersandtuberculosis:anIndian concern
2006 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Vanhoof,J;Landewe,S;Van Wijngaerden,E;Geusens,P
Highincidenceofhepatotoxicityof isoniazidtreatmentfortuberculosis chemoprophylaxisinpatientswith rheumatoidarthritistreatedwith methotrexateorsulfasalazineand anti-tumournecrosisfactorinhibitors
2003 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
DianaMariadeAlmeidaLopes; ValériaGoesFerreira Pinheiro;HelenaSerraAzul Monteiro;JoséAjaxNogueira Queiroz;LucivaldoDos SantosMadeira;Mônica MariadeAlmeidaLopes
Diagnosisandtreatmentoflatent tuberculosisinpatientswithchronic inflammatorydiseases:useof
TNF-alpha-targetingbiologicalproducts
2011 Agerange:≥11years CAPESPortal
Trajman,A.;Steffen,R.E.; Menzies,D.
Interferon-gammareleaseassaysversus tuberculinskintestingforthediagnosis oflatenttuberculosisinfection:an overviewoftheevidence
2013 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Winthrop,KL Updateontuberculosisandother opportunisticinfectionsassociatedwith drugsblockingtumournecrosisfactor␣
2005 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Keane,Joseph;Bresnihan,Barry Tuberculosisreactivationduring immunosuppressivetherapyin rheumaticdiseases:diagnosticand therapeuticstrategies
2008 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Kurt,OzlemKar;Kurt,Bahar; Talay,Fahrettin;Tug,Tuncer; Soy,Mehmet;Bes,Cemal; Hayran,Mutlu
Intermediatetolong-termfollow-up resultsofINHchemoprophylaxispriorto anti-TNF-alphatherapyinahigh-risk areafortuberculosis
2013 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Haroon,Muhammad;Martin, Una;Devlin,Joe
Highincidenceofintoleranceto tuberculosischemoprophylaxis
2013 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Carmona,Loreto; Gómez-Reino,JuanJ; Rodríguez-Valverde,Vicente; Montero,Dolores;
Pascual-Gómez,Eliseo;Mola, EmilioMartin;Carre ˜no,Luis; Figueroa,Manuel
Effectivenessofrecommendationsto preventreactivationoflatenttuberculosis infectioninpatientstreatedwithtumor necrosisfactorantagonists
2005 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Moosig,F.;Dalhoff,K. Infectiouspulmonarycomplicationsof rheumaticdiseases
2009 Articlewrittenin German
CAPESPortal
Nobre,Christiane;Callado, Maria;Lima,José;Gomes, Kirla;Martiniano,Germana; Vieira,Walber
Tuberculosisinfectioninrheumatic patientswithinfliximabtherapy: experiencewith157patients
2012 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Kilic,Omer;Kasapcopur, Ozgur;Camcioglu,Yildiz; Cokugras,Haluk;Arisoy,Nil; Akcakaya,Necla
Isitsafetouseanti-TNF-␣ agentsfor
tuberculosisinchildrensufferingwith chronicrheumaticdisease?
2012 Included CAPESPortal, Medline (Pubmed), Scopus,Webof Science Diel,R.;Hauer,B.;
Loddenkemper,R.;Manger, B.;Krüger,K.
Recommendationsfortuberculosis screeningbeforeinitiationof
TNF-␣-inhibitortreatmentinrheumatic
diseases
2009 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Valls,Victoria;Ena,Javier Short-coursetreatmentoflatent tuberculosisinfectioninpatientswith rheumaticconditionsproposedfor anti-TNFtherapy
2015 Doesnotaddressthe agerangeofinterest ofthestudy
Table2–(Continued)
Authors Title Year Inclusionor
justificationfor exclusion
Database
Bray,Marie-Gaëlle;Poulain, Cécile;Dougados,Maxime; Gossec,Laure
Frequencyandtoleranceof
antituberculosistreatmentaccordingto nationalguidelinesforpreventionofrisk oftuberculosisduetotumornecrosis factorblockertreatment
2010 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Chu,AlvinaD;Polesky,Andrea H;Bhatia,Gulshan;Bush, ThomasM
Activeandlatenttuberculosisinpatients withsystemiclupuserythematosusliving intheUnitedStates
2009 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Dinser,R;Fousse,M;Sester,U; Albrecht,K;Singh,M;Köhler, H;Müller-Ladner,U;Sester,M
Evaluationoflatenttuberculosisinfection inpatientswithinflammatory
arthropathiesbeforetreatmentwith TNF-alphablockingdrugsusinganovel flow-cytometricinterferon-gamma releaseassay
2008 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Xie,Xi;Li,Fen;Chen,Jin-Wei; Wang,Jia
Riskoftuberculosisinfectionin anti-TNF-␣ biologicaltherapy:From
benchtobedside
2014 Doesnotaddressthe agerangeofinterest ofthestudy;limited approachforLTBI treatment
CAPESPortal
He,Dongyi;Bai,Fengmin; Zhang,Shu;Jiang,Ting; Shen,Jie;Zhu,Qi;Yue,Tao; Shao,Lingyun;Gao,Yan; Feng,Yun;Weng,Xinhua; Zou,Hejian;Zhang,Ying; Zhang,Wenhong
Highincidenceoftuberculosisinfection inrheumaticdiseasesandimpactfor chemoprophylacticpreventionof tuberculosisactivationduringbiologics therapy
2013 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal, Medline (Pubmed), Scopus,Webof Science
Bieber,Jeffry;Kavanaugh, Arthur
Considerationoftheriskandtreatment oftuberculosisinpatientswhohave rheumatoidarthritisandreceivebiologic treatments
2004 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Mariette,X;Salmon,D Frenchguidelinesfordiagnosisand treatinglatentandactivetuberculosisin patientswithRAtreatedwithTNF blockers
2003 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Abud-Mendoza,Carlos; Martínez-Martínez,Marco Ulises;DeJesús
Macías-Mendoza,José; Maga ˜na-Aquino,Martín
Shouldtuberculinskintestbepositiveto givelatenttuberculosistreatmentbefore tumornecrosisfactor-alphainhibitorsin selectedpatientsindevelopingcountries?
2010 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Scrivo,Rossana;Armignacco, Orlando
Tuberculosisriskandanti-tumour necrosisfactoragentsinrheumatoid arthritis:acriticalappraisalofnational registrydata
2014 Doesnotaddressthe agerangeofinterest ofthestudy
CAPESPortal
Calzada-HernándezJ(1), Anton-LópezJ(2), Bou-TorrentR(3), Iglesias-JiménezE(4), Ricart-CamposS(5),Martín deCarpiJ(6);CarmenGarcía deVicu ˜naMu ˜nozdelaNava, Torrente-SegarraV(7), Sánchez-ManubensJ(8), Giménez-RocaC(9), Rozas-QuesadaL(10), Juncosa-MorrosMT(11), FortunyC(12),Noguera-Julian A(13)
Tuberculosisinpediatricpatientstreated withanti-TNF␣ drugs:acohortstudy
2015 Included Medline (Pubmed)
SantosMJ(1),FonsecaJE, CanhãoH,CondeM,José VieiraM,CostaL,CostaM, SalgadoM,MeloGomesJÁ
Guidelinesforprescribingandmonitoring biologictherapiesinjuvenileidiopathic arthritis
2007 Verylimited approachofLTBI treatment
Table2–(Continued)
Authors Title Year Inclusionor
justificationfor exclusion
Database
Demir,S.a,SadiAykan,F.a, Öztuna,D.b
Latenttuberculosistreatmentresultsin patientsthattakenTNF-alphablockersat AnkaraNumunetrainingandresearch hospitalchestdiseasesclinicforlast8 years(2006–2013)
2014 Doesnotaddressthe agerangeofinterest ofthestudy
Scopus
NurayAktayAyaz,Erkan Demirkaya,YeldaBilginer,U ˘g urÖzc¸elik,NazanC¸obano ˘glu, NuralKiper,NesrinBesbas, AysinBakkalo, ˘gluSezaÖzen
Preventingtuberculosisinchildren receivinganti-TNFtreatment
2010 Included Author’smanual search
andchesttomography.AnTST≥10mmwasconsidered pos-itive.Atthepre-anti-TNF-␣ therapyassessment,21patients weredetectedwithLTBIandtreatedwithINH(isoniazid)at 10mg/kg/day(maximum,300mg/day)for9monthsstarting1 monthbeforeanti-TNF-␣ therapy.NoTBdiseasewasdetected inthese patientsafter 9months. During follow-up, 7 chil-drenhadpositiveTSTandreceivedtreatmentforLTBIwith INHat 10mg/kg/day (maximum, 300mg/day)for9 months duringanti-TNF-␣ therapy.A13-year-oldfemalepatientwith juvenileidiopathicarthritisandsecondaryuveitisdeveloped positiveIGRAduringtreatmentwithINH.Anti-TBtreatment was started and, after 18 months, TB-related signs disap-peared.
Calzada-Hernándezetal.,17inaprospectivecohortstudy, assessed221childrenandadolescents withrheumatic dis-eases using TNF blockers. Before treatment, patients were submittedtoTSTorQuantiFERONGold-InTube(QTF-G)test and chest X-ray, and an TST ≥5mm was considered posi-tive.Duringtreatment,patientswereassessedevery6months throughclinicalhistoryandphysicalexamination.TST/QTF-G
andchestX-rayswereperformedonlywhennecessary.LTBI wasdiagnosedin3patientswithjuvenileidiopathicarthritis. TheyweretreatedforLTBIandthentheanti-TNF-␣treatment
wasreintroduced.OnepatientreceivedtreatmentwithINH for9monthsand,intwo,thetreatmentconsistedofINHand Rifampicinfor3months.Treatmentadherencewasobserved inallthreecases,andtherewerenoproblemsrelatedto tol-eranceandevidenceofTBactivation.Therewerenochanges inliverenzymelevelsaspartateaminotransferase(AST)and alanineaminotransferase(ALT).
Ayaz et al.,18 in aprospective cohortstudy, followed 36 patients diagnosedwith juvenileidiopathic arthritistaking etanercept.Allchildrenandfamilymemberswerescreened forLTBIandactiveTBpriortoetanercepttherapy.Screening consistedofclinicalhistory,physicalexamination,TST,and chestX-ray.Thechildrenwerere-evaluatedevery3months. SevenpatientshadTST>10mmandwere treatedwithfull doseofINHfor4–8weeks,plus9monthsafterthestartof theanti-TNF-␣therapy.TherewasnoTBactivationinthese
patientsorothercomplicationsduringfollow-up.
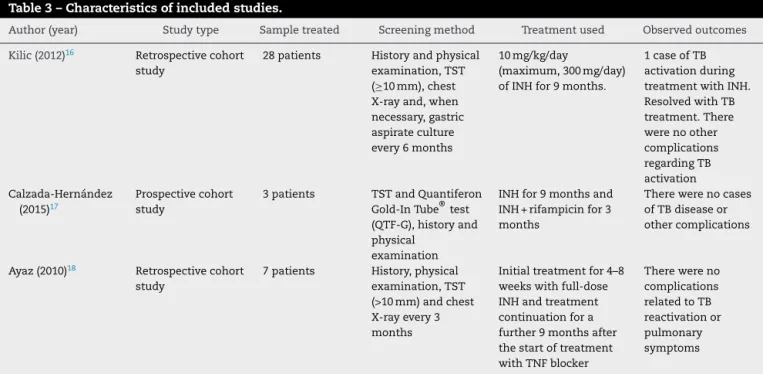
Table3–Characteristicsofincludedstudies.
Author(year) Studytype Sampletreated Screeningmethod Treatmentused Observedoutcomes
Kilic(2012)16 Retrospectivecohort
study
28patients Historyandphysical examination,TST (≥10mm),chest X-rayand,when necessary,gastric aspirateculture every6months
10mg/kg/day
(maximum,300mg/day) ofINHfor9months.
1caseofTB activationduring treatmentwithINH. ResolvedwithTB treatment.There werenoother complications regardingTB activation Calzada-Hernández
(2015)17
Prospectivecohort study
3patients TSTandQuantiferon Gold-InTube®test (QTF-G),historyand physical
examination
INHfor9monthsand INH+rifampicinfor3 months
Therewerenocases ofTBdiseaseor othercomplications
Ayaz(2010)18 Retrospectivecohort
study
7patients History,physical examination,TST (>10mm)andchest X-rayevery3 months
Initialtreatmentfor4–8 weekswithfull-dose INHandtreatment continuationfora further9monthsafter thestartoftreatment withTNFblocker
Discussion
Intheliterature,itispossibletoidentifydifferenttherapeutic regimens forthe treatmentofLTBI.TheMinistry ofHealth recommendsINHatthedoseof5mg/kgto10mg/kgofweight uptoamaximumdoseof300mg/dayforaminimumperiodof 6months.However,thereare4optionsrecommendedbythe WHO:INHfor6or9months;Rifapentineweeklyfor3months plusINH;INHfor3–4monthsplusRifampicin;3–4monthsof rifampicin.8
Duetothegreatersusceptibilityoftheat-riskpopulationto developtheactivedisease,thecorrecttreatmentisvery impor-tant. Inthe caseofnewbornscohabiting withthe infected indexcase,newbornsshouldnotbevaccinatedatbirth.INHis administeredforaperiodofthreemonths,afterwhichtheTST isperformed.Chemoprophylaxisismaintainedforanother3 monthsiftheTSTis≥5mm.Otherwise,INHisdiscontinued andtheindividualisvaccinatedwithBCG.9
For children who are contact with infected individuals, treatmentisindicatedwhen theTST is≥5mminchildren notvaccinatedwithBCG,inchildrenvaccinatedformorethan twoyearsorinthepresenceofanyimmunosuppressive con-dition.ItisalsoindicatedwhentheTST≥10mminchildren vaccinatedwithBCG forlessthan twoyears.Childrenthat acquiredLTBIuptotheageof5areconsideredprioritiesfor LTBItreatment.9
However,the number ofstudies included in the review showsthescarcityofstudiesevaluatingthetreatmentofLTBI inchildrenwithrheumaticdiseases.Thepresenceofthese childreninthe riskgroup,aswell asthedisease mortality, makeitimportanttocarryoutstudiescomparingthe differ-entavailabletherapies.Itisobservedthat,duetothisfact,the besttherapyforthesepatientsisyettobedefined.Onlythe studybyKilicetal.16showedacaseofTBactivation.Thelow incidenceofTBdiseaseinthesepatientsisprobablyduetothe continuousfollow-upandscreeningofthesechildrenduring thechronicdiseasetreatment.
Onthe otherhand,onlyonestudy showeddatarelated toliverenzymealterations duringtreatment. Althoughnot frequentandreversible,INH-inducedhepatotoxicityin chil-drenwithLTBIhasbeendescribedintheliterature.19However, thebehaviorofthehepaticprofileinchildrenwithrheumatic diseases receivingthis medication has notbeen described. Because this is a group of childrenwith chronic systemic diseasesreceivingothermedications,informationabout tol-erabilitymaybevaluableinclinicalpractice.
Goodadherencetotreatmentinallthreestudiesmayalso haveoccurred duetothemedicalmonitoringthesechronic patientsneedtoundergo.Silvaetal.20showedthat,inchildren withoutcomorbidities,8.9%didnotreturnfortheTSTreading and12.65%didnotadheretotreatment.
OfthetherapeuticregimensrecommendedbytheWHO, thefollowingwerenotobserved:INHfor6months;Rifampicin for 3 months weekly plus INH; 3–4 months of rifampicin. The use to these schemes would be important for the comparison.
Thus,thebest therapeuticregimenforthe treatmentof LTBI in pediatric patients with rheumatic diseases cannot yetbedetermined.Morestudiesarerequiredtoevaluatethe
response tothe other available therapeuticregimens,with bettertolerabilityassessmentandlargersamplesize.
Finally, theresultsofthestudies showedthatINH ther-apyfor6–9monthsandINHtherapyplusrifampicinforthree monthsmayhavealowactivation rate;however,the dura-tioniscontroversial,whichemphasizestheneedtoperform furtherstudies.12
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.WorldHealthOrganization.GlobalHealthObservatorydata repository.Availableat:http://apps.who.int/gho/data/ node.main.GHECOD[accessed22.11.15].
2.WorldHealthOrganization.Globaltuberculosisreport2015. Availableat:http://apps.who.int/iris/bitstream/10665/ 191102/1/9789241565059eng.pdf?ua=1[accessed22.11.15]. 3.SistemadeInformac¸ãodeAgravoseNotificac¸ão.Série
históricadacoeficientedeincidênciadatuberculose. SINAN/SES2015.Availableat:http://portalsaude.saude. gov.br/images/pdf/2015/setembro/24/taxa-incid–ncia-tuberculose-1999-2014-base-JUN-2015.pdf[accessed22.11.15]. 4.SistemadeInformac¸õessobreMortalidade–SIM.Série
HistóricadoCoeficientedeMortalidadedeTuberculose. Brasil,RegiõeseUnidadesFederadasderesidênciaporano dediagnóstico.MS/SVS/DASIS.Availableat:http://portalsaude. saude.gov.br/images/pdf/2015/setembro/24/taxa-mortalidade-tuberculose-1999-2014-base-JUN-2015.pdf[accessed22.11.15]. 5.MinistériodaSaúde,SecretariadeVigilânciaemSaúde.
Situac¸ãoepidemiológicadatuberculosenosestadospartese associadosdoMercosul2009a2013–Brasília:MS;2015. Availableat:http://portalsaude.saude.gov.br/images/pdf/ 2015/outubro/07/tuberculose-mercosul-6out15-web.pdf
[accessed22.11.15].
6.DiretoriadeVigilânciaEpidemiológica/SESAB,Boletim epidemiológicoJan/2015.DIVEP2015.Availableat:http:// www.suvisa.ba.gov.br/sites/default/files/BOLETIM%202014 %20editado.pdf[accessed22.11.15].
7.ErnstJD.Theimmunologicallifecycleoftuberculosis.Nat RevImmunol.2012;12:581–91.
8.WorldHealthOrganization.Guidelinesonthemanagement oflatenttuberculosisinfection;2015.Availableat:http://apps. who.int/iris/bitstream/10665/136471/1/9789241548908eng. pdf?ua=1&ua=1[accessed22.11.15].
9.MinistériodaSaúdeSecretariadeVigilânciaemSaúde. DepartamentodeVigilânciaEpidemiológica.Manualde recomendac¸õesparaocontroledatuberculosenoBrasil– Brasília:MS;2011.
10.SociedadeBrasileiradeReumatologia.Preliminaryguidelines oftheBrazilianSocietyofRheumatologyforevaluationand treatmentoftuberculosislatentinfectioninpatientswith rheumatoidarthritis,infaceofunavailabilityofthe tuberculinskintest.RevBrasReumatol.2015;55:390–3.
11.MarquesCDL,DuarteALBP,CavalcantiFS,CarvalhoEMF, GomesYM.Abordagemdiagnósticadatuberculoselatentena artritereumatoide.RevBrasReumatol.2007;47:424–30.
12.CostaAF,LazariCS,LeiteOHM.Tuberculoselatente: rastreamento,métodosdiagnósticosetratamento.RevPaul Reumatol.2014;13:11–6.
sobreastaxasdeinfecc¸ãoempacientescomartrite reumatoide.RevBrasReumatol.2013;53:501–15.
14.TittonDC,RanzolinA,HayataAL,DuarteA,BrenolC,Pinheiro GC,etal.RelatóriodoBiobadaBrasilde30/06/2014.Available at:biobadaser.ser.es/biobadamerica/Brasil[accessed22.11.15]. 15.TittonDC.RegistroBrasileirodeMonetarizac¸ãodeTerapias
BiológicasemDoenc¸asReumáticas–BiobadaBrasil.RevPaul Reumatol.2014;13:17–20.
16.KilicO,KasapcopurO,CamciogluY,CokugrasH,ArisoyN, AkcakayaN.Isitsafetouseanti-TNF-␣agentsfor tuberculosisinchildrensufferingwithchronicrheumatic disease.ReumatolInt.2012;32:2675–9.
17.Calzada-HernándezJ,Anton-LópezJ,Bou-TorrentR, Iglesias-JiménezE,Ricart-CamposS,MartíndeCarpiJ,etal.
Tuberculosisinpediatricpatientstreatedwithanti-TNF␣ drugs:acohortstudy.PediatrReumatol.2015;13:54.
18.AyazNA,DemirkayaE,BilginerY,Özc¸elikU,C¸obano ˘gluN, KiperN,etal.Preventingtuberculosisinchildrenreceiving anti-TNFtreatment.ClinRheumatol.2010;29:
389–92.
19.ChangSH,NahidP,EitzmanSR.Hepatotoxicityinchildren receivingisoniazidtherapyforlatenttuberculosisinfection.J PediatrInfectDis.2014;3:221–7.