w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
article
UHPLC–MS
quantification
of
coumarin
and
chlorogenic
acid
in
extracts
of
the
medicinal
plants
known
as
guaco
(
Mikania
glomerata
and
Mikania
laevigata
)
Lucilia
V.
de
Melo,
Alexandra
C.H.F.
Sawaya
∗DepartamentodeBiologiaVegetal,InstitutodeBiologia,UniversidadeEstadualdeCampinas,Campinas,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:Received16December2014 Accepted6February2015 Availableonline27March2015
Keywords: Mikaniaglomerata Mikanialaevigata
Coumarin Chlorogenicacid UHPLC–MSanalysis
a
b
s
t
r
a
c
t
InBrazil,MikaniaglomerataSpreng.andM.laevigataSch.Bip.exBaker,Asteraceae,knownpopularly asguaco,arewidelyusedforcoldsandasthma.Althoughcoumarinisadoptedasthechemicalmarker ofbothspecies,itwasnotalwaysdetectedinM.glomerata,forwhichchlorogenicacidwasidentified andquantifiedinstead.Thepurposeofthisstudywastodevelopandvalidateamethodtoquantifyboth coumarinandchlorogenicacidandapplyittoextractsofplantsidentifiedasM.glomerata,M.laevigata,or asguaco,todeterminethepatternofcompositionofthesetwospeciesandtoobservedifferencesbetween oven-driedandlyophilizedleaves.Amethodusingultra-highresolutionliquidchromatography–mass spectrometry(UHPLC–MS)inthefullscanmodewasvalidatedforselectivity,matrixeffect,linearity, lim-itsofdetectionandquantification,precisionandaccuracy.Theconcentrationofcoumarinvariedbetween speciesandsamples,thereforethesetwospeciesshouldnotbeusedinterchangeably.Theconcentration ofchlorogenicacidwasalsodeterminedforallsamples.TheUHPLC–MSmethodpermittedthe quantifi-cationofcoumarinandchlorogenicacidin16samplesofguacoandseveralcommercialsampleswere possiblymisidentified.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
The therapeutic use of medicinal plants is part of the
his-toryofhumanity.Frequentlythepopulationof underdeveloped
countriesstilldependsstronglyonmedicinalplantsforspiritual,
culturaloreconomic reasons(Quirozetal.,2014).Furthermore,
evenindevelopedcountries,theuseoftraditionalherbal
reme-diesiswidespread.Herbalremediesareevencrossingborders,for
examplethewidespreaduseof traditionalChinese Medicinein
Europe(Hook,2014).
InBrazil,twospeciesoftheMikaniagenus,Asteraceae,popularly
knownasguaco,areusedinsyruporpreparedasteaforcoldsand
otherrespiratoryproblemsduetotheirbronchodilatoreffect(Silva
etal.,2008).MikaniaglomerataSpreng.andM.laevigataSch.Bip.ex
BakermaybefoundintheAtlanticCoastforest,rangingfromthe
stateofBahiaintheNortheastofBrazil,tothesouthernstatesof
Brazil(Gasparettoetal.,2010)andeveninParaguayandArgentina
(Limaetal.,2003).M.glomeratawasincludedinthefirstBrazilian
Pharmacopeia(Brasil,1929)whileM.laevigatawasonlyincludedin
∗ Correspondingauthor.
E-mail:achfsawa@unicamp.br(A.C.H.F.Sawaya).
thefourtheditionoftheBrazilianPharmacopeia(Brasil,2005).Both
speciesaremorphologicallysimilarandmaybeeasilymistaken;
theyareoftencommercializedandpreparedindistinctly(Anvisa,
2011).
Studiesoftheircomposition haveledtotheidentificationof
phenoliccompounds,di-andtri-terpenes,tanninsandother
com-ponents (Gasparettoet al.,2010).Although coumarinhas been
adopted asthe chemical markerof both species(Anvisa, 2008,
2011)and theirpharmacologicalproperties areoftenattributed
to this substance,several studiespresent conflicting resultson
theconcentrationofcoumarin.Forexample:Santosetal.(2006)
reportedthattheextractofM.glomeratapresentedtwiceasmuch
coumarinasthatM.laevigata,whereasBolinaetal.(2009)
con-cluded that M. laevigata presented a slightly higher coumarin
content(0.43%)thanM.glomerata(0.30%).Athirdstudyreported
thatcoumarinwasnotpresentinM.glomerata,onlyinM.
laevi-gata(Bertoluccietal.,2009)whichisconsistentwithourresults.
These contradictory resultsmay be due to misidentification of
theplantspecies(duetotheirmorphologicalsimilarity)ortothe
diverseanalyticalmethodsemployed.Theuseoffreshleaves,oven
dried leavesor lyophilizedmaterial mayalsohave affectedthe
results(Santosetal.,2006).Furtherpossibilitiesarevariationsin
secondarymetabolitesduetoseasonalorenvironmentalfactors
http://dx.doi.org/10.1016/j.bjp.2015.02.005
(Gobbo-NetoandLopes,2007).Preliminarystudiesbyourgroup
detectedthepresenceofchlorogenicacidinM.glomerataleaves,
whichwasasurprisingasonlyonestudywasfoundrelatingthe
presenceofchlorogenicacidin guacoleaves(Silva etal.,2006).
Thereforeitwasnecessarytobeginthisinvestigationbydefining
theplantsamples,thedryingmethodandtheanalyticalmethod
tobeused.Thismethodshouldbeselectiveenoughtoquantify
coumarinand chlorogenicacidcorrectly,but shouldalsodetect
other,stillunidentified,componentsintheextracts.
Severalmethodshave beenfoundinliteratureforthe
quan-tificationof coumarins;fromsimplethin layerchromatography
(Alvarenga et al., 2009) to modern electrochemical methods (Miyanoetal.,2014).Celeghinietal.(2001)quantifiedcoumarin
inextractsofM.glomerataleavesbyhighresolutionliquid
chro-matographywithUVdetection(HPLC–UV),whileMuceneekietal.
(2009)quantifiedo-coumaricacid,coumarinandsyringaldehyde
byHPLC–UV.Parketal.(2009)andChenetal.(2012)usedHPLC
coupledtomassspectrometry(HPLC–MS)intheMRMmodeto
quantifycoumarins.Whilethismethodishighlyselectiveand
sen-sitive,itonlypermitstheanalysisoftheselectedcomponents.In
fact,allthemethods citedabovewouldnothave allowedusto
observethepresenceofchlorogenicacidinM.glomeratasamples,if
theyhadbeendevelopedforcoumarin.Thesamecanbesaidofthe
HPLC–MS(MRM)methodusedtoquantifydicaffeoylquinicacids
(Cliffordetal.,2008)andchlorogenicacidmetabolites(Santosetal.,
2005).However,byusingMSdetectioninfullscanmodeitis
pos-sibletoquantifyselectedionsaswellasdetectothercomponents
oftheextract.
Inordertoevaluatetheconcentrationofcoumarinand
chloro-genicacidinleavesofM.glomerataandM.laevigata,whileallowing
thedetectionoftheothersamplecomponents,a methodusing
ultra-highresolution liquidchromatography–massspectrometry
(UHPLC–MS)wasdevelopedandvalidated.Thismethodwasused
toanalyzetheethanolic extractsof ovendried and lyophilized
leavesofbothspecies,aswellasleavescommercializedsimplyas
guaco.Themethodwasdevelopedforethanolicextractsofleaves
oftwoplantsidentifiedbyspecialistsasM.laevigataandM.
glom-erata;andthenappliedtoothersamplesoffreshanddryleaves
(commercializedasguaco).
Materialsandmethods
Plantsamples
MikaniaglomerataSpreng.andM.laevigataSch.Bip.exBaker,
Asteraceae,plants weredonatedand identified byCPQBA,
Uni-camp(Paulínia,SP)andvoucherspecimensdepositedattheState
UniversityofCampinasHerbarium(UEC)number102046forM.
laevigataandnumber102047forM.glomerata.Theseplantsare
growingintheExperimentalFieldoftheInstituteofBiology.For
thisstudy,leavesofbothspecieswerecollectedthesamemorning
anddriedbytwodifferentmethodsbeforegrindingand
extrac-tion.
Othercommercialsamplesfreshanddryleaveslabeledasguaco,
M.laevigataorM.glomeratawereboughtfromlocalmarketsor
collectedfrominstitutional (CPQBA-Unicamp)or homegardens
(Table2).Allsampleswereextractedwithindaysoftheir
acqui-sition. The dry leaves were extracted in the same way as the
identifiedplant samples;thefresh leaveswerelyophilized and
thenextracted.OneplantwhichwascollectedinthestateofSão
PauloandidentifiedasM.glomeratabyProf.GeorgeY.Tamashiro
oftheBiologyInstituteofUnicamp,presentedconcentrationsof
coumarinandchlorogenicacidsbelowquantificationlevelandwas
thereforedriedandextractedtobeusedasablankplantmatrix
(BPM)forcalibrationcurves.
Dryingandextraction
Approximatelyhalfoftheleavescollectedfromeachplantwere
driedinanovenwithaircirculationat40◦Cfor50h;theotherhalf
wasdriedbylyophilizationfor50h.Thematerialwasthenground
inamortar,passedthroughasievewith0.84mmspaces,andplaced
in67%ethanol(Ecibra,Brazil)toextract,followingtheproportionof
200gofleavestoatotalof1.0lofsolvent(Brasil,1929).After
filter-ing,a10mlaliquotwastakentoevaluatethetotalsolidsextracted
bydyingthesolventinanovenat105◦Cuntilconstantweight.
Chromatographicmethod
A chromatographic method was developed and validated
using an ultra-high performance liquid chromatographer
cou-pled toa triple quadrupolemass spectrometer.Theequipment
used was an Acquity UPLC-TQD (Micromass, Waters,
Manch-ester,England)and thecolumnwasa C18BEHAcquityWaters
(1.7m×2.1mm×50mm),oventemperatureof 30◦C.The
elu-tionwascarriedoutwithaflowof200l/min,SolventA–purified
water(Milli-Q)with0.1%formicacidandSolventB–HPLCgrade
acetonitrile(JTBaker,PA,USA),underagradientstartingwith10%B,
rampingto25%Bin4min,thento100%Bin8min,heldat100%B
until8.5minthereturningtotheinitialconditionsandstabilizing
until10min.
MSdetection wasperformedwithelectrosprayionizationin
bothpositiveandnegativeionmodes,underthefollowing
con-ditions:capillary±3000V,cone±35V,sourcetemperature150◦C
and desolvation temperature of 300◦C. Due to their structure,
chlorogenicacidionizedwellinnegativeionmodeandcoumarin
inthepositiveionmode.
Priortoinjectiontheextractswerefurtherdilutedinpurified
water(Milli-Q)intheproportionof1partextractto2partswater,
2lofeachsamplewereinjected.Theconcentrationofcoumarin
andchlorogenicacidintheplantextractswasquantifiedby
com-parisontoexternalcalibrationcurvesofcoumarin(Sigma–Aldrich)
andchlorogenicacid(Sigma–Aldrich)insolutionsof70%ethanol
andinBPM.Themethodwasvalidatedaccordingtotheparameters
describedbelow.
Selectivity.Solutionsofthestandardsofcoumarinand
chloro-genic acid, plant extracts and plant extracts spiked with the
standardswereinjected,evaluatingretentiontimesand
fragmen-tationspectra(MS/MS)oftheionofm/z147inthepositiveionmode
(coumarin)andorm/z353inthenegativeionmode(chlorogenic
acid).
Matrixeffect.ThiseffectwascalculatedaccordingtoEconomou etal.(2009)usingtheformula:C%=100×(1−Sm/Ss);whereC%is
thepercentageofincreaseorsuppressionofthesignal,Smisthe
angularcoefficientofthecalibrationcurveusingBPMandSsisthe
angularcoefficientofthecalibrationcurveusingasolutionof70%
ethanol.
Linearity.Thisparameterwasevaluatedbythecorrelation
coef-ficientofthecurvesofcoumarinandchlorogenicacidinBPM.
Limitofdetection(LD)andquantification(LQ).These
parame-tersweredeterminedbytheinjectionofa seriesofdilutionsof
coumarinandchlorogenicacidinBPM,withLDdeterminedasthe
concentrationthatresultedinapeakareathreetimesgreaterthan
thenoiselevelandLQaconcentrationthatresultedinapeakarea
tentimesgreaterthanthenoiselevel.
Precision.Thisparameterwasevaluatedfor fiveinjectionsof
extractsofM.laevigataandM.glomerataleavesalongonedayfor
theconcentrationofcoumarinandchlorogenicacid.
Accuracy.Asnocertifiedmaterialwasavailable,this
parame-terwasevaluatedbytheaddition(fortification)ofcoumarinand
chlorogenicacidinthreelevelsofconcentration:low(30g/ml),
tothepreparationofthecalibrationcurve.Recovery(R%)was
calcu-latedaccordingtothefollowingequation:R%=(C1−C2)/C3×100;
whereC1istheconcentrationwhichwasdeterminedforthe
forti-fiedsample,C2istheconcentrationofthenon-fortifiedsampleor
matrixandC3istheconcentrationofstandardwhichwasadded
(fortification).
Resultsanddiscussion
Dryingmethod
Leafextractsofbothspecies(driedintheovenorby
lyophiliza-tion)presentedsimilaramountsofsolid residue:1.2%(m/v)for
ovendriedM.laevigataand1.3%forlyophilizedM.laevigata;1.3%
forovendriedM.glomerataand1.4%forlyophilizedM.
glomer-ata.TheUHPLC–MSprofileoftheleafextractsofeachspecieswas
different(Fig.1)butthedryingproceduredidnotaffectthe
gen-eralprofileofthespecies.Howevertheamountofcoumarinand
chlorogenicacidwaslowerfortheovendriedleavesofbothspecies
(Table1),showingthatheataffectedthecontentsofthebioactive
compoundsevaluated.Forthis reason,alltheotherfreshleaves
collectedduringthisstudy(Table2)werelyophilized,ratherthan
ovendried,beforeextraction.
ValidationofUHPLC–MSmethod
Analyticalcurvesofcoumarinandchlorogenicacidstandards
withconcentrationsbetween1ng/mland 800g/mlwerebuilt
in 70% ethanol/waterand in BPM tovalidate themethod.This
rangeof concentrationswasnecessarydue tothevariable
con-tentofcoumarinandchlorogenicacidinbothplantspeciesandin
theextractsthatwereanalyzed.Furthermoreallparameterswere
successfullyvalidatedforthisrangeofconcentrations.
Selectivity. This parameter was determined comparing the
retentiontime(RT)andthefragmentationofcoumarinstandard
(positiveionmodem/z147)andchlorogenicacidstandard
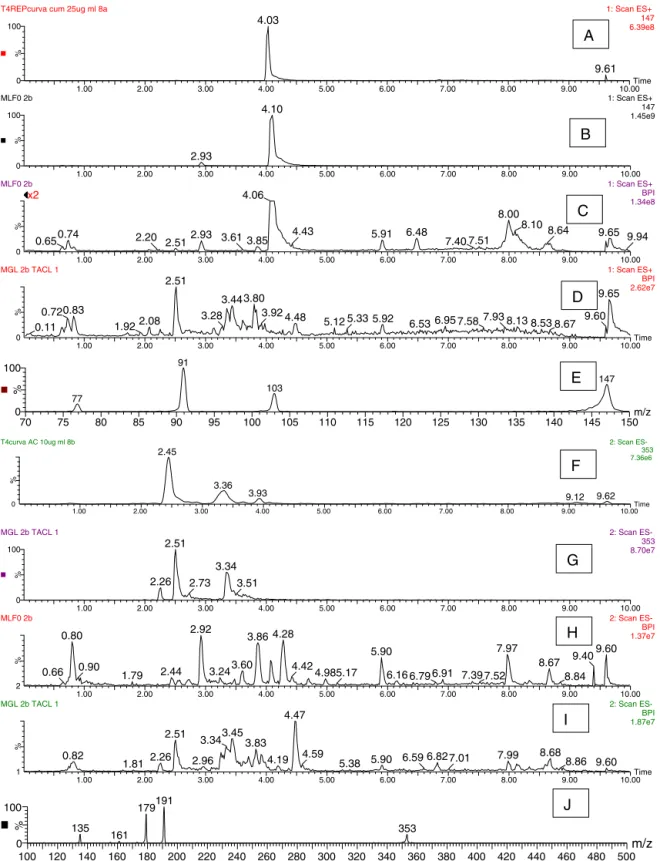
(nega-tiveionmodem/z353)withthesameionsinthesamples.Fig.1A
showstheselectedionchromatogram(m/z147positiveionmode)
ofthecoumarinstandardandFig.1Bshowstheselectedion
chro-matogram(m/z147)oftheM.laevigataextract,theretentiontimes
arepracticallyidenticalandbothpresentthesameMS/MS(Fig.1E).
Fig.1Fshowstheselectedionchromatogramofm/z353negative
ion mode.Although threepeaksare present,thefirst retention
time(2.45min)correspondstochlorogenicacidandtheothersare
isomers,presentasimpuritiesinthestandard.Fig.1Gshowsthe
selectedionchromatogram(m/z353)oftheM.glomerataextract,
thepeak atretention timeof 2.51showedthesameMS/MSas
chlorogenicacid(Fig.1J).Smallvariationsin theretention time
wereduetothecomplexmatrixoftheplantextracts.Thesolvent
didnotpresentpeaksofthesecompoundsandinBPM(Fig.2)the
areasofpeaksofthesecompoundswerebelowtheLD.
Matrixeffect.Thisparameterwasevaluatedcomparingcurvesof
bothstandardsinsolventwithcurvesINBPM.Theresultsshowed
thattheplantmatrixresultedinareductionofpeakareaof4.37%
forcoumarinandof19.20%forchlorogenicacidinrelationtothe
sameconcentrationsinsolvent.Thereforeallsubsequentanalytical
curvesusedinthisstudywerebuiltusingBPM.
Linearity.AnalyticalcurvesofbothstandardsinBPMwerebuilt
between1ng/mland800g/ml,withtriplicateinjectionsofeach
point. The ideal parameter of linearity (R2>0.99) couldnot be
attainedduetotheinterferenceofthematrixandthewiderangeof
concentrationsused.Theanalyticalcurveforcoumarin(m/z147in
thepositiveionmode)waslinear(R2=0.9718)forconcentrations
between1.5g/mland730.0g/ml,andthecurveforchlorogenic
acid(m/z353inthenegativeionmode)waslinear(R2=0.9831)
forconcentrationsbetween10.0g/mland550.0g/ml.Onlyone
sampleofdryleaves(sample8,Table2)felloutsidetherangeof
thesecurvesandtheconcentrationofcoumarinwasobtainedby
extrapolationofthecurve.
Limitofdetection(LD)andquantification(LQ).Forcoumarinthe
LDwas0.32g/mlandtheLQwas3.30g/mlinBPM.For
chloro-genicacidtheLDwas4.18g/mlandtheLQwas20.38g/mlin
theblankplantmatrix.TheLDandLQwerehigherforchlorogenic
acid,possiblybecauseoflessionizationinthenegativeionmode.
Precision.Theareaof thepeaksoffivereplicateinjectionsof
theextractsofM.glomeratapresentedvariationof5%forcoumarin
(m/z147inthepositiveionmode)and4%forchlorogenicacid(m/z
353inthenegativeionmode).FortheextractsofM.laevigatathe
variationwasof1%forcoumarinand4%forchlorogenicacid.
Accuracy.Therecuperation(R)valuesforthethreelevelsof
for-tificationofcoumarinwereintheextractofM.laevigata75.15%
(low),84.60%(medium)and67.04%(high).Forthefortificationwith
chlorogenicacidintheextractofM.glomeratatheRwas:106.42%
(low),88.56%(medium)and95.74%(high).Asonlytheresultforthe
highestconcentrationofcoumarinfellslightlyoutsidetheaccepted
recuperationparameter(70–120%)(Ribanietal.,2004).
AnalyticalresultsforplantsamplesOneadvantageofthemethod
presentedhereinisthatacquisitioninbothpositiveandnegative
modesisobtainedinthesameshortchromatographicrun.This
chromatographic methodwasfirst usedtoanalyzetheextracts
oftheovendriedandlyophilizedleavesofM.glomerataandM.
laevigata.Theextractswerepreparedusing200gofleaves/lof
sol-vent;thereforetheresultsshowninTable1ing/mlcorrespondto
200mgofdriedleaves.Inthismannerweobservethattheleavesof
theidentifiedM.glomerataplantcontainedpracticallynocoumarin.
Chlorogenicacidcontentwasbetween0.67%(m/m)forovendried
leavesand0.82%(m/m)forlyophilizedleaves.Incomparison,M.
laevigataovendriedleavescontained0.37%(m/m)coumarinand
lyophilizedleavescontained0.57%(m/m)coumarin.Thisresultis
inagreementwiththecoumarincontentpresentedbyBolinaetal.
(2009)forM.laevigataleaves,andalsowiththeresultspresentedby
Bertoluccietal.(2009)whostatedthatnocoumarinwasdetected inM.glomerata.Furthermore,theUHPLC–MSchromatogramsshow
thatthetwospeciesofguacopresentdistinctchromatographic
pro-filesinbothpositive(Fig.1CandD)andnegative(Fig.1HandI)ion
modes.
The peak of coumarin(RT 4.06) is clearly seenin the
chro-matogramoftheM.laevigataextract (Fig.1C)butabsentinthe
chromatogramoftheM.glomerataextract(Fig.1D).Inversely,the
peakofchlorogenicacid(RT2.51)isabsentinthechromatogram
of theM. laevigata extract (Fig. 1H) but is clearly seen in the
chromatogramoftheM.glomerataextract(Fig.1I).Furthermore,
thechromatographicprofilesofbothspeciesareclearlydifferent.
Althoughclimaticandseasonalvariationscouldaffecttheseresults,
bothspeciesareplantedside-bysideintheExperimentalFieldin
Unicampandcollectedatthesametime,sosubjecttothesame
influences.
Inordertocheckifotherguacosampleswouldbehaveinthe
samemanner,eightsamplesoffreshleavesandeightsamplesofdry
leaveswereacquired,extractedandanalyzedusingthesame
vali-datedchromatographicmethod.TheresultsareshowninTable2.
Freshleaves,samples9–12,identifiedasM.glomerataand
col-lected at CPQBA-Unicamp, presented the same pattern as our
originalM.glomerataleaves,withvaryingamountsofchlorogenic
acidandnodetectablelevelsofcoumarin.Sample13,identifiedas
M.laevigataandcollectedatCPQBA-Unicamp,presented
approx-imately the same amount of coumarin as our original sample
(Table1)andchlorogenicacidbelowtheLQ.Thisconfirmedthe
expectedpattern ofcompoundsexpectedforthesespecies.The
freshleavesofbothspecieswerecollectedseveralmonthsafterthe
Time
1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
%
0 100
T4REPcurva cum 25ug ml 8a 1: Scan ES+
147 6.39e8
4.03
9.61
Time
1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
%
0
1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
%
0
1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
%
0 100
MLF0 2b 1: Scan ES+
147 1.45e9
4.10
2.93
MLF0 2b 1: Scan ES+
BPI 1.34e8
x2 4.06
0.74
0.65 2.20 2.512.93 3.613.85
8.00 6.48
5.91 4.43
7.51 7.40
8.10
9.65 8.64
9.94
MGL 2b TACL 1 1: Scan ES+
BPI 2.62e7
2.51
0.83 0.72
0.11 1.922.08
9.65 3.80
3.44
3.28 3.92 4.48 5.92
5.12 5.33 6.536.957.587.938.138.53 8.67 9.60
m/z 70 75 80 85 90 95 100 105 110 115 120 125 130 135 140 145 150
%
0
100 91
77
147 103
Time
1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
%
0
T4curva AC 10ug ml 8b 2: Scan ES-
353 7.36e6
2.45
3.36
3.93 9.12 9.62
Time
1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
%
1
1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
%
2
1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
%
0 100
MGL 2b TACL 1 2: Scan ES-
353 8.70e7
2.51
2.26
3.34 2.73 3.51
MLF0 2b 2: Scan ES-
BPI 1.37e7
2.92 0.80
0.66 0.90 1.79 2.44
4.28 3.86
3.60 3.24
7.97 5.90
4.42
4.985.17 6.16 6.796.91 7.39 7.52
9.60 9.40 8.67
8.84
MGL 2b TACL 1 2: Scan ES-
BPI 1.87e7
4.47 3.45
2.51 0.82 2.26
1.81
3.34 2.96
3.83
4.19 4.59 5.38 5.90 6.596.827.01 7.99 8.688.86 9.60
m/z
100 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 460 480 500
%
0
100 179191 135
161 353
A
J
I
H
G
F
E
D
C
B
Fig.1.PositiveionmodeESI-MSUHPLCof:(A)coumarinstandardm/z147RT4.03min,(B)extractedionm/z147M.laevigataextract,(C)chromatogramofM.laevigata extract,(D)chromatogramofM.glomerataextract,(E)MS/MSofcoumarinm/z147.NegativeionmodeESI-MSUHPLCof:(F)chlorogenicacidstandardm/z353RT2.45min, (G)extractedionm/z353M.glomerataextract,(H)chromatogramofM.laevigataextract,(I)chromatogramofM.glomerataextract,(J)MS/MSofchlorogenicacidm/z353.
coumarinthanchlorogenicacidforM.laevigataandtheopposite,
morechlorogenicacidthancoumarin,forM.glomerata.
Driedleafsamples2,3,5,6,7and8,presentedapattern
simi-lartoM.laevigataleaves,withvaryingconcentrationsofcoumarin
andlessornochlorogenicacid.Thewaythesecommercial
sam-plesweredriedisunknown,butpossiblyaffectedthecontentsof
coumarin/chlorogenicacid.Thesesampleswerelabeledsimplyas
guaco(sample2)orM.glomerataandmayhavebeenmisidentified.
Driedleafsample4,labeledasguacoandacquiredintheAmazon,
presentedanapproximatelyequalconcentrationofcoumarinand
chlorogenicacid,whichisdifferentfromthepatternspreviously
encounteredandcouldevenbelongtoadifferentspeciesof
Mika-nia.Sample1,alsolabeledasguaco,presentedseveralmoldyleaves
andstemsinthepackageandnodetectablelevelsofcoumarinor
chlorogenicacid.Thereforeitisimpossibletoascertainifthis
Table1
Concentrationofcoumarinandchlorogenicacidinhydro-ethanolicextractsofovendriedandlyophilizedleavesofM.glomerataandM.laevigataplantedintheexperimental field(IB,UNICAMP),triplicateextractions.
Leavesof Coumarincontentinextract (g/ml)
CV(%) Chlorogenicacidcontentinextract (g/ml)
CV(%)
OvendriedM.glomerata * – 1348 9.94
LyophilizedM.glomerata * – 1634 5.79
OvendriedM.laevigata 775 3.26 * –
LyophilizedM.laevigata 1131 0.61 * –
*BelowLD.
Table2
Concentrationofcoumarinandchlorogenicacidinhydro-ethanolicextractsofdryandfreshleaveslabeledasM.glomerata,M.laevigataorguaco.
Samplelabeledas Form Boughtorcollectedin Coumarin (g/ml)
Chlorogenicacid(g/ml)
1.Guaco Dryleaves PortoAlegre–RS a a
2.Guaco/Mikania Dryleaves PortoAlegre–RS 155 b
3.M.glomerata Dryleaves Florianópolis–SC 170 64
4.Guaco Dryleaves Manaus–AM 128 156
5.M.glomerata Dryleaves Brasília–DF 375 b
6.M.glomerata Dryleaves Paulínia–SP 254 a
7.M.glomerata Dryleaves Paulínia–SP 316 a
8.M.glomerata Dryleaves Paulínia–SP 2794 a
9.M.glomerata(1)Id Freshleaves CPQBA,UNICAMPPaulínia–SP a 529
10.M.glomerata(2)Id Freshleaves CPQBA,UNICAMPPaulínia–SP a 211
11.M.glomerata(3)Id Freshleaves CPQBA,UNICAMPPaulínia–SP a 786
12.M.glomerata(4)Id Freshleaves CPQBA,UNICAMPPaulínia–SP a 260
13.M.laevigata(1)Id Freshleaves CPQBA,UNICAMPPaulínia–SP 1024 b
14.Guaco Freshleaves Guarapuava–PR 172 b
15.Guaco Freshleaves JoãoPessoa–PB 365 b
16.Guaco Freshleaves SãoPaulo–SP 354 a
a:BelowLD,b:BelowLQ,Id–botanicalidentificationbyCPQBA-UNICAMP.
badconservation.Itisworryingthatthissamplewasbeingsold
atamarketandcouldhavebeenconsumedbychildrenoradults
wishingtoalleviatethesymptomsofacold!
Freshleafsamples14–16,knownsimplyasguacobytheirusers,
presentedvaryingconcentrationsofcoumarinbutchlorogenicacid
contentsbelowLDorLQ,whichissimilartotheresultsofM.
laevi-gatavoucherplantandfreshplantfromCPQBA.
Theonlysampleswhichpresentedconcentrationsofcoumarin
and chlorogenic acid compatible with theidentified sample of
M.glomeratawerethosefreshleavesidentifiedand collectedat
CPQBA-Unicamp.Mostothersamples,regardlessoftheirlabels,had
concentrationsofcoumarinandchlorogenicacidwhichwere
sim-ilartothosefoundinleavesoftheM.laevigatavoucherplant.The
onlytwoguacosampleswhichdidnotfollowthispatternwere
pos-siblydegraded(sample1)orbelongedtoanotherspeciesofMikania
(sample4).
The variation in theconcentration of coumarin and
chloro-genic acid found in identified specimens of M. glomerata and
M.laevigatademonstratesthatthesetwospeciesdonotcontain
similaramounts ofcoumarinand thereforeshould notbeused
interchangeably. Furthermore commercialsamples also contain
variablecontentsofcoumarinandchlorogenicacidandmaybe
misidentifiedordegraded.Furtherstudiesregardingthe
morpho-logicaldescriptionofplantsknownasguacothroughoutBrazilare
underwaytoascertainwhichspeciesisbeingusedregionallyand
ifenvironmentalfactorsaffecttheconcentrationofcoumarinand
chlorogenicacidintheseplants.
By using UHPLC–MS in the full scan mode, not only were
coumarinandchlorogenicacididentifiedandquantified,butalso
othercompounds,whichmake upthecomplexchemicalprofile
oftheseplantextracts,weredetected.AlthoughotherHPLC–MS
methodsintheMRMmodemaybemoresensitive,theydonot
pro-videuswithapanoramicviewofsamplecomposition,whichleads
tonewdiscoveries,suchastheimportanceofchlorogenicacidin
M.glomeratasamples.Thischromatographicmethodallowedus
todistinguishbetweensamplesofleavesfromtwo
morpholog-ically similarspeciesandcan beused forthequalitycontrolof
thedryleavesand extractsofthesespecies.Thisinformationis
paramountforthecorrect useofthesemedicinalplantsby the
population.
Conflictofinterest
Theauthorshavenonetodeclare.
Authors’contributions
ACHFSplannedtheresearch,LVMperformedtheexperiments
validatedthemethod.Bothauthorshelpedwithwritingthispaper.
Acknowledgements
This study was partially financed by CNPq grant number
473597/2013-6. We would like to thank P. Mazzafera for the
useof theUHPLC–MSequipment(BIOEN-FAPESP grant number
2008/58035-6)andDr.GlynM.Figueira(CPQBA)fortheM.
glom-erataandM.laevigataplantsamples.
References
Alvarenga,F.C.R.,Garcia,E.F.,Bastos,M.A.F.,Grandi,T.S.M.,MariaGorette,R.,Duarte, M.G.R.,2009.Evaluationofthequalityofcommercialsamplesofleavesand tincturesofguaco.Rev.Bras.Farmacogn.19,442–448.
Anvisa,2008.Instruc¸ãoNormativaN◦5de11dedezembrode2008.Listade
Medica-mentosFitoterápicosdeRegistroSimplificado,Brasilia,DF.
Anvisa, 2011. Formulário de Fitoterápicos da Farmacopéia Brasileira, 1st ed, http://www.anvisa.gov.br/hotsite/farmacopeiabrasileira/conteudo/Formulario de FitoterapicosdaFarmacopeiaBrasileira.pdf(accessed30.06.14).
Bertolucci,S.K.,Pereira,A.B.,Pinto,J.E.,Ribeiro,J.A.A.,deOliveira,A.B.,Braga,F.C., 2009.DevelopmentandValidationofanRP-HPLCmethodforquantificationof cinnamicacidderivativesandkaurane-typediterpenesinMikanialaevigataand
Mikaniaglomerata.PlantaMed.75,280–285.
Bolina,R.C.,Garcia,E.E.,Duarte,M.G.R.,2009.Estudocomparativodacomposic¸ão químicadasespéciesvegetaisMikaniaglomerataSprengeleMikanialaevigata SchultzBip.Rev.Bras.Farmacogn.19,294–299.
Brasil,1929.FarmacopéiadosEstadosUnidosdoBrasil,firsted.CompanhiaEditora Nacional,SãoPaulo.
Brasil,2005.FarmacopéiaBrasileira,Fourthed.,PartII,6thfasc.Atheneu,SãoPaulo.
Celeghini,R.M.S.,Vilegas,J.H.Y.,Lanc¸as,F.M.,2001.ExtractionandquantitativeHPLC analysisofcoumarininhydroalcoholicextractsofMikaniaglomerataSpreng: (guaco)leaves.J.Braz.Chem.Soc.12,706–709.
Chen,H.,Zhang,W.,Yuan,J.,Li,Y.,Yang,S.,Yang,S.,2012.Simultaneous quantifica-tionofpolymethoxylatedflavonesandcoumarinsinFructusaurantiiandFructus
aurantiiimmaturususingHPLC–ESI–MS/MS.J.Pharm.Biomed.Anal.59,90–95.
Clifford,M.N.,Kirkpatrick,J.,Kuhnert,N.,Roozendaal,H.,Salgado,P.R.,2008.LC–MSn analysisofthecisisomersofchlorogenicacids.FoodChem.106,379–385.
Economou,A.,Botitsi,H.,Antoniou,S.,Tsipi,D.,2009.Determinationofmulti-class pesticidesinwines bysolid-phaseextractionand liquid chromatography-tandemmassspectrometry.J.Chromatogr.A1216,5856–5967.
Gasparetto,J.C.,Campos,F.R.,Budel,J.M.,Pontarolo,R.,2010.Mikaniaglomerata
SprengeM.laevigataSch.Bip.exBaker,Asteraceae:estudosagronômicos, genéticos,morfoanatômicos,químicos,farmacológicos,toxicológicoseusonos programasdefitoterapiadoBrasil.Rev.Bras.Farmacogn.20,627–640.
Gobbo-Neto,L.,Lopes,N.P.,2007.Medicinalplants:factorsofinfluenceonthe con-tentofsecondarymetabolites.Quim.Nova30,374–381.
Hook,I.L.I.,2014.DangguitoAngelicasinensisroot:arepotentialbenefitstoEuropean womenlostintranslation?Areview.J.Ethopharmacol.152,1–13.
Lima,N.P.,Biasi,L.A.,Zanette,F.,Nakashima,T.,2003.Produc¸ãodemudaspor estaquiadeduasespéciesdeguaco.Hortic.Bras.21,106–109.
Miyano,D.M.,Lima,T.,Simões,F.R.,La-Scalea,M.A.,Hueder,L.A.,Oliveira,P.M., Codognoto,L.,2014.ElectrochemicalStudyofsimplecoumarinandits determi-nationinaqueousinfusionofMikaniaglomerata.J.Braz.Chem.Soc.25,602–609.
Muceneeki,R.S.,Amorim,C.M.,Cesca,T.G.,Biavatti,M.W.,Bresolin,T.M.B.,2009.A simpleandvalidatedLCmethodforthesimultaneousdeterminationofthree compoundsinMikanialaevigataextracts.Chromatogr.S69,S219–S223.
Quiroz,D.,Towns,A.,Legba,S.I.,Swier,J.,Brière,D.,Soswf,M.,vanAndel,T.,2014.
QuantifyingthedomesticmarketinherbalmedicineinBenin,WestAfrica.J. Ethopharmacol.151,1100–1108.
Park,A.Y.,Park,S.Y.,Lee,J.,Jung,M.,Kim,J.,Kang,S.S.,Youma,J.,Hana,S.B.,2009.
SimultaneousdeterminationoffivecoumarinsinAngelicaedahuricaeRadixby HPLC/UVandLC-ESI-MS/MS.Biomed.Chromatogr.23,1034–1043.
Ribani,M.,Bottoli,G.,Collins,C.H.,Jardim,I.C.S.F.,Melo,L.F.C.,2004.Validac¸ãoem métodoscromatográficoseeletroforéticos.Quím.Nova27,771–778.
Santos,S.C.,Krueger,C.L.,Steil,A.A.,Kreuger,M.R.,Biavatti,M.W.,Wisniewski,A., 2006.Characterisationofguacomedicinalextracts,MikanialaevigataandM. glomerataandtheirEffectsonAllergicPneumonitis.PlantaMed.72,679–684.
Santos,M.D.,Martins,P.R.,dosSantos,P.A.,Bortocan,R.,Iamamoto,Y.,Lopes,N., 2005.Oxidativemetabolismof5-o-caffeoylquinicacid(chlorogenicacid),a bioactivenaturalproduct,bymetalloporphyrinandratlivermitochondria.Eur. J.Pharm.Sci.26,62–70.
Silva,A.I.,Santana,C.S.,Pivato,S.C.L.,deMaria,C.A.B.,Moreira,R.F.A.,2006. Chloro-genicacidprofileofcommercialBrazilianherbalinfusions.Sci.Aliment.26, 187–194.