PHYSICO-CHEMICAL ASSESSMENT
OF AGRICULTURAL POLLUTION ON
GROUNDWATER AND SOIL QUALITY
IN AN AGRICULTURAL FARM (NORTH
EASTERN MOROCCO)
S. Fetouani a,b,*, B. Bendra a,b , M. Vanclooster c , M. Sbaa a,b a
Laboratory of Hydrobiology and General Ecology, University Mohamed First, LP 524, CP 60000, Oujda, Morocco
b
Eastern Center of Sciences and Technologies of Water, University Mohammed First, LP 524, CP 60000, Oujda, Morocco
c
Universite´ catholique de Louvain, Department of Environmental Sciences and Land Use Planning, Unite´ GERU, Belgium
ABSTRACT
To ensure sustainable food security, Morocco gives priority to agricultural and rural development by promoting investment in agricultural sector and use of intensification factors to improve incomes in rural areas.
The Triffa irrigated perimeter is one of the oldest and the most productive in the country thanks to the Mohammed the V dam activity and the beginning of agricultural development intensification. Although this intensification has a positive effect on agricultural yields, it has negative impacts on soil and generates groundwater quality degradation. Indeed, recent studies performed in this area by us and Bendra (Fetouani et al., 2008; Bendra et al, 2012) have mentioned the existence of salinity problems, nitric groundwater pollution and soils salinization. This degradation is caused essentially by intensive use of agrochemicals, including nitrogen fertilizers and pesticides, and non-control of irrigation and cultivated plots drainage. However, a degradation of groundwater and soil quality is not without risk to Human health.
Having a global vision about situation of groundwater and soil quality in the Triffa plain we have decided to deepen this theme to a local scale and to study in details the impact of intensive agriculture on groundwater and soil quality in a farm, located in the centre of the Triffa plain.
To sum up the results of this study the state of soil quality in the farm is not alarming. However, the groundwater quality is mainly dramatic, because it is a receptacle of all the nutrients applied on the surface, especially nitrates.
Key words: groundwater, soil, physicochemical assessment, farm, Triffa, north eastern Morocco.
I-INTRODUCTION
Awareness of public opinion to the environmental problems tends to generate number of preserving actions. Among these different problems, agricultural pollution is growing due to agriculture intensification and agricultural practices.
In the previous decades, the main objective of agriculture was to reach the highest production levels in order to feed a growing world population. To ensure this, governments set different technical ways such as fertilization, mechanization, irrigation and phytosanitary treatments that actually allows to obtain yields never achieved before (Gardner, 1944).
In fact, the agriculture intensification has increased production; it permits to reach highest levels production but it causes negative effects on the environment. Water and soil resources are subject to overexploitation, mismanagement and pollution (De Fraiture, 2010). In fact, more than 1/6 lands in the world are affected by degradation and deforestation; 6.4% of the land would be affected by salinity and alkalinity phenomena, which is an area of approximately 10 million km2 (FAO, 2000). Consequently, the average arable area per capita worldwide continues to decrease from 0.38 hectares in 1970 to 0.28 hectares in 1990 (Ghassimi, 1995).
and drainage of cultivated plots (Soudí et al, 1999). Because of its diffuse nature, pollution remains difficult to resolve; its evaluation and treatment are particularly difficult problems.
In arid and semi-arid zones, high agricultural potential surfaces are progressively rare and irrigated surfaces are increasing. It is therefore imperative to give great importance to the irrigated areas. In an arid climate dominance as it is in Morocco, irrigation is undoubtedly necessary to improve agricultural productivity and to ensure population needs in terms of food.
The development of agricultural intensification in arid and semi-arid irrigated zones is strongly accentuated and it generates a deterioration of soil and groundwater quality. The most prominent and most common degradation processes are soils salinization (Laudelout and Chiang, 1995; Badraoui et al., 1998 and Lahlou et al., 1998) and groundwater nitric pollution (Dakak, 1996). A significant loss of soil organic stocks increases the deterioration process of soil quality.
Intensification, in search for a growing productivity, has as a secondary consequence the disappearance of small farmers who generally exist in marginal areas; this leads to economic, social and ecological fragilization of concerned regions.
II-MATERIALS AND METHODS 1. The case study region
1.1. Triffa plain
Located in north-eastern Morocco, and sheltered by three important wadis (the Moulouya Wadi and its affluent, the Cheraa Wadi, in the west, and the Kiss Wadi in the east along the Moroccan–Algerian border) and three mountainous ridges (the Kebdana chain in the west, the Beni Snassen in the south, and the Oulad Mansour in the north along the Mediterranean Sea), the Triffa Plain covers a total area of about 750 km2 (Fig.1). The Altitude varies from 50 to 150 m.
The climate regime of the southern part of the Triffa plain is warm semi-arid, while the north-western part is cold semiarid (Haloui, 1991). Rainfall diminishes from the west to the east and from the south to the north. Mean temperature is slightly higher in the south as compared to the north.
The study area is the most fertile and productive agricultural region of north-east Morocco. Land use within the area is very heterogeneous, which reflects the diversity of soil types, local climate conditions and agronomic practices.
Rainfall is insufficient to support modern agriculture. Therefore, 39060 ha of the plain is irrigated. Modern irrigation started from 1954 onwards when the reservoir of Mohammed the V (total storage capacity of 730 Mm3) and the reservoir of Mechra-Hommadi (total storage capacity of 42 Mm3) were constructed. These reservoirs regulate flow in the Moulouya basin.
From a geological point of view, the Triffa plain is a large and flat quaternary dejection plain, situated at the footside of the Beni Snassen mountains. The upper aquifer below the quaternary deposits is situated in tertiary formations and limited on its base by marl. Its southern limit is constituted by the anticlinal of Beni Snassen, while the northern limit is within the pliocene formation of the anticlinal of Oulad Mansour (Fig. 2). Primary trias and lias formations outcrop on the highest spots of the area.
The upper aquifer of the plain is very heterogeneous, is unconfined and is composed of different lithological facies: sandstone, chalky gravel stone, conglomerates and calcareous sandstone. The flow of the aquifer is directed in the south-east north-west direction, and is influenced by the Hassi Smia discontinuity (El Mandour, 1998). The transmissivity coefficients of the aquifer are situated between 10-2 and 5×10-2 m2/s (DGH, 1997), while the storage coefficient equals 2.5% (Chettouani and Damou, 1993).
According to legend of the FAO-UNESCO (soil map of the world 1974), the soils of the Triffa plain are rendzinas, xerosols luvic and calcic, luvic and calcic yermosols, calcic and chromic luvisols, calcic and chromic cambisols.
1.2. Farm
The studied area is a farm located about 10 km from north of Berkane city, in the centre of the Triffa irrigated perimeter. It is situated in Madarh commune (493800 m N longitude, 783200m S latitude) (Fig.3).
The farm is equipped with drip irrigation system and covers a total area of 75 hectares divided into 5 sectors, each sector divided in plots, the agricultural surface is mainly covered by orange with some rotation crops (potatoes, pea, beans, melon...) (Fig.4).
The studied farm is located in the central area of the Triffa characterized by a semi-arid Mediterranean-type climate, low rainfall (average 300 mm); two periods are to be distinguished:
-A rather rainy period with relatively abundant rainfall from October until February with a maximum in February (from 45 mm to 67 mm on average).
Temperature varies from 4.7 ° C in January (the coldest month minimum average) at 37.5 ° C in August (average of the maxima of the warmest months) with an annual average of 18.2 ° C.
The present farm is equipped by an irrigation basin of 16.000 m3 of storage capacity, it receives superficial water from Mohammed the V dam in addition to pumped groundwater from 9 wells.
2. Sampling strategy and soil analysis
Sixty-two sampling locations were selected for soil quality determination in October 2006 (Fig.5). The sampling points were distributed all over the study farm to ensure appropriate spatial coverage of the entire agricultural area.
For each plot, six single samples were taken from the top layer of soil (0–30 cm) and mixed for analysis. The samples were air-dried and sieved to 2mm diameter. The sieved fraction was then stored in plastic boxes at room temperature before analysis.
Soil organic matter (SOM) was estimated by multiplying the percentage of organic carbon by a factor of 1.724 according to Nelson and Sommers (1982). Total reduced nitrogen (total-N) in soil fine fraction was determined by the standard Kjeldhal method (Jackson, 1958). The pH of the soil was measured in the soil solution (1:2.5) using a digital pH-meter (WTW model 530; Lutron Electronic Co Inc.; Coopersburg, Pennsylvania, USA). Total phosphorus (TP) was determined by two methods depending on total lime content: (i) for non-calcareous or slightly calcareous soils, with a total free lime content of less than 3%, extraction was carried out by sulphuric acid 0.002N according to the Truog method (Truog, 1930), and (ii) for soils with a total free lime content of more than 3%, extraction by ammonium oxalate (0.2N) was preferred (Joret and Hebert, 1955). The PO4-P was determined in acidic conditions using orthophosphate and ammonium molybdate reagents (approved standard method AFNOR norm T90-023) (AFNOR, 1992).
Heavy metals were analyzed by ICP after mineralization with aqua regia (HNO3 and HCl with a ratio of 1/3) (EPA - ROC, 1994). Only 40/62 soil samples were selected to analyze their content of heavy metals.
3. Sampling strategy and groundwater analysis
The studied farm is equipped with 9 wells distributed in all over the farm; each well is provided with a pump and has been monthly or bi-monthly monitoring of their physicochemical quality from October 2006 until October 2007(Fig.6).
Pumped groundwater samples were collected in polyethylene bottles of 1L. Before sampling, the recipient was cleaned several times using the pumped water. Flushing allowed also to clean the adductions and tubing within the well and pumping system. Recipients were gradually filled to avoid turbulences and aeration during the sampling. To avoid sampling artefacts and analytical artefacts, in particular the gain of dissolved gas and microbiological activity, water samples were immediately cooled at 4°C using portable icebox. Analysis was further performed as fast as possible and this within 24 h after sampling.
The analytical method for NO3-N dosage is the method of sodium salicylate (cited in Afnor T90-012). For the determination of PO4-P, use was made of the colorimetric method (Afnor T90-023). The quantity of dissolved organic matter was determined through the reduction of KMnO4 (Afnor T90-18).
The pH is measured directly in water by a pH-meter (WTW530, LUTRONTM).
Soluble heavy metals in the water were determined after filtration, by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP - AES) using a spectrometer of type JY. Only samples from October campaigns 2006, February and April 2007 were subject to analysis their content of heavy metals.
III. RESULTS AND DISCUSSION
1.Physico-chemical assessment of soil quality 1.1. Soil pH
The pH of soil in the study farm is situated between 8.60 and 9.59 (Fig.7). According to the French Association of Soil Studies (AFES, 1992), the majority of parcels are basic to very basic. The micronutrient deficiencies are usually due to alkaline pH of soil. The soil alkalinity reduces availability of essential nutrients to plants (wholesale, 1979) and (Morel, 1996) in (Bensaadi, 2004) and contributes frequently to the formation of insoluble hydroxides and (Heller et al., 1998); (FAO, 2003).
1.2. Soil organic matter (SOM)
Soil organic matter (SOM) varied between 1.5% and 10%. High SOM concentration in sampled parcels suggests probable measurement of residue content which is not yet thoroughly humified.
According to the pedological reference (AFES, 1992) all sampled soils are qualified as organic type (Fig.8). Our results are consistent with those found by Bendra et al (2012).
Soil organic matter (SOM) is a relevant indicator of soil quality (Larson and Pierce, 1991; Rosell et al., 2001) as SOM plays an essential role in cropping systems since it is involved in the physical and chemical characteristics of the soil, and also in biological processes (Baldwin, 2006). SOM improves the nutrient-holding capacity of soils (resilience) (Baldock and Skjemstad, 2000), and contributes to the plant growth suitability of the soil’s physical environment. Its management is of prime importance to maintain soil fertility and ensuring sustainable agriculture (Martin et al., 1990). The decomposition of SOM is mainly a bacterial and macrofauna-dependent process, thus all parameters that control these activities (soil pH, soil humidity, soil temperature, soil nutrient status, etc.), interact indirectly in SOM mineralization.
The SOM content in the studied farm appears independent of culture type; there is no distinction between orange with intermediate crops and cereals. The SOM spatial distribution is more affected by agricultural practices than culture type.
The semi arid climate to witch belong the farm slows probably mineralization process of SOM. Water is a key element in understanding the soil organic matter destination, a soil with frequent and massive water flux has necessarily fragile organic matter status, opposed to the soil where drought is over ruling (Campbell et al., 1996).
The irrigation technique used in the farm maintains the soils organic matter status, contrasting gravity irrigation which accelerates the mineralization process and therefore weakens the soil organic matter (Benlaghlid, 1995). Organic manure is often imported from other farms where sheeps, goats and cattle livestock. Spreading organic manure is done anarchically especially in soils with orange and intermediate crops, in the aim to fortify soil organic matter status and to make soil more fertile.
1.3. Soil total nitrogen content and C/N ratio
The soil nitrogen reserves are found in the organic state as humus containing about 5% nitrogen. Every year, in a Mediterranean climate, 1 to 2% of organic nitrogen reserves pass to the nitric state available to the plant: it is mineralization (Namam et al., 2001). Many factors influence positively the net nitrogen mineralization: the soil temperature and humidity (Nadelhoffer et Fry, 1988; Nadelhoffer et al., 1991; Sierra, 1997; Jussy, 1998; Joshi et al., 2003), the organic nitrogen concentration in the humus (Pastor et al., 1984; Van Cleve et al., 1993; Vervaet et al., 2002), and pH (Falkengren-Grerup et al., 1998; Li et al., 2007; Pietri et Brookes, 2008). Plant species can also influence the nitrogen mineralization by secretion of specific compounds by leaves (Northup et al., 1995); (Hattenschwiler and Vitousek, 2000) or roots (Subbarao et al., 2007).
The types of irrigation can affect water drainage which consequently causes significant leaching of nitrogen at depth. Excessive irrigation led to drainage toward the aquifer and contributed to N leaching losses (Stevenson and Neilson, 1990). However nitrogen leaching of localized irrigation is relatively insignificant; soils maintain rich in organic nitrogen and mineralization is even lower.
The total nitrogen of the studied farm soil varies between 0.06% and 0.29% (Fig.9). Following AFES norms, most of the farm soils (61% of sampled soils) are considered as rich, and 39% of soils sampled as poor in total nitrogen content.
The C/N ratio of the farm soils ranges from 5.23 to 53.43. In cultivated soils, C/N ratios over 12 indicate a lack of mineralization (either anaerobic conditions or excessive acidity or both) (Baize, 2000). The C/N ratio decreases with increasing biological activity (or increases when biological activity is limited). SOM decomposition led to C-losses by CO2 releases that are greater than N-losses. As N is recycled into the SOM, the C/N ratio has a tendency to decrease toward a value characteristic of a given humus type (Soltner, 2000). In our study, 77.41% of the sampling soils have a C/N ratio above 12 indicating low mineralization in most of the soils. This is a cause of localized irrigation which interposes a biological activity due to lack of water necessary for the organic matter decomposition. In calcareous soils, as the case in the studied farm, calcium carbonate inhibits humified organic matter biodegradation. Its intervention is manifested by forming a CaCO3 crystalline coating around organic matter (Muller, 1972 in Oustani, 1993).
1.4. Total-P and PO4-P soil content
The PO4-P concentrations level in farm soils varies between 63.42 and 232.24 mg/ Kg (Fig.10).
al., 1999). The use of P-fertilizers on calcareous soils has often been described as problematic due to P fixation problems (Olsen et al., 1954; Sample et al., 1980). For orange production the most important crop in the studied farm, P is applied only once, generally during the months of February and March but in highly varying quantities.
1.5. Heavy metals Status in the farm soils
The statistical description of heavy metals contents in farm soils is presented in Table I. The mean concentrations of trace metals were found in the order of: Fe> Mn> Pb> Cr> Cu> As> Ni> Cd> Zn.
Soil heavy metals content is not the only parameter for an environmental risk estimation. The mobility, which usually depends on their chemical form, is an important factor for evaluating their distribution and toxicity degree since complex forms are much less available than the free ions (Forstner, 1979). The chemical speciations are closely related to parameters as pH which plays important role in the partition of an element between the dissolved and particle phase. So, the water pH used for irrigation and the pH on sol-residual system are often the most important chemical properties governing trace element sorption, precipitation, solubility and consequently the bioaccumulation and availability of heavy metals pollutants (Kabala and Singh, 2001). The composition and salinity of different waters used for irrigation can play non negligible role in the adsorption or fixing of the pollutants (Forstner, 1979). Different parameters of soil matrix as chemical composition, mineralogy, texture and organic matter yield control, too, the heavy metals bioactivity (Tam and Wong, 1999). The farm soils pH oscillates between 8.60 and 9.59 this alkaline pH restricts heavy metals passage in the solid phase to soil solution and the plant (Thornton 1996). High levels of organic matter promote the heavy metals setting in farm soil by the insoluble organometallic complexes formation and consequently decrease their mobility and phytotoxicity (Paré et al., 1999; Udom et al., 2004).
On nine analyzed elements (As, Cd, Cr, Cu, Ni, Pb, Zn, Mn, Fe), only the nickel (Ni) and lead (Pb) exceeded the maximum allowable concentrations in agricultural soils (Kabata-Pendias and Pendias, 2001). However 12/40 soils samples studied have exceeded the reference value for nickel, while 4/40 have exceeded the reference value for lead (Tab. I).
In agricultural soils, high Pb concentrations may be the over-fertilization result. According to Gimeno-Garcia (1996) who worked on Valencia cultivated soils, a slight increase in Pb soil content is the result of fertilizers intensive use (for example urea and phosphate). Concentrations of Pb soil exceeding the 100 mg kg-1 characterize potentially contaminated soils (Kabata-Pendias and Pendias, 2001). Some pesticides long used in orchards, basis of arsenate of lead or organomercury compounds, also leads to Pb soil pollution (Robert and Just 1998).
Comparing our results with those found for agricultural soils in other countries (France, Spain and Haut-Rhin) (Tab. II) shows that farm soils studied are characterized by higher content of: Cd, Cr, Cu, Ni, Pb, Mn and Fe than those found in French soils (Baize, 1997) Spanish soils (Micó, 2005) and Haut-Rhin soils (MRA68. 1999).
2. Physico-chemical assessment of farm quality 2.1. groundwater pH
Mean pH values are within the norms recommended by Morocco (1993) in all the wells during all the sampling campaigns. They do not descend below 6.5 value obtained at P1 and not exceed 8 obtained at the P7 (Fig.11). No correlation between pH values and the main soil uses. They are determined by aquifer geology or more by soils carbonate content in the basin catchment.
In irrigation water the pH value influences form and availability of nutrients. pH levels in farm irrigation water are situated between 5.5 and 6.5. With these values, most trace elements solubility are optimal (Couture, 2004).
2.2. Dissolved organic matter content
Dissolved organic matter (DOM) encompasses living or dead biomass as well as all organic molecules from decomposing biomass. In addition, it includes organic molecules from anthropogenic origin such as for example residues of plant protection products and their metabolites. Values are situated between 0.23 and 2.08 mg/l and do not exceed WHO drinking water norms (WHO, 2000). The minimum value is registered in P6 well during September 2006 and maximum was recorded at the P9 well during May 2007 (Fig.12).
Low spatial variation for the different wells in organic matter concentrations is shown, while temporal variation for every station is considerable. They increase significantly between February and May 2007, then decrease by half at the end of the from study cycle, a slight increase in organic matter content of groundwater is also observed early in the study cycle of (December 2006).
through infiltrating water to increase levels of groundwater organic matter, this infiltration was facilitated by : heavy rains experienced by the studied area during this period, the permeable sandy loam and the low depth. At the level of the studied firm the owner practice a manure application between the end of October and December which explains the observed increases in the concentrations of organic matter in groundwater in the study cycle.
Losses organic matter in manure by leaching during study period is due to the absence of precipitation and localized irrigation system.
2.3. Nitrate content
Nitrate-N presents the greatest problems in agriculture. Highly soluble and mobile in soil solution nitrates are easily leached into groundwater thus constituting a potential source of nitrates pollution (Zolle, 1994, Soudi, 1995).
Various aspects of farming contribute to groundwater nitric contamination: the difficulty of adjusting nitrogen fertilization (Kucke and Kleeberg. 1997), non-use of intercropping (Webster et al., 1999) and application of traditional tillage (Mehdi and Madramootoo, 1999).
The result of studied farm groundwater analyzes shows that nitrate values obtained have significant spatial and temporal fluctuations during the study cycle. They increase significantly during May 2007, and then decrease towards the end of the study cycle, an increase of nitrate in groundwater is also early observed. Spatiotemporal distribution of nitrate groundwater studied concentrations is shown in (Fig.13).
Data analysis shows that mean levels of nitrate in all studied wells exceed WHO recommended standards during all campaigns. The mean values vary between 51 mg/l stored at P4 during September 2007 and 145 mg/l recorded in May 2007 at P6.
The studied groundwater have high nitrate levels due to agricultural pollution caused mainly by overuse and not rational nitrogen fertilizer applied to citrus fruit into two fractions the first at the end of March the second in July and August. The intermediate crops nitrogen fertilization applies only once early of August.
The increases nitrate in groundwater observed in early cycle of study at the farm level can be explained by a spreading of manure between the end of October and December.
The aquifer driven mainly by the return of irrigation water coupled to excessive doses of fertilizers applied by farmers can cause important drainages and consequently potential risk of nitric pollution. This risk depends of climate and the year water availability. It is strongly attenuated in dry years.
The nitrogen leaching in the soil as nitrate ion, is easy, especially as the soil is permeable sandy clay and by the low depth of aquifer in the study area.
The present situation of groundwater nitric pollution, as has been demonstrated in this study is dramatic, which therefore necessary to adopt urgently an action plan to prevent pollution, restoration and preservation of the aquifer quality.
2.4. Phosphorous content
Orthophosphate in the soil and groundwater may result from the decomposition of the organic matter and the leaching of phosphorous fertilizer residues. In addition, non-agricultural sources such as detergents may contribute to the presence of orthophosphate in the soil (Bremond and Perrodon, 1979). In soil systems, orthophosphate has a low mobility, as it readily sorbs on Fe and Al oxides (Rodier, 1984; BGS, 1996).
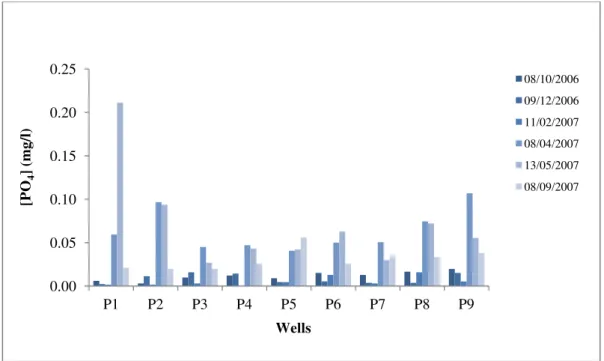
The contamination level in all well water studied for this parameter is generally low and varies between 0 and 0.21 mg/l (Fig.14). This could be explained by the orthophosphate fixation in the soil (Rodier, 1984) and their low mobility in the soil–groundwater system (BGS et al., 1996).
Phosphorus in the farm level studied is brought at one time, usually during March and is often brought as superphosphate (18%), and sometimes triple superphosphate.
Between April 2007 and May 2007, the orthophosphates concentration in groundwater increases probably following the application of phosphate fertilizer, and then decreases during the September. From October 2006 to February 2006 the well water orthophosphate concentrations drop in a very remarkable.
For agricultural use, orthophosphates concentration in groundwater should not exceed 65mg/l (Ministry of Environment, Morocco, 2001). All analyzed groundwater does not exceed a concentration of 0.21 mg/l, so the farm groundwater studies designed for irrigation are long way from posing problems for crops.
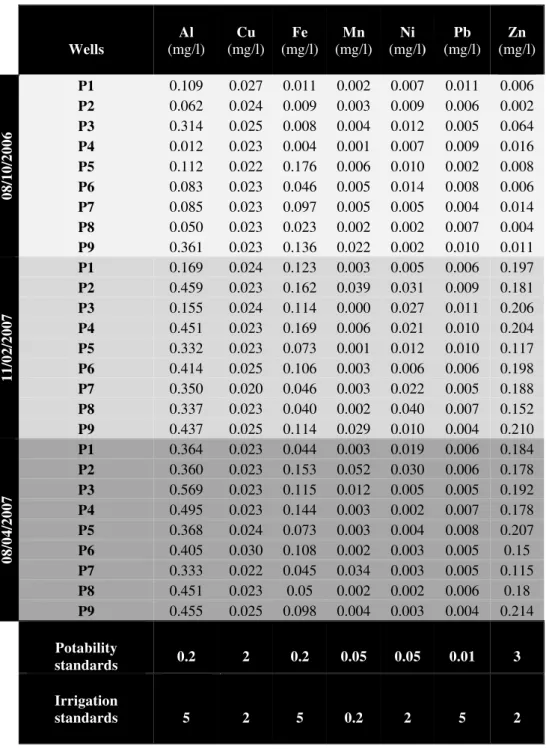
2.5. Heavy metals Status in the farm groundwater
metal levels relatively lower than the WHO potability standards and Moroccan water quality standards for irrigation.
The low heavy metals concentrations in the groundwater can be explained by alkaline pH ranged between 8.60 to 9.59 this alkaline tendency restricts the passage of heavy metals from the solid phase to the soil solution (Paré et al., 1999). Levels of organic matter in the farm soil promotes the fixing of heavy metals in the soil by the formation of insoluble organometallic complexes and therefore decreases their mobility to the aquifer (Paré et al., 1999;) (Udom et al., 2004).
IV-CONCLUSION
The present paper research is a contribution to the physicochemical assessment of groundwater and soil quality, in private farm located in vulnerable zone of agricultural pollution in Triffa plain, physicochemical result reflect a bed quality of groundwater which is generally considered not suitable for drinking and not adaptable for irrigation.
Agricultural practices are potentially responsible of groundwater pollution especially by nitrates. The excessive application of fertilizer, repeated applications of the high doses of manure and pesticides, intensive irrigation and precipitation have all contributed to the increase in the concentration of nitrates in the Triffa groundwater. The heavy metals concentrations are low generally in groundwater and without any toxic effects for humans and crops.
The C/N ratio reveals that 77.41% of soil parcels present a low mineralization rate (C/N< 12).
Of the nine elements analyzed heavy metals (As, Cd, Cr, Cu, Ni, Pb, Zn, Mn, Fe), only the Ni and Pb have concentrations exceeding the permissible maximum values in the farm soils.
To conclude soil quality results in the farm are not alarming contrasting to groundwater quality which is mainly dramatic. In fact groundwater is a receptacle of all applied nutrients on the surface, especially nitrates. Strategies need therefore to be developed to reduce the pressure of irrigated agriculture on groundwater quality. These measures should envisage to control the fertilizer management within the area and hence to implement a more sustainable agriculture. Possible strategies may include the control of the timing and dosage of mineral and organic fertilizer in terms of the crop nutrient demand, the valorisation of harvest residues as organic fertilizer, the modification of crop rotation in particular the use of catch crops or green manuring crops.
REFERENCES
[1] AFES. (1992). Référentiel pédologique 1992. principaux sols d’Europe. INRA Paris. 222p.
[2] AFNOR – ISO. (1992). Projet de norme ISO/CD/11259. Qualité du sol. Description des sols et des sites. Version n°8 de février 1992. [3] Amrani M., Westfall DG., Mughli L. (1999). Evaluation of residual and cumulative phosphorus effects in contrasted Moroccan
calcareous soils. Nutrient Cycling in Agro Ecosystems 55(3): 231–238. doi: 10.1023/A:1009855609746.
[4] Badraoui M., Soudi B., Merzouk A., Farhat A., M’hamdi A. (1998). Changes of soil qualities under irrigation in the Bahira region of Morocco: Salinization. Advances in GeoEcology 31, 503-508.
[5] Baize D. (1997). Teneurs totales en éléments traces métalliques dans les sols. INRA, Paris. [6] Baize D. (2000) Guide des analyses en pédologie. Techniques et pratiques. INRA Editions. 257 p.
[7] Baldock JA., Skjemstad JO. (2000). Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Organic Geochemistry 31: 697–710. doi: 10.1016/S0146–6380(00)00049–8.
[8] Baldwin KR. (2006). Soil Quality Considerations for Organic Farmers. CEFS-USDA. North Carolina Cooperative Extension Service; 14 pp.
[9] Bendra B., Fetouani S., Laffray X., Vanclooster M., Sbaa M., Aleya L. (2012). Effects of irrigation on soil physico-chemistry: a case study of the Triffa plain (Morocco). Irrigation and Drainage. DOI: 0.1002/ird.688.
[10] Benlaghlid M.L. (1995). Analyse du sol et raisonnement de la fertilisation « principes généraux de la fertilisation ». Ecole nationale d’agriculture de Meknès (CNERV). 180p.
[11] Bensaadi A. (2004). Contribution à l'étude de l'état nutritionnel des vergers de pommier dans la région d'Ychemoul., Thèse ing, Agro, Univ Batna., 88 P.
[12] BGS/ODA/PNUE/OMS (1996) - Characterisation and assessment of groundwater quality concerns in Asia-Pacific Region. Doc. PNUE/DEIA/AR.96-1. Nairobi: PNUE.
[13] Bremond R., Perrodon C. (1979). Paramètres de la qualité des eaux. Minist, de l’environnement Paris, 259p.
[14] Campbell C.A., McConkey B.G., Zentner R.P., Selles, F., Curtin, D. (1996). Long-term effects of tillage and crop rotations on soil organic C and total N in a clay soil in southwestern Saskatchewan. Can. J. Soil Sci. 76, 396–401.
[15] Chetouani B., Damou S. (1993). Diagnostic des problèmes d’engorgement des sols, de drainage et de la qualité des eaux et des sols dans la plaine des Triffa (Basse Moulouya). Mémoire de troisième cycle en agronomie. I.A.V Hassan II. Rabat. Volume 1. 171p et volume 2. 127p.
[16] Couture I. (2004). Analyse d’eau pour fin d’irrigation. MAPAQ Montérégie-Est. AGRI-VISION 2003-2004. 8p.
[17] Dakak H. (1996). Etude de la pollution nitrique des eaux souterraines du périmètre de Tadla : vulnérabilité des aquifères et impacts des activités agricoles. Thèse de 3éme cycle, Géologie appliquée à l’environnement. Université Mohammed V, Rabat, Maroc.
[18] De Fraiture C., Wichelns D. ( 2010). Satisfying future water demands for agriculture. Agric Water Manage ; 97 : 502-11.
[19] DGH (Direction Générale de l’Hydraulique). 1997. Etude de l’aquifère superficiel de la plaine de Triffa. Mission I, description et analyse. Rapport définitif, p. 64.
[20] El Mandour A. (1998). Contribution hydrogéologique de la plaine des Triffa : salinisation et modélisation, thèse d’état. Univ.Mohamed Ier, Fac.Sci, Oujda. 206p.
[22] Falkengren-Grerup U., Brunet J., Diekmann M. (1998). Nitrogen mineralization in deciduous forest soils in south Sweden in gradients of soil acidity and deposition. Environmental Pollution 102, 415-420.
[23] FAO. (2000). Land resources: Potential and constraints at regional and country levels. World Soil Resources report n° 90. Rome : FAO, 2000.
[24] FAO. (2003). Les engrais et leurs applications, Précis à l'usage des agents de vulgarisation agricole., Quatrième édition, Editions F.A.O., I.F.A. (Paris, France) et IMPHOS (Casablanca, Maroc)., 84 P.Pare T., Dinel H., Moulin A.P., Townley-Smith L. (1999). Organic matter quality and structural stability of a Black Chernozemic soil under different manure and tillage practices. Geoderma 91. [25] FAO-UNESCO. (1974). Legend of the soi1 map of the world. UNESCO, Paris, p. 59.
[26] Fetouani S., Sbaa M., Vanclooster M., Bendra B. (2008). Assessing ground water quality in the irrigated plain of Triffa (north-east Morocco). Agricultural water management, 95: 133-142.
[27] Forstner U. (1979). Metal pollution assessment from sediment during hydrus analisis.in Forstner U. and whitmann GTW, eds Matalpollution in the aquatic environment Springer-Verlag, New Yorkn pp. 110-196.
[28] Gardner B.L. (1994). Commercial agriculture in metropolitan areas: Economics and regulatory issues. Agricultural and Resource Economics Review, April. 201–209.
[29] Ghassimi F., Jakeman AJ., Nix HA. (1995). Salinization of land and water resources. Canberra(Australia) : University of New South Wales Press Ltd, 1995.
[30] Gimeno-Garcia E., Andreu V., Boluda R. (1996). Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environmental pollution 92:19-25.
[31] Haloui B. (1991). La végétation du Maroc oriental : phytoécologie, phytomasse, minéralomasse et productivité des principaux écosystèmes forestiers. Thèse és-science.Fac Sci. Oujda, 180p.
[32] Hamdaoui F. (1996). Caractérisation actuelle de la qualité des sols et des eaux dans le périmètre irrigué des Doukkala. Mém. 3ème Cycle, Dept. Sci. Du Sol, IAV Hassan II, Rabat.
[33] Hättenschwiler S., Vitousek P.M. (2000). The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends in Ecology & Evolution 15, 238-243.
[34] Heller A., Feldman B.J., Say J., Vreeke, M.S., Tomasco M.F. (1998). Small volume in vitro analyte sensor. PCT Int. Appl. WO9835225 A1 (US Pat. Appl. WO -US2652).
[35] Jackson ML. (1958). Nitrogen determination for soils and plant tissue. In Jackson ML (ed.). Soil Chemical Analysis, Prentice-Hall: New Jersey; 183–204.
[36] Joret G., Hebert J. (1955). Contribution à la détermination du besoin des sols en acide phosphorique. Annals of Agronomy 2: 233–299. [37] Joshi A.B., Vann D.R., Johnson A.H., Miller E.K. (2003). Nitrogen availability and forest productivity along a climosequence on
Whiteface Mountain, New York. Canadian Journal of Forest Research 33, 1880-1891.
[38] Jussy J.H. (1998). Minéralisation de l'azote, nitrification et prélèvement radiculaire dans différents écosystèmes forestiers sur sol acide. Effet de l'essence, du stade de développement du peuplement et de l'usage ancien des sols. In. Université Henri Poincaré, Nancy - I, p. 161.
[39] Kabala C. and Singh B.R. (2001). Fractionnement and mobility of copper, Lead and Zinc in soil profile in vicinity of a copper smelter, J. Environ. Qual. 485-492.
[40] Kabata-Pendias A., Pendias H. (2001). Trace Elements in Soils and Plants. 3rd Edn., CRC Press, Boca Raton, Florida, USA., ISBN-13: 9780849315756, Pages: 413.
[41] Kücke, M., Kleeberg P. (1997). Nitrogen balance and soil nitrogen dynamics in two areas with different soil, climatic and cropping conditions. Europ. J. Agron. 6, 89 – 100.
[42] Lahlou M., Badraoui M. et Soudi. (1998). SMSS : un logiciel du simulation du mouvement de sels dans le sol. Etude et gestion des sols, 4, 5,247-256.
[43] Larson WE., Pierce FJ. (1991). Conservation and enhancement of soil quality. In Evaluation for Sustainable Land Management in the Developing World. Vol. 2: Technical Papers. Dumanski J, Pushparajah E, Latham M, Myers RJK (eds). Proceedings of International Workshop, 15–21 Sept 1991, Chiang Raï, Thailand. International Board for Soil Research and Management: Bangkok; 175–203. [44] Laudelout H., Chiang N.C. (1995). Modélisation du mouvement des sels dans les sols du Maroc. Homme Terre et Eaux, vol.25, 100,
57-61.
[45] Li X.-G., Rengel Z., Mapfumo E., Singh B. (2007). Increase in pH stimulates mineralization of 'native' organic carbon and nitrogen in naturally salt-affected sandy soils. Plant and Soil 290, 269-282.
[46] Martin A., Mariotti A., Balesdent J., Lavelle P., Voattoux R. (1990). Estimate of organic matter turnover rate in a savannah soil by 13 C natural abundance measurements. Soil Biology and Biochemistry 22: 517–523. doi: 10.1016/0038–0717(90)90188–6.
[47] Mehdi B., Madramootoo C. A. (1999). Soil nitrate distribution under grain and silage corn using three tillage practices on a loamy sand in southwestern Quebec. Soil & Tillage Research 51 (1-2), 81-90.
[48] MICÓ C. (2005). Estudio de metales pesados en suelos con cultivos hortícolas de la provincia de Alicante. Doctoral Thesis. University of Valencia, Valencia, Spain.
[49] Ministère de l’environnement-Maroc. (2001). Rapport sur l’état de l’environnement du Maroc, MATUHE, département de l’environnement. 255p.
[50] MRA68. (1999). Les métaux lourds parlons-en! Tabou(e) story 13p.
[51] Nadelhoffer K.J., Giblin A.E., Shaver G.R., Laundre, J.A. (1991). Effects of temperature and substrate quality on element mineralization in six arctic soils. Ecology, 72, 242–253.
[52] Nadelhoffer KJ., Fry B. (1988). Controls on natural nitrogen-15 and carbon-13 abundances in forest soil organic matter. Soil Sci Soc Am J 52:1633-1640.
[53] Naman F., Soudi B., Chiang C. (2001). Impact de l’intensification agricole sur le statut de la matière organique des sols en zones irrigues semi-arides au Maroc. Etude et Gestion des Sols 2001 ; 8 : 269-77.
[54] Nelson DW., Sommers LE. (1982). Total carbon, organic carbon and organic matter. In Page AL, Miller RH, Keeney DR (eds).Methods of Soil Analysis. Part 2. Chemical and Microbiogical Properties, Agronomy Monograph 9, 2nd edn. ASA/CSSA/SSSA: Madison, WI, USA; 539–579.
[55] Northup R.R., Yu Z.S., Dahlgren R.A., Vogt K.A. (1995). Polyphenol control of nitrogen release from pine litter. Nature 377, 227-229.
[56] Olsen SR., Cole C., Watanabe FS., Dean LA. (1954). Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. US Department of Agriculture, Circular 939: 67–96.
[57] Oustani M. (1993).Contribution à l'étude de l'influence certains amendements organiques (palmes de dattier, crottes du dromadaire) sur les propriétés biologiques et chimiques d'un sol salé de la région de Ouargla. Thèse d'Ing. INFSAS. Ouargla. 128p.
[59] Pastor J., Aber J.D., McClaugherty C.A., Melillo J.M. (1984). Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65, 256-268.
[60] Pietri J.C.A., Brookes P.C. (2008). Nitrogen mineralization along a pH gradient of a silty loam UK soil. Soil Biology & Biochemistry 40, 797-802.
[61] Robert M., Juste C. (1997). Stocks et flux d'éléments traces dans les sols du territoire in "Aspects sanitaires et environnementaux de l'épandage agricole des boues d'épuration urbaines, ADEME Journées techniques des 5 et 6 juin 1997", ADEME éd., 320p.
[62] Rodier J. (1984). L’analyse de l’eau, eaux naturelles, eaux résiduaires, eau de mer. 7éme édition, Dunod, Paris, 1353p.
[63] Rosell RA., Gasparoni SC., Galatini SA. (2001). Soil organic matter evaluation. In Lal R, Kimble JM, Follette RF, Stewart BA (eds). Management of Carbon Sequestration in Soils, Advances in Soil Science. CRC Press: Boca Raton, FL; 311–322.
[64] Sample EC., Soper RJ., Racz GJ. (1980). Reactions of phosphate fertilizers in soils. In Khasawneh FE, Sample EC, Kamprath EJ (eds). The Role of Phosphorus in Agriculture, ASA/CSSA/SSSA: Madison, WI, USA; 263–310.
[65] Secrétariat d’état chargé de l’environnement du Maroc /Direction de la Recherche et de la Planification de l'Eau. (2007). Etat de la qualité des ressources en eau du Maroc.
[66] Sierra J (1997). Temperature and soil moisture dependance of N mineralization in intact soil
[67] Soltner D. (2000). Les bases de la production végétale, T I : le sol et son amélioration., 22e Edition, Editions Sciences et techniques agricoles "Le Clos Lorelle"- 49130 Saint-Gemmes-Sur-Loire., 472 P.
[68] Soudi B., Rahoui M., Chiang C., Badraoui M., Aboussaleh A. (1999). Eléments méthodologiques de mise en place d'un système de suivi et de surveillance de la qualité des eaux et des sols dans les périmètres irrigués. Hommes Terre et eaux, Vol. 29, n°111, juin 1999, pp. 13-22.
[69] Soudi, B. et al. (1995). Mise en place des réseaux de suivi de la nappe phréatique et de la qualité des sols et des eaux du périmètre de Tadla Rapport M.R.T N° 608-0213-3-20014.
[70] Stevenson D.S. et Nelson G.H. (1990) – Nitrogen additiony and loses to drainage in orchard-type irrigated lysimeters. Can. J. Soil sci.70.11.19.
[71] Subbarao G.V., Rondon M., Ito O., Ishikawa T., Rao I.M., Nakahar K., Lascano C., Berry W.L. (2007). Biological nitrification inhibition (BNI) - is it a widespread phenomenon? Plant and Soil 294, 5-18.
[72] Tam N.F.Y. and Wong Y.S. (1999). Mangrove soil in remouving pollutants frommunicipal wastewater of different salinities. J. Environ. Qual, 28 556-564.
[73] Thornton I. (1996). Risk assessment related to metals: the role of the geochemist. Report of the International Workshop on Risk Assessment of Metals and their Inorganic Compounds, Angers, France, November 1996. International Council on Metals and the Environment.
[74] Truog E. (1930). Determination of readily available phosphorus of soils. American Society of Agronomy 22: 874–882.
[75] Udom B.E., Mbagwu J.S.C., Adesodun J.K., Agbin N.N. (2004). Distribution of Zn, Cd, Cu and Pb in a tropical Ultisol after long term disposal of sewage sludge. Environ. Int., 30: 467-470.
[76] Van Cleve K., Yarie J., Erickson R., Dyrness C.T. (1993). Nitrogen mineralization and nitrification in successional ecosystems on the Tanana River floodplain, interior Alaska. Canadian Journal of Forest Research 23, 970-978.
[77] Vervaet H., Massart B., Boeckx P. (2002). Use of principal component analysis to assess factors controlling net N mineralization in deciduous and coniferous forest soils. Biology and Fertility of Soils 36, 93-101.
[78] Webster C.P., Bedford R.K., Cannell R.P. (1999). Crop uptake and leaching losses of labelled fertilizer nitrogen in relation to waterlogging of clay and sandy soils. Plant Soil, 92, 89-110.
[79] WHO. (2006). Directives de la qualité pour l’eau de boisson, troisième édition, volume 1, recommandation, (3éme éd). Genève. [80] WHO. (2000). Global Water Supply and Sanitation Assessment 2000 report. OMS, UNICEF, p. 77.
[81] Zolle, I. (1994) .Non-ionic surfactants in reused water: are actived sludge/soil aquifer treatements sufficient. Wat. Res. 28 (7), pp : 1625-1629.
Fig.4. Map of soil use at the studied farm level
Fig.6. Groundwater sample locations within the studied farm
Fig.8. Spatial distribution of the soil organic matter (SOM)
Fig.10. Spatial distribution of the soil orthophosphate (P-PO4)
Fig.11. pH water wells variations
0 1 2 3 4 5 6 7 8 9
P1 P2 P3 P4 P5 P6 P7 P8 P9
pH
Wells
Fig.12. Variation of the organic matter water wells concentration [OM]
Fig.13.Variation of the nitrate water wells concentration [NO3]
0.00 0.50 1.00 1.50 2.00 2.50
1 2 3 4 5 6 7 8 9
[0M] (mg/l)
Wells
08/10/2006
09/12/2006
11/02/2007
08/04/2007
13/05/2007
08/09/2007
0 20 40 60 80 100 120 140 160
P1 P2 P3 P4 P5 P6 P7 P8 P9
[NO
3
] (mg/l)
Wells
Fig.14. Variation of the orthophosphate water wells concentration [P-PO4]
Tab. I. Heavy metals soil samples of the farm
Tab. II. Comparison of the trace metals levels in Triffa soil farm with French, Spanish and Haut-Rhin soil
0.00 0.05 0.10 0.15 0.20 0.25
P1 P2 P3 P4 P5 P6 P7 P8 P9
[PO 4 ] (mg/l) Wells 08/10/2006 09/12/2006 11/02/2007 08/04/2007 13/05/2007 08/09/2007
Number of values
mg.Kg-1 of dry matter
Cr (40) Cd (40) Cu (40) Ni (40) Pb (40) Zn (40) As (40) Fe (40) Mn (40)
Minimum 54.28 0.41 21.08 22.95 43.33 0.01 19.81 19100 600
Median 69.78 0.64 37.05 35.50 81.24 0.02 35.93 32900 1200
Maximum 85.60 0.98 57.22 308.48 107.55 4.48 44.11 39100 1700
Standards of agricultural soils - Kabata-Pendias and Pendias
(2001)
100 5 100 100 100 300 50 - -
number of values > Standards of agricultural soils -
Kabata-Pendias and Kabata-Pendias (2001) 0 0 0 12 4 0 0 - -
Number of values
Cr (40) Cd (40) Cu (40) Ni (40) Pb (40) Zn (40) As (40) Fe (40) Mn (40)
Our study 69.78 0.64 37.05 35.50 81.24 0.02 35.93 32900 1200
French soils 10-90 0.05-0.45 2-20 2-60 9-50 10-100 - - -
Spanish soils 28.3 0.38 21.6 23.7 19.6 57.8 - 15.27 320
Tab. III. Heavy metals groundwater concentrations of the farm. Water potability and irrigation standards (WHO, 2006) (Secretary of State for the Environment of Morocco, 2007)
Wells
Al
(mg/l) (mg/l) Cu (mg/l) Fe (mg/l) Mn (mg/lNi ) Pb
(mg/l) (mg/l) Zn
0
8
/1
0
/2
006
P1 0.109 0.027 0.011 0.002 0.007 0.011 0.006 P2 0.062 0.024 0.009 0.003 0.009 0.006 0.002 P3 0.314 0.025 0.008 0.004 0.012 0.005 0.064 P4 0.012 0.023 0.004 0.001 0.007 0.009 0.016 P5 0.112 0.022 0.176 0.006 0.010 0.002 0.008 P6 0.083 0.023 0.046 0.005 0.014 0.008 0.006 P7 0.085 0.023 0.097 0.005 0.005 0.004 0.014 P8 0.050 0.023 0.023 0.002 0.002 0.007 0.004 P9 0.361 0.023 0.136 0.022 0.002 0.010 0.011
1
1
/0
2
/2
007
P1 0.169 0.024 0.123 0.003 0.005 0.006 0.197 P2 0.459 0.023 0.162 0.039 0.031 0.009 0.181 P3 0.155 0.024 0.114 0.000 0.027 0.011 0.206 P4 0.451 0.023 0.169 0.006 0.021 0.010 0.204 P5 0.332 0.023 0.073 0.001 0.012 0.010 0.117 P6 0.414 0.025 0.106 0.003 0.006 0.006 0.198 P7 0.350 0.020 0.046 0.003 0.022 0.005 0.188 P8 0.337 0.023 0.040 0.002 0.040 0.007 0.152 P9 0.437 0.025 0.114 0.029 0.010 0.004 0.210
0
8
/0
4
/2
007
P1 0.364 0.023 0.044 0.003 0.019 0.006 0.184 P2 0.360 0.023 0.153 0.052 0.030 0.006 0.178 P3 0.569 0.023 0.115 0.012 0.005 0.005 0.192 P4 0.495 0.023 0.144 0.003 0.002 0.007 0.178 P5 0.368 0.024 0.073 0.003 0.004 0.008 0.207 P6 0.405 0.030 0.108 0.002 0.003 0.005 0.15 P7 0.333 0.022 0.045 0.034 0.003 0.005 0.115
P8 0.451 0.023 0.05 0.002 0.002 0.006 0.18
P9 0.455 0.025 0.098 0.004 0.003 0.004 0.214
Potability
standards 0.2 2 0.2 0.05 0.05 0.01 3
Irrigation