Brazilian
Journal
of
Hematology
and
Hemotherapy
w w w . r b h h . o r g
Review
article
Klotho:
its
various
functions
and
association
with
sickle
cell
disease
subphenotypes
Ana
Paula
Almeida
de
Souza
Pacheco
a,
Marilda
Goncalves
a,b,∗aFundac¸ãoOswaldoCruz(FIOCRUZ),Salvador,BA,Brazil bUniversidadeFederaldaBahia(UFBA),Salvador,BA,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received19March2014 Accepted21July2014
Availableonline5October2014
Keywords:
VitaminD Oxidativestress Sicklecelldisease Endothelium Genepolymorphism
a
b
s
t
r
a
c
t
TheKlothoprotein,whosegenehaspredominantrenalexpression,actsinthecontrolof serumphosphorusand1,25-dihydroxyvitaminD3andregulatesthefunctionofion chan-nels.Italsoparticipatesinthemechanismofprotectionagainstoxidativestressandactson thevascularendotheliumbyinducingtheproductionofnitricoxide.Mutationsthatreflect defectsintheKlothogeneexpressionmaybeimplicatedintheonsetofosteonecrosis, pri-apism,andlegulcersinpatientswithsicklecelldisease,asaresultofoxidativestressand endothelialimpairment,importantfactorsinthedevelopmentandseverityofthisdisease. PreviousreportsregardingtheassociationofKlothosinglenucleotidepolymorphismswith sicklecelldiseasesubphenotypeshavefoundthatthesepolymorphismsareimportantto identifygeneticmarkersofriskintheseindividualsandallowearlyandmoreeffective therapeuticintervention.
©2014Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
Introduction
Klotho is a gene that consistsof five exons and is located onchromosome13q12inhumans.Itsexpressionoccurs pre-dominantlyinthekidneydistalconvolutedtubulesandthe choroidplexusofthebrain.Endocrineorgans(pituitarygland, parathyroidgland,pancreas,ovary,testisandplacenta),the heartandpancreaticcellsalsoexpressKlotho.1–7
Klotho generates twotranscripts, a transmembrane pro-teinandasecretedprotein,resultingfromalternativesplicing tothethirdexon.Additionally, thetransmembrane protein
∗ Correspondingauthorat:CentrodePesquisasGonc¸aloMoniz,Fundac¸ãoOswaldoCruz(FIOCRUZ),RuaWaldemarFalcão,121,Candeal,
40296-710Salvador,BA,Brazil.
E-mailaddress:mari@bahia.fiocruz.br(M.Goncalves).
canbecleavedby␣-and-secretasestogenerateasecreted protein, which is, two-times longer than the alternatively splicedtranscript.4,6,8–11So,theeffectsoftheKlothoprotein
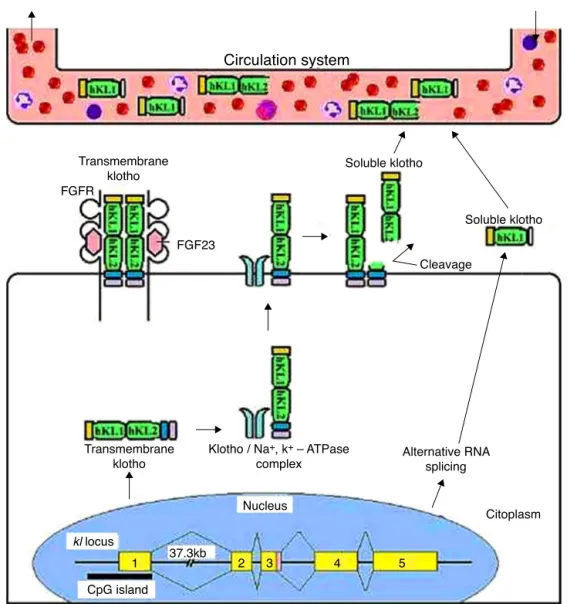
extendbeyondthetissuesthatexpressthegenebecauseofits humoralfactorfunction.Klothoreachesthesystemic circula-tionbysecretedfractions,andisreleasedintotheextracellular spaceandsubsequentlyintocirculation;itisfoundinblood, urineandcerebrospinalfluid(Figure1).11–13
Multiple aging phenotypes result from defects in gene expression of Klotho,11 such as growth retardation,
hyper-phosphatemia, moderate hypercalcemia, vascular and soft tissuecalcification,andhighlevelsof1,25-dihydroxyvitamin
http://dx.doi.org/10.1016/j.bjhh.2014.07.022
Circulation system
Transmembrane klotho
Transmembrane klotho
Alternative RNA splicing
Citoplasm Nucleus
37.3kb
CpG island kl locus
4 5
3 2 1
Soluble klotho
FGF23
Klotho / Na+, k+ – ATPase
complex FGFR
Soluble klotho
Cleavage
Figure1–TransmembraneandsecretedKlothoproteinprocessing.ThetransmembraneKlothoproteinactsasaco-receptor
forfibroblastgrowthfactor23signaling.ThistransmembraneproteinformscomplexeswithNa+/K+-ATPasechannelsand
isrecruitedtothecellsurface.Onceonthesurface,theKlothoproteiniscleavedbysecretasesformingasolubleformof
Klotho.Thisformentersthecirculatorysystemsimilartotheformproducedbyalternativesplicing,andactsonother
organsasahumoralfactor.
D3(1.25(OH)2D3)andfibroblastgrowthfactor23(FGF23).These
defectsinanimalsresultinashortenedlifeexpectancywhen comparedtowild-typephenotypes.Additionally,thekl−/−
ani-malexhibitshypokinesia, gaitchanges,andatrophyofthe genitals,skinand thymus.3,14 Klothosinglenucleotide
poly-morphisms(SNPs)havebeenassociatedwithsubphenotypes ofsicklecelldisease(SCA),15–19amonogenicautosomal
reces-sivedisorder,characterizedbya-globingene(HBB)mutation whichresultsintheformationoftheSvarianthemoglobin (HbS).
GiventhepossibleroleofKlothoasoneofthegenes respon-sible for the phenotypic variability between patients with sicklecell disease(SCD), theaimofthispaperistoreview thevariousfeaturesoftheKlothoprotein,aswellasdiscuss currentliteratureabouttheassociationbetweenKlotho poly-morphismsandSCDsubphenotypes.
Klotho
participates
in
the
regulation
of
the
bone-kidney
endocrine
axis
The phenotypic similarity between kl−/− mice and mice
with reduced Fgf23 expression (Fgf23−/− mice) suggests a
functional relationship between Klotho and FGF23. The Klotho transmembrane protein is a required co-factor/co-receptorforFGF23 signaling(Figure1),and theinactivation of the Klotho-FGF23 axis in kl−/−/Fgf23−/− mice results in
increased expression of co-transporter sodium-phosphate type-2a (NaPi-2a).14 FGF23, in turn, is a bone-derived
FGF23
VDR RXR
Fgf23
Cyp27b1
FGFR
Na
NaPi-2a Na
Transmembrane klotho
Vitamin D
Basal Apical
Figure2–PhosphateandvitaminDhomeostasis
regulation.VitaminDuponbindingtoitsvitaminD
receptor(VDR)inosteocytesinducesVDR-retinoidX
receptor(RXR)heterodimerbinding,whichactivatesFgf23
expression.Phosphatealsoinducesasimilarreaction.
FGF23thenbindstotheFGFR-Klothocomplexinrenal
tubularcells,blockingphosphatereabsorptionbyNaPi-2a
transportersandvitaminDsynthesisbysuppressingthe
Cyp27b1expressionandupregulatingCyp24expression.
ThismechanismcontrolsthephosphateandvitaminD
levelsinthebody;theFgf23expressionincreaseswhenthe
levelsofthesetwofactorsareelevated.After
re-establishingnormallevels,Fgf23expressionis
downregulatedandmaintainsphosphatereabsorptionand
vitaminDsynthesis.
Klotho acton the bone-kidney endocrine axisto maintain phosphatehomeostasis(Figure2).4,14,20,21
Thisbone-kidneyendocrineaxisalsoregulatesvitaminD levels. Highserum phosphate levels increaseFgf23 expres-sion inthe bone. FGF23 represses Cyp27b1 expressionand increasesCyp24expression,reducing1␣-hydroxylaselevels. Theend resultisthe reductionof1.25(OH)2D3 serum
syn-thesis(Figure2).Thesemechanismsareessentialforvitamin D homeostasis, preventing hypervitaminosis D.It has also beenfoundthattheadministrationof1.25(OH)2D3inducesthe
expressionofKlothointhekidney,whichreinforcesthe inte-gratedmechanismbetweenFGF23andKlothointhisaxis.4,22
Disturbancesofthesenegativefeedbackloopscanalsolead toahypercalcemicstatebecausevitaminDalsopromotesgut calciumabsorption.4,23,24
From observations ofFgf23−/− mice, there is additional
phosphaturic activity from secreted Klotho proteinthat is FGF23independent.TheKlothoglycosidaseactivityactsonan unknownsubstratethatispresentinthebrushborderof kid-neyproximaltubularcells.Thismodificationisaccompanied byproteolyticcleavage(thisstepoccurs,butitisnotrequired toinactivatethetransporter)andNaPi-2aco-transporter inter-nalization(Figure3).2
Klothocan also inhibitphosphate transport invascular smoothmusclecellsthroughNaPiclass3transporters, Pit-1andPit-2,preventingvascularcalcificationevents,because excessivephosphateinfluxinthesecellspromotesacascade ofeventsresponsibleforthecalciumandphosphate mineral-izationintheirinterior.25
Acute regulation Chronic regulation
Unidentified substrate
NaPi-2a 4
Figure3–SolubleKlothoproteinactsonphosphate
transportintheproximaltubules.Inacuteregulatory
conditions,Klothomodifiesanunknownsubstrate,glycan
(1),reducingthephosphatetransportercoupledtosodium
(2).Additionally,thecarrierundergoesproteolyticcleavage
(3)andsubsequentendocytosis(4).Uponsustained
elevatedsolubleKlotholevelsorchronicregulation
conditions,thereisongoingreductionofthesetransporters
atthecellsurface.
AdaptedfromHuetal.2
Effect
of
the
Klotho
protein
on
ion
channel
regulation
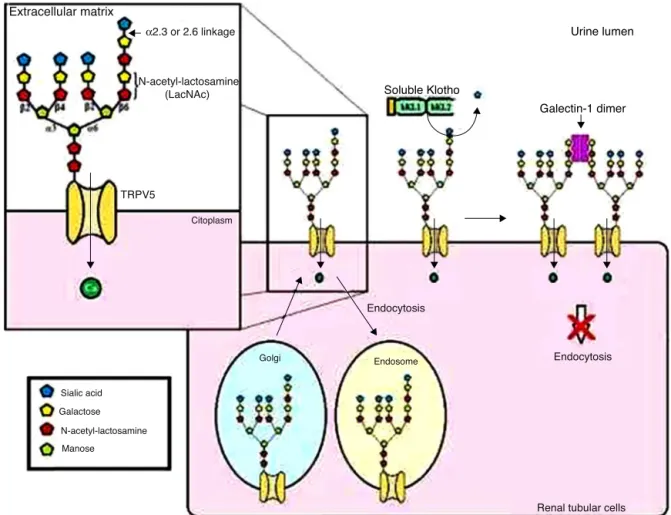
ThesecretedKlothoproteinregulatesotherionchannels,such asthetransientreceptorpotentialcationchannel,member5 ofsubfamilyV(TRPV5),whichisprimarilyresponsibleforCa2+
entry during kidney transepithelial reabsorption. Secreted KlothoproteininhibitsTRPV5internalizationthroughits siali-daseactivityonthisionchannel,increasingTRPV5cellsurface levelsandwiththistheCa2+inflowandkidneyreabsorption
(Figure4).4,26,27
Theretainingmechanismofthecellsurfaceionchannel occursduringtheregulationofpotassiumchannelsinthe kid-neyoutermedulla(ROMK1),resultinginanincreaseinROMK1 intheplasmamembraneofrenaltubularcells,withincreased potassiumsecretioninurine.28
Klotho
as
an
insulin/growth
factor
insulin-like
1
signaling
mechanism
regulator
Extracellular matrix
α2.3 or 2.6 linkage
N-acetyl-lactosamine (LacNAc)
N-acetyl-lactosamine
Manose
TRPV5
Endocytosis
Endosome Golgi
Citoplasm
Sialic acid
Galactose
Endocytosis
Renal tubular cells Urine lumen
Galectin-1 dimer Soluble Klotho
Figure4–GeneralmodelforTRPV5regulationbyKlotho.SolubleKlothoremovesthesialicacidresidueattachedby␣2.6
bondingintheN-acetyl-lactosamine(LacNAc)repeatsoftheTRPV5N-glycans.TheLacNAc,whenexposed,canbindtoits
ligand,thegalectin-1dimer,intheextracellularmatrix.Thismechanismreducescarrierinternalization.
regulatedbyenablinginsulin/IGF-1signaling,whichpromotes the serine-threonine kinase Akt phosphorylation. The Akt hastheabilitytophosphorylateFOXO,resultinginits exclu-sionfrom thenucleusandinactivation.Klothoproteinacts byinhibitinginsulin/IGF-1signaling,decreasingFOXO phos-phorylationandincreasingSOD2expressionandminimizing oxidativestress.11,29
Effect
of
Klotho
protein
on
the
vascular
endothelium
BothsecretedKlothoproteinshaveanti-apoptoticand anti-aging activity on vascular endothelial cells. These cells arecontinuouslyexposed toKlotho.Thevascular endothe-lium, due to the release of nitric oxide (NO) in response tospecific agonists suchas acetylcholine, plays an impor-tantrole invasculartone maintenance.Ithasbeenshown that mutations in the Klotho gene significantly attenuate endothelium-dependentvasodilationoftheaortaand arteri-olesinresponsetoacetylcholine.30,31Thereisevidencethat
Klothointhesecretedandmembraneboundformscan up-regulateNOproduction,althoughthemechanisms arestill unknown.4,11 It is believed that defects in the Klotho gene
down-regulateendotheliumNOsynthase(eNOS).Theeffects ofKlothoonthevascularendotheliumareprotectiveagainst endothelialdysfunction.
Kl−/− mice subjected to the induction of lower limb
ischemiaexhibitedpersistentbloodflowlossanddecreased capillarycapacity,incontrasttothehighperfusionobserved in heterozygous Klotho animals (kl+/−) and wild-type
ani-mals.Kl−/−animalspresentwithdeficientangiogenesisand
reduced levels of urinary NO and tissue cyclic guanosine monophosphate (cGMP), with high rates of progression to spontaneousamputation.32 Moreover,the Klothoproteinis
capableofincreasingangiotensin-convertingenzyme-I activ-ityinendothelialcellsbytheactivationofthecyclicadenosine monophosphate (cAMP)-protein kinase A (PKA) pathway,33
suggestinginvolvementoftherenin-angiotensinsystemand NOinvasculartonemaintenance.
TheKlothoproteinhasananti-apoptoticeffectonhuman umbilical vein endothelial cells, with decreased caspase-3 andcaspase-9activity,thereforeactingasahumoralfactor. Itretardscellularagingbymechanismsinvolvingp53/p21.34
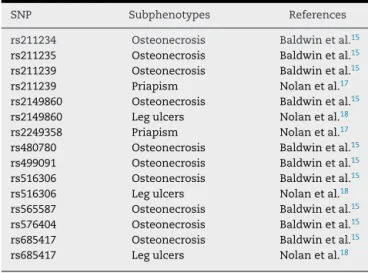
SNP Subphenotypes References
rs211234 Osteonecrosis Baldwinetal.15
rs211235 Osteonecrosis Baldwinetal.15
rs211239 Osteonecrosis Baldwinetal.15
rs211239 Priapism Nolanetal.17
rs2149860 Osteonecrosis Baldwinetal.15
rs2149860 Legulcers Nolanetal.18
rs2249358 Priapism Nolanetal.17
rs480780 Osteonecrosis Baldwinetal.15
rs499091 Osteonecrosis Baldwinetal.15
rs516306 Osteonecrosis Baldwinetal.15
rs516306 Legulcers Nolanetal.18
rs565587 Osteonecrosis Baldwinetal.15
rs576404 Osteonecrosis Baldwinetal.15
rs685417 Osteonecrosis Baldwinetal.15
rs685417 Legulcers Nolanetal.18
TheKlothoproteinhasbeenassessedasatherapeutictool inparticularinrespecttopreventingactivityofage-related phenotypes.30,35,36Themainaimofthisreviewistodescribe
thepossibleroleoftheKlothoproteinasabiomarkerinsickle celldisease.
Klotho
protein
in
sickle
cell
disease
SomestudieshaveevaluatedtheassociationofKlothoSNPs with subphenotypes of individuals with SCA, one type of SCD.15–19 Itisadisorderofmonogenicautosomalrecessive
inheritance,characterized by a mutationin the HBB gene, wherevalinereplacesglutamicacidinthe-globin polypep-tidechain.Thismutationresultsinthevarianthemoglobin namedS(HbS),which,underdeoxygenatedconditions,tends to polymerizeinside the red blood cell conferringa sickle shape.
These individuals exhibit a chronic inflammatory and hemolyticstate,withsignificantproductionofROS,increased molecule adhesion of endothelial cells and blood cells, and reduced NO production resulting in vaso-occlusive phenomena; all of theseare importantevents that trigger thevariedsubphenotypesofthedisease.37Thelarge
pheno-typicvariabilityobservedinSCD(includingpaincrises,stroke, priapism, osteonecrosis, leg ulcers, bacteremia, pulmonary hypertension,acutechestsyndrome,andgallstones) demon-stratesgeneinteractionsinsubphenotypedevelopment.38
IndividualswithSCAandosteonecrosisofthehipor shoul-der,withorwithout␣-thalassemia,showedthat10KlothoSNPs were significantly associated with these subphenotypes.15
Additionally,twoSNPshavebeenassociatedwithpriapism17
andthreewithlegulcers18(Table1).Theseassociationsmay
beunderestimated,giventhatcontrolgroupindividualscould developsuchsubphenotypesinthefuture.
However, other studies have not succeeded in replicat-ingtheseresults.Ulugetal.19testedKlothoSNPsin39SCD
patientswhohadfemoralandhumeralheadavascular necro-sis,evidencedbysymptomsandimagingstudies.Thecontrols were 205 individuals with SCD without symptoms of this subphenotype.Therewasnoreproductionoftheassociation
• Hemolysis
• Increases oxidative stress
• Decreases NO production
• Chronic inflammation
• Homeostatic imbalance
• Vasoocclusive phenomena
• Increases oxidative stress
• Decreases NO production
Osteonecrosis priapism leg ulcer
Figure5–SummaryoftheroleofKlotho SNPstoestablish
SCDsubphenotypes.
foundbyBaldwinetal.15whichmaybeexplainedbythe
inad-equate samplesizeand bythe absence ofany radiological investigationofcontrolsasit isnotpossibletoensurethat the control individuals were notasymptomatic patientsin earlystagesofavascularnecrosis.Elliotteetal.16testedthe
associationofKlothoSNPsandpriapisminSCDpatients,but were unsuccessfulinfinding theassociation betweenSNPs and subphenotypesreportedbyNolanetal.17probablydue
todifferencesinthedefinitionofpriapism,thepatients’ages andadjustmentsmadeinthetests.
Osteonecrosis,associatedwithvaso-occlusiveeventsand increased blood viscosity, is a common clinical manifesta-tionofSCD.Bonemicrocirculationisafavorableenvironment for sickledredblood cells, leukocytes,and platelet deposi-tion, leading to infarctionand necrosis ofbone tissue.38,39
So,despitethemethodologicaldifferencesthatdidnotallow reproducibilityoftheresultsofBaldwinetal.15itmaybethat
endothelial dysfunction is exacerbated in individuals with SCDandKlothoSNPsduetothelossoftheprotectiveeffect againstoxidativestressaswellasreductionsintheproduction ofNOthataffectvasculartone.
Priapism is a prolonged penile erection irrespective of sexual interest, and has a direct relation with intravas-cular hemolysis.38 Normal erection is dependent on the
activation ofguanylate cyclase byNO for the synthesisof cGMP,whichgeneratestherapidrelaxationofpenilesmooth muscle. Erection regulationis drivenbyphosphodiesterase type 5, which controls the production of cGMP.40,41
How-ever,theexpressionofphosphodiesterasetype5isreduced when there is NO depletion. Both hemolysis and genetic alterations inKlothoexpressioncontribute tothereduction in NO synthesis, which affects the erection control mech-anisms by phosphodiesterase type 5, thereby prolonging erection.
Similartopriapism,legulcersarerelatedtotheseverityof hemolysis,andbothNOdepletionandoxidativestressappear toplayaroleinthedevelopmentofulcers.18,38However,the
pathophysiologyofthisclinicaleventstillneedstobe eluci-datedinordertoclarifywhetherthesemechanismscauseleg ulcerswhenKlothoSNPsarepresent.
Conclusion
The Klotho gene has a wide range of functions in several structuresofthebody,increasingphosphaturicactivityand reducing1.25(OH)2D3 synthesis, regulatingionchannel
lev-els on the cell surface with anti-aging and anti-apoptotic effects,reducing oxidative stressand inducingthe produc-tionofNO.SNPsinthisgenehavereportedlybeenassociated withsubphenotypesofSCD,howeverthisdatawasnot repro-ducedprobablyduetomethodologicaldifferences.Giventhe endothelialinvolvement of the Klotho protein and knowl-edgeaboutSCDpathophysiology(markedlycenteredaround hemolysisandvaso-occlusivephenomena),itisessentialto conduct furtherstudieswith thepower needed totestthe associations betweenthe Klotho gene and SCD,in order to identifygeneticmarkersofriskintheseindividualsandallow earlierandmoreeffectivetherapeuticinterventions.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
This work was supported by grants from the Brazilian NationalCouncilofResearch(CNPq)(311888/2013-5)(M.S.G.); theFoundationofResearchandExtensionofBahia(FAPESB) (3626/2013)(M.S.G.); PPSUS/FAPESB (020/2013EFP00007295), (M.S.G.),andCNPq(402022/2010-6)(coordinatedbyF.F.C.).The sponsorsofthisstudyarepublicornonprofitorganizations thatsupportscienceingeneral,andtheyhadnorolein gath-ering,analyzing,orinterpretingthedata.
r
e
f
e
r
e
n
c
e
s
1. Ben-DovIZ,GalitzerH,Lavi-MoshayoffV,GoetzR,Kuro-oM, MohammadiM,etal.Theparathyroidisatargetorganfor FGF23inrats.JClinInvest.2007;117(12):4003–8.
2. HuMC,ShiM,ZhangJ,PastorJ,NakataniT,LanskeB,etal. Klotho:anovelphosphaturicsubstanceactingasan autocrineenzymeintherenalproximaltubule.FASEBJ. 2010;24(9):3438–50.
3. Kuro-oM,MatsumuraY,AizawaH,KawaguchiH,SugaT, UtsugiT,etal.Mutationofthemouseklothogeneleadstoa syndromeresemblingageing.Nature.1997;390(6655):45–51.
4. Kuro-oM.Klotho.PflugersArch.2010;459(2):333–43.
5. LiSA,WatanabeM,YamadaH,NagaiA,KinutaM,TakeiK. ImmunohistochemicallocalizationofKlothoproteininbrain, kidney,andreproductiveorgansofmice.CellStructFunct. 2004;29(4):91–9.
6. MatsumuraY,AizawaH,Shiraki-IidaT,NagaiR,Kuro-oM, NabeshimaY.Identificationofthehumanklothogeneandits twotranscriptsencodingmembraneandsecretedklotho protein.BiochemBiophysResCommun.1998;242(3):626–30.
7. TakeshitaK,FujimoriT,KurotakiY,HonjoH,TsujikawaH, YasuiK,etal.Sinoatrialnodedysfunctionandearly unexpecteddeathofmicewithadefectofklothogene expression.Circulation.2004;109(14):1776–82.
8.BlochL,SineshchekovaO,ReichenbachD,ReissK,SaftigP, Kuro-oM,etal.Klothoisasubstrateforalpha-,beta-and gamma-secretase.FEBSLett.2009;583(19):3221–4.
9.ChenCD,PodvinS,GillespieE,LeemanSE,AbrahamCR. Insulinstimulatesthecleavageandreleaseofthe
extracellulardomainofKlothobyADAM10andADAM17.Proc NatlAcadSciUSA.2007;104(50):19796–801.
10.Shiraki-IidaT,AizawaH,MatsumuraY,SekineS,IidaA, AnazawaH,etal.Structureofthemouseklothogeneandits twotranscriptsencodingmembraneandsecretedprotein. FEBSLett.1998;424(1–2):6–10.
11.WangY,SunZ.Currentunderstandingofklotho.AgeingRes Rev.2009;8(1):43–51.
12.ImuraA,IwanoA,TohyamaO,TsujiY,NozakiK,Hashimoto N,etal.SecretedKlothoproteininseraandCSF:implication forpost-translationalcleavageinreleaseofKlothoprotein fromcellmembrane.FEBSLett.2004;565(1–3):143–7.
13.LiuH,FergussonMM,CastilhoRM,LiuJ,CaoL,ChenJ,etal. AugmentedWntsignalinginamammalianmodelof acceleratedaging.Science.2007;317(5839):803–6.
14.NakataniT,SarrajB,OhnishiM,DensmoreMJ,TaguchiT, GoetzR,etal.Invivogeneticevidenceforklotho-dependent, fibroblastgrowthfactor23(Fgf23)-mediatedregulationof systemicphosphatehomeostasis.FASEBJ.2009;23(2):433–41.
15.BaldwinC,NolanVG,WyszynskiDF,MaQL,SebastianiP, EmburySH,etal.Associationofklotho,bonemorphogenic protein6,andannexinA2polymorphismswithsicklecell osteonecrosis.Blood.2005;106(1):372–5.
16.ElliottL,Ashley-KochAE,DeCastroL,JonassaintJ,PriceJ, AtagaKI,etal.Geneticpolymorphismsassociatedwith priapisminsicklecelldisease.BrJHaematol.
2007;137(3):262–7.
17.NolanVG,BaldwinC,MaQ,WyszynskiDF,AmiraultY,Farrell JJ,etal.Associationofsinglenucleotidepolymorphismsin klothowithpriapisminsicklecellanaemia.BrJHaematol. 2005;128(2):266–72.
18.NolanVG,AdewoyeA,BaldwinC,WangL,MaQ,Wyszynski DF,etal.Sicklecelllegulcers:associationswithhaemolysis andSNPsinKlothoTEKandgenesoftheTGF-beta/BMP pathway.BrJHaematol.2006;133(5):570–8.
19.UlugP,VasavdaN,AwogbadeM,CunninghamJ,MenzelS, TheinSL.Associationofsickleavascularnecrosiswithbone morphogenicprotein6.AnnHematol.2009;88(8):803–5.
20.KurosuH,OgawaY,MiyoshiM,YamamotoM,NandiA, RosenblattKP,etal.Regulationoffibroblastgrowthfactor-23 signalingbyklotho.JBiolChem.2006;281(10):6120–3.
21.UrakawaI,YamazakiY,ShimadaT,IijimaK,HasegawaH, OkawaK,etal.KlothoconvertscanonicalFGFreceptorintoa specificreceptorforFGF23.Nature.2006;444(7120):770–4.
22.TsujikawaH,KurotakiY,FujimoriT,FukudaK,NabeshimaY. Klotho,agenerelatedtoasyndromeresemblinghuman prematureaging,functionsinanegativeregulatorycircuitof vitaminDendocrinesystem.MolEndocrinol.
2003;17(12):2393–403.
23.HuangCL,MoeOW.Klotho:anovelregulatorofcalciumand phosphorushomeostasis.PflugersArch.2011;462(2):185–93.
24.YoshidaT,FujimoriT,NabeshimaY.Mediationofunusually highconcentrationsof1,25-dihydroxyvitaminDin
homozygousklothomutantmicebyincreasedexpressionof renal1alpha-hydroxylasegene.Endocrinology.
2002;143(2):683–9.
25.LauWL,FestingMH,GiachelliCM.Phosphateandvascular calcification:emergingroleofthesodium-dependent phosphateco-transporterPiT-1.ThrombHaemost. 2010;104(3):464–70.
activatestheTRPV5channel.Science.2005;310(5747):490–3.
28.ChaSK,HuMC,KurosuH,Kuro-oM,MoeO,HuangCL. Regulationofrenaloutermedullarypotassiumchanneland renalK(+)excretionbyKlotho.MolPharmacol.
2009;76(1):38–46.
29.YamamotoM,ClarkJD,PastorJV,GurnaniP,NandiA,Kurosu H,etal.Regulationofoxidativestressbytheanti-aging hormoneklotho.JBiolChem.2005;280(45):38029–34.
30.NagaiR,SaitoY,OhyamaY,AizawaH,SugaT,NakamuraT, etal.Endothelialdysfunctionintheklothomouseand downregulationofklothogeneexpressioninvariousanimal modelsofvascularandmetabolicdiseases.CellMolLifeSci. 2000;57(5):738–46.
31.SaitoY,YamagishiT,NakamuraT,OhyamaY,AizawaH,Suga T,etal.Klothoproteinprotectsagainstendothelial
dysfunction.BiochemBiophysResCommun. 1998;248(2):324–9.
32.ShimadaT,TakeshitaY,MuroharaT,SasakiK,EgamiK, ShintaniS,etal.Angiogenesisandvasculogenesisare impairedintheprecocious-agingklothomouse.Circulation. 2004;110(9):1148–55.
33.YangJ,MatsukawaN,RakugiH,ImaiM,KidaI,NagaiM,etal. UpregulationofcAMPisanewfunctionalsignalpathwayof Klothoinendothelialcells.BiochemBiophysResCommun. 2003;301(2):424–9.
Klothoonvascularendothelialcells.BiochemBiophysRes Commun.2006;339(3):827–32.
35.MasudaH,ChikudaH,SugaT,KawaguchiH,Kuro-oM. Regulationofmultipleageing-likephenotypesbyinducible klothogeneexpressioninklothomutantmice.MechAgeing Dev.2005;126(12):1274–83.
36.SaitoY,NakamuraT,OhyamaY,SuzukiT,IidaA,Shiraki-Iida T,etal.Invivoklothogenedeliveryprotectsagainst endothelialdysfunctioninmultipleriskfactorsyndrome. BiochemBiophysResCommun.2000;276(2):767–72.
37.MorrisCR,KatoGJ,PoljakovicM,WangX,BlackwelderWC, SachdevV,etal.Dysregulatedargininemetabolism, hemolysis-associatedpulmonaryhypertension,and mortalityinsicklecelldisease.JAMA.2005;294(1):81–90.
38.SteinbergMH.Geneticetiologiesforphenotypicdiversityin sicklecellanemia.ScientificWorldJournal.2009;9:46–67.
39.BennettOM,NamnyakSS.Boneandjointmanifestationsof sicklecellanaemia.JBoneJointSurgBr.1990;72(3):494–9.
40.ChampionHC,BivalacquaTJ,TakimotoE,KassDA,Burnett AL.Phosphodiesterase-5Adysregulationinpenileerectile tissueisamechanismofpriapism.ProcNatlAcadSciUSA. 2005;102(5):1661–6.
41.NolanVG,WyszynskiDF,FarrerLA,SteinbergMH.